Tournefortia sarmentosa Inhibits the Hydrogen Peroxide-Induced Death of H9c2 Cardiomyocytes
Abstract
Tournefortia sarmentosa is a traditional Chinese medicine used to reduce tissue swelling, to exert the antioxidant effect, and to detoxify tissue. T. sarmentosa is also used to promote development in children and treat heart dysfunction. However, many of the mechanisms underlying the effects of T. sarmentosa in the treatment of disease remain unexplored. In this study, we investigated the antioxidant effect of T. sarmentosa on rat H9c2 cardiomyocytes treated with hydrogen peroxide (H2O2). T. sarmentosa reduced the cell death induced by H2O2. T. sarmentosa inhibited H2O2-induced changes in cell morphology, activation of cell death-related caspases, and production of reactive oxygen species. In addition, we further analyzed the potential active components of T. sarmentosa and found that the compounds present in the T. sarmentosa extract, including caffeic acid, rosmarinic acid, salvianolic acid A, and salvianolic acid B, exert effects similar to those of the T. sarmentosa extract in inhibiting H2O2-induced H9c2 cell death. Therefore, according to the results of this study, the ability of the T. sarmentosa extract to treat heart disease may be related to its antioxidant activity and its ability to reduce the cellular damage caused by free radicals.
1. Introduction
Oxidative stress caused by free radicals is associated with many cardiovascular diseases, including ischemic heart disease, atherosclerosis, hypertension, and cardiomyopathies [1–4]. Free radicals are generated in the human body during the process of energy production. When the body suffers from inflammation, such as inflammation elicited by infection or injury, many free radicals are produced to remove foreign substances [5]. However, if the host does not have a sufficient protective mechanism to remove excess free radicals after infection, these free radicals will cause cell damage (including lipid peroxidation of the cell membrane and DNA damage) [6, 7]. In addition, environmental factors such as ultraviolet radiation, chemical drugs, and even excessive pressure result in a substantial increase in the production of free radicals in the body [8]. When cardiac muscle cells are attacked by free radicals, the free radicals-induced injury can damage nuclear DNA and lead to permanent DNA damage. The lipids in the cell membrane can be oxidized, altering cell membrane fluidity, preventing nutrients from entering the cell, and eventually causing necrosis. Free radicals attack the side chains of amino acids, causing proteins to lose their function and disrupting the normal functions of cells [5].
Several antioxidant enzymes in the human body, such as superoxide dismutase, glutathione peroxidase, or catalase, counteract free radicals. These enzymes quickly convert the free radicals produced by the body into less toxic or nontoxic substances through redox reactions [9, 10]. However, when the activity of such antioxidant enzymes is insufficient due to insufficient intake of cofactors (superoxide dismutase requires metal ions, such as Cu2+ or Zn2+) or differences in genetic background, cells are unable to avoid free radicals attack and subsequent damage [11]. When a tissue is damaged and dies, it can be restored by the proliferation of stem cells present in the tissue in order to maintain the normal operation of the organ or tissue. However, the existence of cardiac stem cells remains controversial [12]. Therefore, methods to prevent cardiomyocyte damage by free radicals or to reduce cells damage caused by the oxidative pressure generated by free radicals are important topics at present.
Tournefortia sarmentosa is often mentioned in reports that evaluate its antioxidant activity in Chinese medicine [13, 14]. T. sarmentosa also exerts the effects of relieving wind, detoxifying, reducing swelling, treating aching muscles and bones, and promoting childhood development [15–17]. In addition to being used as a specific therapeutic drug in traditional Chinese medicine, T. sarmentosa has recently been promoted as a health food. However, although several studies have investigated the antioxidant effect of T. sarmentosa, researchers have not determined whether T. sarmentosa protects cardiomyocytes from oxidative stress. In this study, we investigated the protective effect of T. sarmentosa on hydrogen peroxide- (H2O2-) induced myocardial cell death. In addition to the protective effect of T. sarmentosa on cardiomyocytes, we also analyzed the potential active components of T. sarmentosa that protect against cardiomyocyte death.
