Derrone Inhibits Platelet Aggregation, Granule Secretion, Thromboxane A2 Generation, and Clot Retraction: An In Vitro Study
Abstract
Cudrania tricuspidata (C. tricuspidata) is widespread throughout East Asia and in China and Korea, and it is widely used as a traditional remedy against eczema, mumps, and tuberculosis. With regard to the aforementioned medical efficacy, various studies are continuously being conducted, and it has been reported that C. tricuspidata extract has various actions against inflammation, diabetes, obesity, and tumors. Therefore, we evaluated antiplatelet effects using derrone in C. tricuspidata. We examined the effect of derrone on the phosphorylation of vasodilator-stimulated phosphoprotein (VASP) and inositol 1, 4, 5-triphosphate receptor I (IP3RI), and on the dephosphorylation of cytosolic phospholipase A2 (cPLA2), mitogen-activated protein kinases p38 (p38MAPK), and Akt, which affects platelet function and thrombus formation. Various agonists-induced human platelets were inhibited by derrone without cytotoxicity, and it also decreased the intracellular calcium level through the signaling molecule phosphorylations. In addition, derrone inhibited glycoprotein IIb/IIIa (αIIb/β3) affinity. Thus, in the present study, derrone suppressed human platelet aggregation and thrombin-induced clot formation.
1. Introduction
In normal circulation of blood, platelets are necessary for hemostasis and thrombosis. [1]. The cardiovascular diseases (CVDs) are becoming a critical threat to our lives in these years. It is now widely accepted that platelets play an important role in cardiovascular disease as they have a fundamental role in thrombosis. Therefore, many drugs or natural substances have been developed to treat CVDs. [2, 3]. Inhibition of platelets has influence on the CVDs; however, the survival rate is still low. Thus, discovery of various substances is required [4]. In addition, there is an ever-increasing interest toward different traditional medicines (mainly, herbal remedies) in different diseases, including CVDs [5–7]. Various ginsenosides have been used as a traditional herbal medicine against several diseases, especially CVDs [8]. Reviewing these options could enable scientists to examine and then introduce novel efficacious medicines.
In normal circulation, synthesized cyclic AMP (cAMP) and cyclic GMP (cGMP) in platelets downregulate platelet function through cAMP/cGMP-dependent kinases, protein kinase A (PKA), and protein kinase G (PKG) [9]. Vasodilator-stimulated phosphoprotein (VASP) contributes to αIIb/β3 activation, but its phosphorylation suppressed its activity [10, 11]. Moreover, PKA and PKG can phosphorylate IP3RI [12], and its phosphorylation suppresses [Ca2+]i mobilization [13, 14].
Regarding the effects of Cudrania tricuspidata (C. tricuspidata) extracts for improving blood circulation, research has reported that the C. tricuspidata extract has antiplatelet effects on rat platelet aggregation [15]. Therefore, in this study, we evaluate the potential efficacy of derrone from unripe fruits of C. tricuspidata.
2. Materials and Methods
2.1. Reagents
Derrone was purchased from ChemFaces (Wuhan, China). Collagen was purchased from Chrono-Log Co. (Havertown, PA, USA). Fura 2-AM (2-acetoxymethyl) and Alexa Fluor 488-conjugated fibrinogen were obtained from Invitrogen (Eugene, OR, USA). Serotonin ELISA kit was purchased from Labor Diagnostika Nord GmbH and Co. (Nordhorn, Germany). Bicinchoninic acid protein assay kit was purchased from Pierce Biotechnology (IL, USA). Cayman chemical (Ann Arbor, MI, USA) offered thromboxane B2 assay kit, cAMP, cGMP enzyme immunoassay kit, and U46619. Cell Signaling (Beverly, MA, USA) supplied all antibodies. The adhesion kit (fibronectin coated) was obtained from Cell Biolabs (San Diego, CA, USA).
2.2. Human Platelets Suspension
Korean Red Cross Blood Center (Suwon, Korea) supplied human platelet-rich plasma (PRP) for research, and study protocols were approved by the Public Institutional Review Board at the National Institute for Bioethics Policy (PIRB-P01-201812-31-007, Seoul, Republic of Korea). The platelets in suspension were adjusted to 5 × 108/mL concentration according to the previous research [16, 17].