2. Materials and Methods
2.1. Preparation of an Aqueous Extract of T. sarmentosa and Reagents
The preparation of the aqueous extract of T. sarmentosa was described in a previous study [14]. The various components of the T. sarmentosa extract, including caffeic acid, rosmarinic acid, salvianolic acid A, and salvianolic acid B, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cell Culture
Rat H9c2 (2-1) cardiomyocytes were purchased from Bioresource Collection and Research Center (Hsinchu city, Taiwan). The cells were cultured in 90% Dulbecco’s modified Eagle’s medium (DMEM) containing 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum at 37°C in a 5% carbon dioxide environment. During cell culture, mycoplasma contamination was regularly evaluated, and mycoplasma contamination was not detected.
2.3. Detection of Cell Viability and Cell Death
WST-1 reagent (Roche Diagnostics, Mannheim, Germany) and a cytotoxicity detection kit (Roche Diagnostics) were used to measure cell viability and cell death, respectively [18]. H9c2 cells were plated in a 6-well plate in triplicate at a density of 105 cells/well and cultured overnight. The medium was replaced with serum-free DMEM, and various doses of T. sarmentosa or 100 μm solutions of the components present in T. sarmentosa were added and incubated for another 24 h. After incubation with WST-1 for 3 h and 100 μM H2O2 for another 1 h, the supernatants were collected, and the absorbance values were measured at 450 nm and 650 nm using a multifunctional microplate instrument (Infinite F200, Tecan, Durham, NC, USA). Alternatively, cells growing in the serum-free medium were cultured with various concentrations of T. sarmentosa or 100 μm solutions of the components present in T. sarmentosa for 24 h, followed by incubation with 100 μM H2O2 for 1 h. The samples were then centrifuged, and the supernatant of each sample was collected. The amounts of lactate dehydrogenase present in the supernatants were detected with a cytotoxicity detection kit.
2.4. Western Blotting
H9c2 cells were plated in a 10 cm dish at a density of 106 cells/dish and cultured overnight. After the cells were cultured in the serum-free medium supplemented with various doses of T. sarmentosa or 100 μm components present in T. sarmentosa for 24 h, the cells were treated with 100 μM H2O2 for 1 h, and the cell extracts were collected with mammalian protein extraction buffer (GE Healthcare Bio-Sciences, MJ, USA). The expression of protein in each sample was analyzed using Western blotting, as described in the previous literature [19]. Caspase-8, caspase-9, and caspase-3 antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). The actin antibody was purchased from Sigma-Aldrich.
2.5. Reactive Oxygen Species (ROS) Measurement
Dihydrorhodamine 123 (DHR123) dye was used to detect ROS levels in cells as previously described [14]. Briefly, H9c2 cells were plated in a 10 cm dish at a density of 106 cells/dish and cultured overnight. After the cells were cultured in serum-free medium supplemented with various doses of T. sarmentosa or 100 μm components present in T. sarmentosa for 24 h, the cells were treated with 100 μM H2O2 and 2.5 μM DHR123 for 1 h. The cells were then trypsinized and passed through a 30 μm filter, and the ROS contents in the cells were analyzed with a flow cytometer (FACScan, Becton Dickinson, Franklin Lakes, NJ, USA).
2.6. Statistical Analysis
The experimental data are presented as the average value ± SD of triplicate samples from each independent biological sample. Statistical analysis was analyzed using Student’s t-test, and p values less than 0.05 were considered significant.
3. Results
3.1. T. sarmentosa Prevented the H2O2-Induced Death of H9c2 Cardiomyocytes
H9c2 cells were selected as the experimental material and treated with 100 μM H2O2 for one hour to induce cell death as described in a previously published study in order to determine whether ROS caused cardiomyocyte death [20]. As shown in Figures 1(a) and 1(b), H2O2 significantly reduced the cell survival rate and increased the number of dead H9c2 cells. However, when the cells were treated with increasing doses of T. sarmentosa, the H2O2-induced decrease in the cell survival rate and increase in the cell death rate were significantly inhibited (Figures 1(a) and 1(b)). When the cells were treated with different doses of T. sarmentosa alone, no significant changes in cell survival or cell death were observed, suggesting that T. sarmentosa is not cytotoxic to H9c2 cells. In addition, the morphology of the cells was observed directly under a microscope. When the cells were treated with H2O2, the spindle-like morphology of the cells was not maintained, and the cells detached. When the cells were pretreated with T. sarmentosa, especially at a high dose of 50–100 μg/mL, the cell morphology recovered noticeably. Based on these results, T. sarmentosa inhibited the changes in cell morphology and cell death induced by H2O2 (Figure 1(c)).
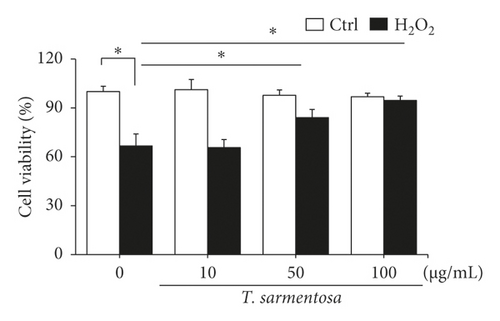
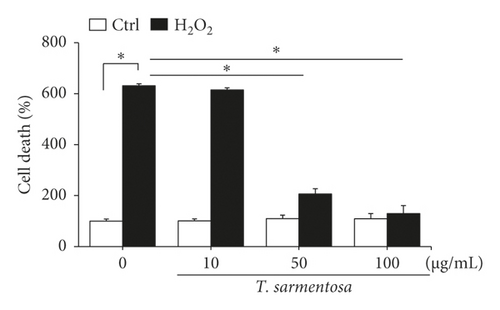
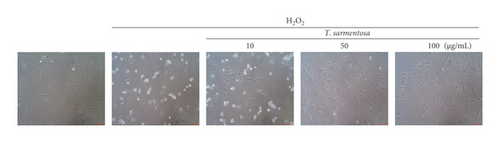
3.2. T. sarmentosa Reduced the Levels of Apoptosis-Related Caspases and Increased the Production of ROS Induced by H2O2
Since T. sarmentosa inhibited the H2O2-induced death of H9c2 cells, we further explored whether T. sarmentosa altered the H2O2-induced activation of cell death-related signal transduction pathways. In the cells treated with different doses of T. sarmentosa, no caspase-8, caspase-9, or caspase-3 expression was detected. However, when the cells were treated with H2O2, the levels of caspase-8, caspase-9, and caspase-3 significantly increased. When the cells were pretreated with high doses of 50–100 μg/mL T. sarmentosa, the H2O2-induced increases in the levels of the death-related caspases mentioned above were significantly reduced (Figure 2(a)).
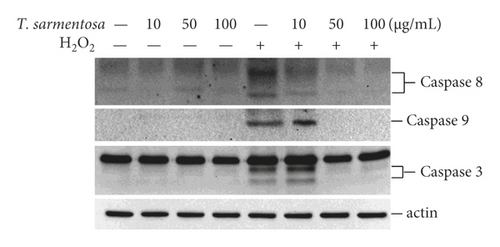
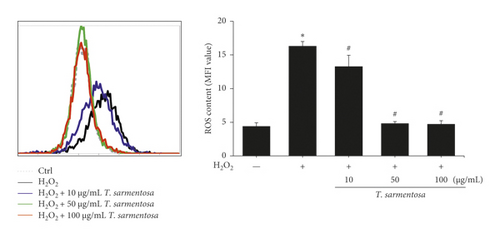
Cell death caused by H2O2 is closely related to ROS production. Therefore, we further verified whether the inhibition of H2O2-induced cell death by T. sarmentosa is related to ROS production. As shown in Figure 2(b), H2O2 significantly increased the intracellular ROS levels, and the H2O2-induced increase in the intracellular ROS content was significantly decreased by treatment with increasing doses of T. sarmentosa.