2.3. Platelet Aggregation Analysis
For in vitro platelet aggregation, platelets suspension (108/mL) was preincubated with derrone (15 to 60 μM) at 37°C for 2 min, and then agonists were added for stimulation. Derrone was dissolved in 0.1% dimethyl sulfoxide. Platelet aggregation was measured for 5 minutes. The change in light transmission is calculated into the aggregation rate (%). Platelet aggregation was conducted using an aggregometer under stirring condition (Chrono-Log, Havertown, PA, USA).
2.4. Cytotoxicity Analysis
Cytotoxicity of derrone was conducted through lactate dehydrogenase leakage assay. Human platelets (108/mL) was incubated with derrone (15 to 60 μM) for 1 hour and centrifuged at 12,000 g. The supernatant was used to detect the lactate dehydrogenase using ELISA reader (TECAN, Salzburg, Austria).
2.5. Intracellular Calcium Level
The Fura 2-AM loaded platelet suspension was preincubated with derrone (15 to 60 μM) at 37°C for 2 min. After incubation, we added collagen (2.5 μg/mL) for calcium release from endoplasmic reticulum in platelets. The calcium mobilization was measured using a spectrofluorometer (Hitachi F-2700, Tokyo, Japan), and Grynkiewicz method was used for calculating the [Ca2+]i concentration [18].
2.6. Detection of Thromboxane B2
Because synthesized thromboxane A2 (TXA2) is converted into thromboxane B2 (TXB2) quickly, the TXA2 generation was measured by detecting TXB2. Platelets suspension (108/mL) was preincubated with derrone (15 to 60 μM) at 37°C for 2 min, and then collagen (2.5 μg/mL) was added for stimulation. The reaction was stopped by adding indomethacin (0.2 mM) in EDTA solution (5 mM). The TXB2 was detected using ELISA reader (TECAN, Salzburg, Austria).
2.7. Detection of Serotonin
Platelet aggregation was conducted for 7 min at 37°C with derrone (15 to 60 μM), and then reaction cuvette was placed onto ice in order to terminate serotonin release for 3 min. After termination, the reaction mixture was centrifuged, and the supernatant was used. The serotonin was detected using ELISA reader (TECAN, Salzburg, Austria).
2.8. Immunoblotting
After platelet aggregation, platelets are dissolved using lysis buffer. The amount of dissolved protein was calculated, and proteins (15 μg) were divided by 8% SDS-PAGE. After electrophoresis, proteins are transferred onto membranes and treated primary (1 : 1,000) and secondary antibodies (1 : 10,000). Western blotting analysis was conducted by Quantity One program (BioRad, Hercules, CA, USA).
2.9. Fibrinogen Binding to αIIbβ3
After platelet aggregation for 7 min, the reaction mixture was incubated with fibrinogen (Alexa flour 488-conjugated) for 5 mins. After incubation, 0.5% paraformaldehyde in PBS was added to fix the binding between platelet integrin and fibrinogen marker. All procedures of fibrinogen binding assay were conducted in the dark condition. The flow cytometry measures the binding (BD Biosciences, San Jose, CA, USA).
2.10. Fibronectin Adhesion
Human platelet suspension (108/mL) was placed in fibronectin coated wells (bovine serum albumin coated well is used as a negative control) and preincubated with derrone (15 to 60 μM) and collagen (2.5 μg/mL) for 1 h at 37°C. After incubation, wells were washed using PBS buffer and added cell stain solution for 10 min. After that, extraction solution was added, and each extraction was measured by ELISA reader (TECAN, Salzburg, Austria).
2.11. Fibrin Clot Retraction
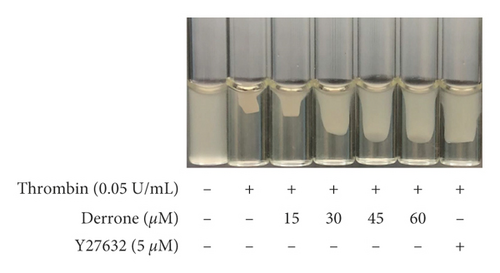
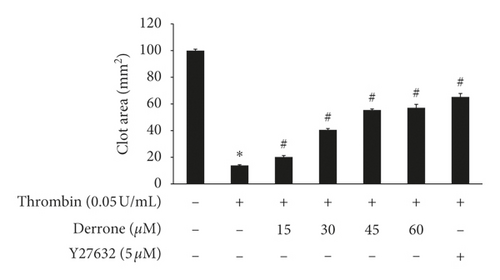
Human platelet-rich plasma (300 μL) was incubated with derrone (15 to 60 μM) for 30 min at 37°C, and thrombin (0.05 U/mL) triggers the clot retraction. After reacting for 15 min, pictures of fibrin clot were taken using a digital camera. ImageJ (v1. 46) was used to convert to clot area (National Institutes of Health, USA).
2.12. Statistical Analyses
All data are presented as the mean ± standard deviation with various numbers of observations. To determine major differences among groups, analysis of variance was performed, followed by the Tukey–Kramer method. SPSS 21.0.0.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis, and p < 0.05 was considered statistically significant.
3. Results
3.1. Inhibitory Action of Derrone on Platelet Aggregation and Cytotoxicity
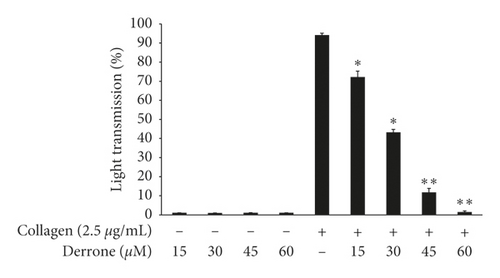
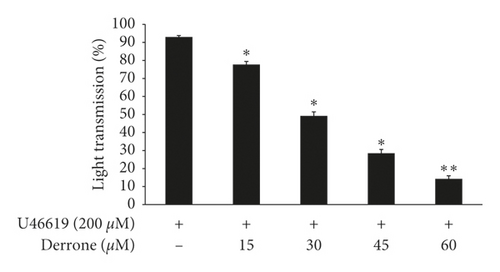
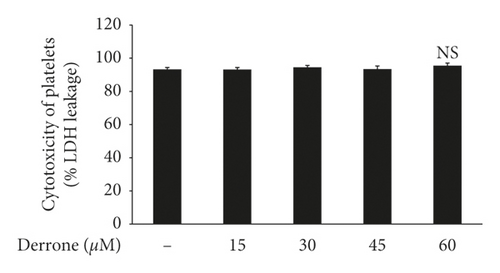
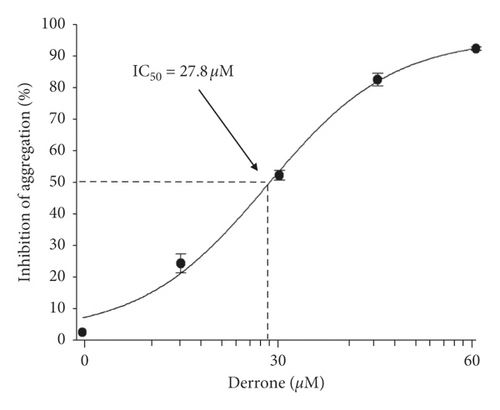
To evaluate the antiplatelet effects of derrone, Figure 1 we used agonists: U46619, collagen, and thrombin. Collagen (2.5 μg/mL), thrombin (0.05 U/mL), and U46619 (200 nM) were used for the full aggregation of human platelets (Figures 2(a)–2(c)). However, collagen-induced platelets treated with derrone (15, 30, 45, and 60 μM) were the most strongly suppressed (23.3%, 54.1%, 87.5%, and 98.4%) (Figure 2(a)) without cytotoxicity (Figure 2(d)), and the half maximal inhibitory concentration (IC50) was 27.8 μM (Figure 2(e)). DMSO 0.1% seemed to have no effect on platelet aggregation [19].
3.2. Inhibitory Action of Derrone on Intracellular Calcium Concentration, IP3RI Phosphorylation, Serotonin Secretion, and ERK Dephosphorylation
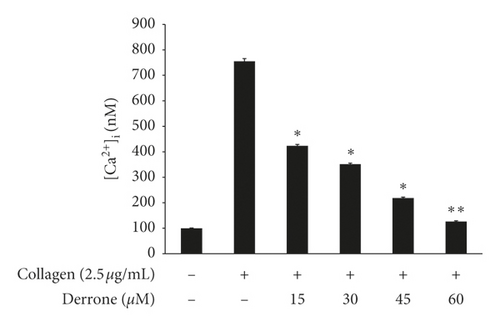
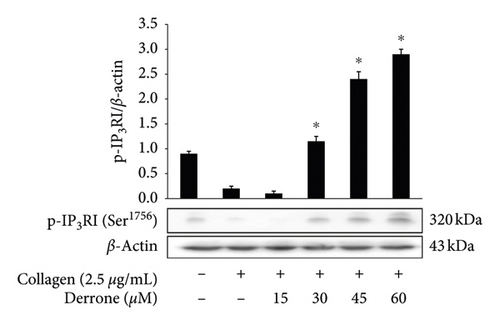
As shown in Figure 3(a), the intracellular calcium level ([Ca2+]i) was elevated from 100.5 ± 0.4 nM to 755.5 ± 10.2 nM by collagen (2.5 μg/mL). However, derrone (15 to 60 μM) reduced the collagen-increased [Ca2+]i levels (Figure 3(a)). Next, we investigated the phosphorylation of calcium-mobilization signaling molecules. As shown in Figure 3(b), derrone (15 to 60 μM) increased inositol 1, 4, 5-triphosphate receptor type I (IP3RI) phosphorylation. This shows that derrone decreased [Ca2+]i levels through phosphorylation of IP3RI. In addition, we explored whether derrone is involved in the inhibition of dense granule secretion. Serotonin is not synthesized in megakaryocytes or platelets, but is taken up from plasma and stored in dense granules in platelets. Upon stimulation by agonists, high [Ca2+]i levels facilitate the myosin light chain and pleckstrin phosphorylation to trigger δ-granule and α-granule release, and the serotonin released by exocytosis acts as a platelet agonist on the 5-hydroxytryptamine 2 (5-HT2) receptor on platelets [20]. We evaluated δ-granule release, and, as shown in Figure 3(c), derrone (15 to 60 μM) dose-dependently inhibited collagen-stimulated serotonin secretion. It is known that ERK phosphorylation is involved in influx from extracellular Ca2+ [21]; therefore, we tested whether derrone is involved in ERK phosphorylation. As shown in Figure 3(d), collagen potently phosphorylated ERK2 (42 kDa) as compared to unstimulated platelets. However, derrone (15–60 μM) inhibited collagen-induced phosphorylation of ERK2 (42 kDa).
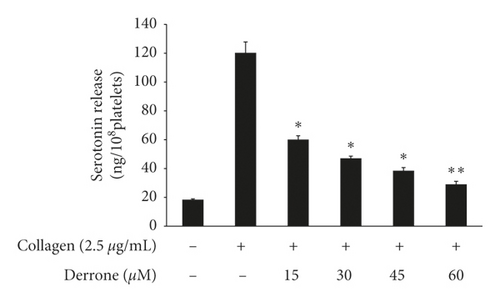
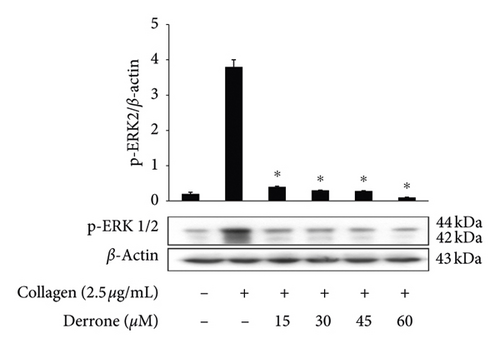
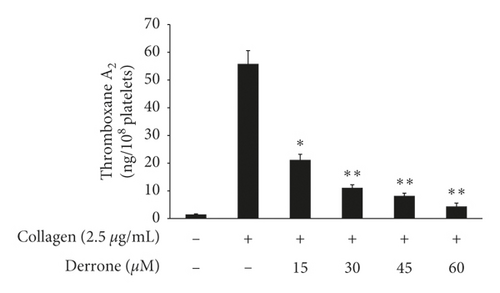
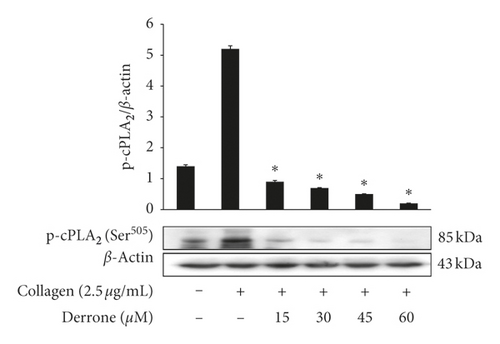
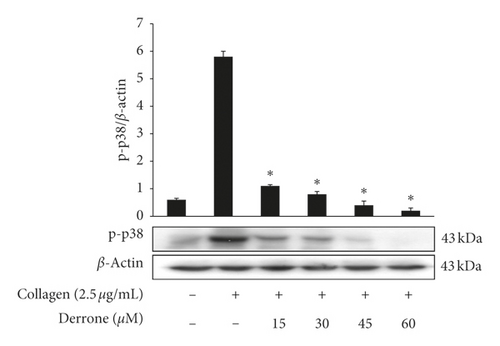
3.3. Inhibitory Action of TXB2, cPLA2, and p38MAPK-Dephosphorylation
As shown in Figure 4(a), collagen (2.5 μg/mL) increased the TXA2 generation to 55.8 ± 4.8 ng/108 platelets from 1.5 ± 0.2 ng/108 platelets. However, derrone inhibited TXA2 production (Figure 4(a)). To identify the inhibitory action of derrone on TXA2 generation, associated signaling molecules, cPLA2, and mitogen-activated protein kinase p38 (p38MAPK), were investigated. The cPLA2 has been reported to hydrolyze arachidonic acid from membrane of platelets, and p38MAPK activates cPLA2 to act as an enzyme. As shown in Figures 4(b) and 4(c), derrone suppressed cPLA2 and p38MAPK phosphorylation in a dose-dependent manner.
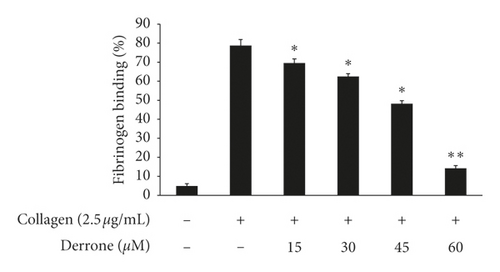
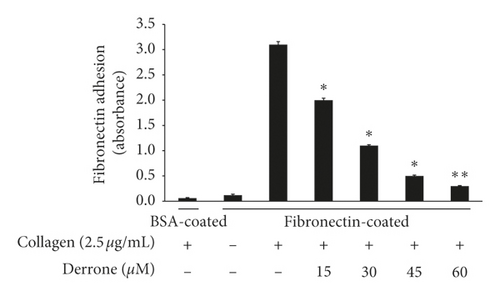
3.4. Inhibitory Action of Derrone on Fibrinogen Binding and Fibronectin Adhesion
Collagen elevated αIIb/β3 affinity, the binding of fibrinogen to αIIb/β3 is increased (Figures 5(a) and 5(b)), and the binding rate is 78.8 ± 3.1%. However, derrone attenuated fibrinogen interaction with αIIb/β3 significantly (Figures 5(a) and 5(b)). αIIb/β3 can bind to fibronectin, which is essential for platelet adhesion and spreading. Thus, we examined whether derrone-treated platelets can adhere to fibronectin-coated well. As shown in Figure 5(c), derrone suppressed fibronectin adhesion.
3.5. Inhibitory Action of Derrone on VASP and Cyclic Nucleotides
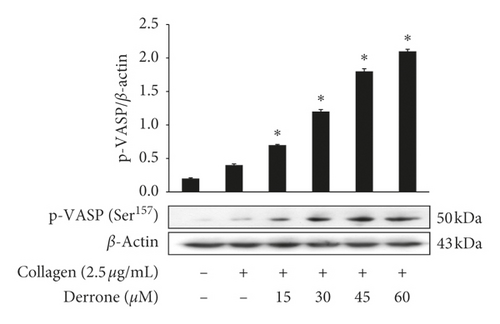
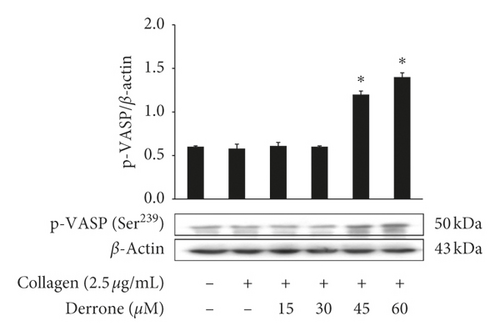
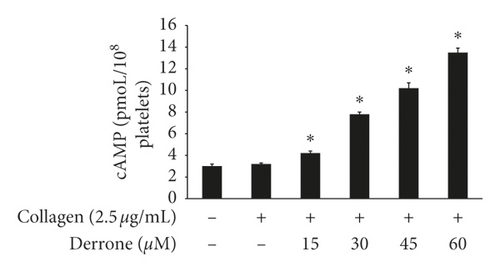
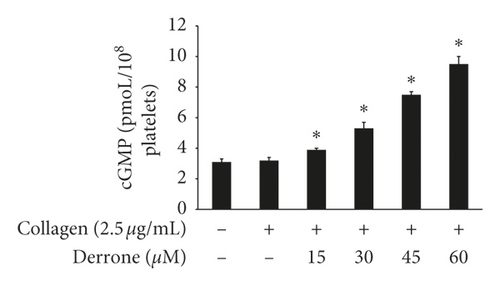
Vasodilator-stimulated phosphoprotein (VASP) regulates actin, but its phosphorylation by kinases inhibits αIIb/β3 affinity [10, 11]. As derrone inhibited collagen-induced αIIb/β3 affinity (Figures 5(a) and 5(c)), we investigated the effect of derrone on VASP phosphorylation. Derrone significantly upregulated VASP phosphorylation at Ser157 and VASP at Ser239 (Figures 6(a) and 6(b)). Next, we investigated whether derrone regulates cAMP and cGMP production in human platelets. As shown in Figures 6(c) and 6(d), derrone significantly increased cAMP and cGMP concentration.
3.6. Inhibitory Action of Derrone on Thrombin-Induced Clot Retraction
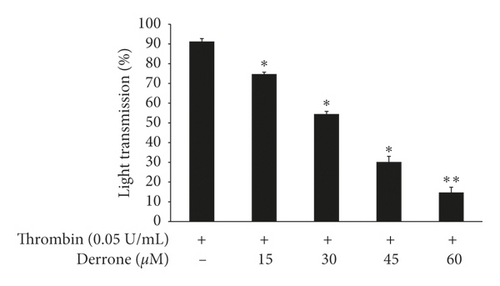
Because derrone inhibited αIIb/β3 in the previous experiment, we examined whether derrone affects fibrin clot retraction using PRP. As shown in Figure 7(a), thrombin stimulated fibrin clot, and the clot was contracted over time. The inhibition rate was 86.1%. However, derrone (15 to 60 μM) effectively delayed the thrombin-stimulated fibrin clot, with inhibitory degrees of 79.8%, 59.4%, 44.5%, 42.8%, and 34.7%, respectively (Figure 7(b)).
4. Discussion
C. tricuspidata is a perennial plant of the Moraceae family, and its roots, leaves, bark, stems, and fruits contain various physiological substances. Among the phytochemicals, xanthones and flavonoids are the major constituents of C. tricuspidata and have antiobesity, antidiabetic, and antitumor effects [22]. A study involving platelets, cudratricusxanthone A from C. tricuspidata extract has antiplatelet activity in thrombin-induced mouse platelets and anticoagulation activity [23]. Because the C. tricuspidata extract showed antiplatelet effects, we recently searched for a new candidate and confirmed that derrone has antiplatelet effects.
We examined whether derrone affects collagen-stimulated platelet activities and associated signaling molecules. Derrone suppressed [Ca2+]i levels (Figure 3(a)) through phosphorylation of IP3RI. In addition, derrone inhibited serotonin release (Figure 3(c)), and ERK2 phosphorylation affected calcium influx (Figure 3(d)). Next, we confirmed that derrone suppressed TXA2 generation (Figure 4(a)). TXA2 is released from platelets and acts a strong agonist that affects platelet-mediated hemostasis and thrombosis. Signaling molecules involved in the production of TXA2 include cPLA2 and p38MAPK. p38MAPK phosphorylates cPLA2 for full catalytic activity, and cPLA2 binds with Ca2+ [24, 25]. Derrone inhibited the phosphorylation of p38MAPK and cPLA2 (Figures 4(b) and 4(c)), which influenced TXA2 generation (Figure 4(a)).
On the platelet surface, αIIb/β3 is the most abundant receptor and is an important binding and adhesion molecule for fibrin-platelet mesh construction and platelet-monocyte interaction. The activation of αIIb/β3 on the platelet membrane leads to its rapid conformational change, allowing binding to adhesive molecules. Derrone suppressed αIIb/β3 affinity, leading to binding and adhesion (Figures 5(a)–5(c)) by upregulating the phosphorylation of VASP (Figures 6(a) and 6(b)) and elevating cAMP and cGMP levels (Figures 6(c) and 6(d)). Moreover, inside-out signaling pathway activates αIIb/β3 and leads to clot retraction. In our experiment, derrone suppressed αIIb/β3 affinity, and clot retraction (Figure 7(a)), through VASP phosphorylation (Ser157 and Ser239). These data indicate that regulation of signaling molecules such as IP3RI (Ser1756) and VASP (Ser157, Ser239) influences the clot retraction delay.
According to the results of studies on the antiplatelet effects of C. tricuspidata, administration of extract of C. tricuspidate (50 and 100 mg/kg) reduced hyperaggregated platelet aggregation without any hepatotoxicity in high-fat diet- (HFD-) fed rats. Moreover, a decrease in thromboxane A2 production was observed in vivo [15]. In addition, we confirmed the antiplatelet effects of cudraxanthone L, euchrestaflavanone A, and cudraxanthone B [26–28]. Their inhibitory mechanism was similar to that of derrone, but cudraxanthone L and euchrestaflavanone A increased only the cAMP levels.
Our study had some limitations, in that it was conducted in vitro and did not confirm the antiplatelet effect in vivo. Although the experiment was conducted using a low concentration (15–45 μM) of derrone in vitro, it is difficult to reach this concentration in blood by ingestion. Moreover, since our study is not an in vivo study using the human body, it is difficult to prove its effect in the human body; thus, we cannot prove that these effects would be the same in people with high and low platelet counts or cardiovascular disease. However, based on the in vitro effect, we suggest that derrone has the potential to inhibit thrombosis-mediated CVDs.
In conclusion, we confirmed that derrone suppressed collagen-stimulated platelet aggregation through downregulation of intracellular calcium concentration, αIIb/β3 affinity, and clot retraction, which were achieved by the regulation of IP3RI (Ser1756), VASP (Ser157 and Ser239), cPLA2 (Ser505), and p38MAPK. In addition, derrone increases cAMP and cGMP levels in human platelets. These two cyclic nucleotides are key mediators of the antiplatelet effects. Thus, we confirmed that derrone could be a potential phytochemical for the prevention of platelet-mediated illnesses.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was supported by a grant (2018R1C1B5083580) from the Basic Science Research Program via the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology, Korea.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