3.3. All the Compounds Present in the T. sarmentosa Extract Inhibited H2O2-Induced H9c2 Cell Death
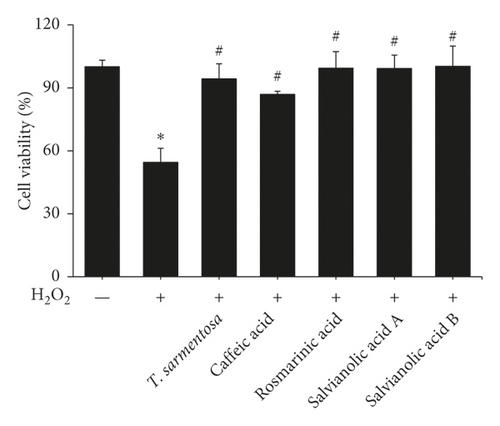
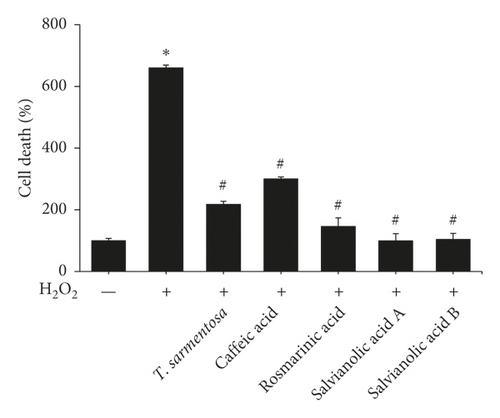
We selected several compounds present in the T. sarmentosa extract that are commercially available to further explore the components of T. sarmentosa that effectively inhibit H2O2-induced H9c2 cell death [21]. As shown in Figures 3(a) and 3(b), caffeic acid, rosmarinic acid, salvianolic acid A, and salvianolic acid B exhibited effects similar to those of T. sarmentosa, as they significantly reversed the H2O2-induced decrease in the H9c2 cell survival rate and increase in the cell death rate.
3.4. All Compounds Present in the T. sarmentosa Extract Decreased the H2O2-Induced Increases in the Levels of Apoptosis-Related Caspases and Production of ROS
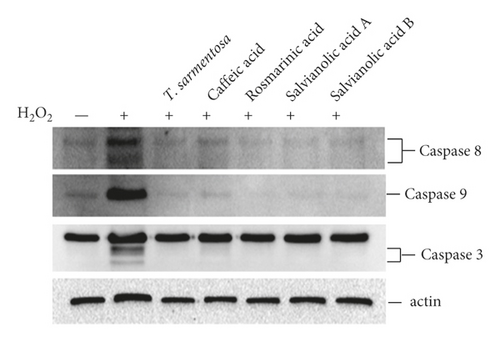
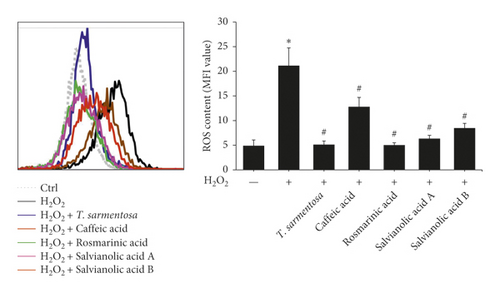
We further analyzed whether the compounds present in the T. sarmentosa extract also altered the H2O2-induced expression of caspases and production of ROS. H2O2 induced the expression of caspases, and except for caffeic acid, which had only a slight effect on the expression of caspase-3, the H2O2-induced expression of caspase-8, caspase-9, and caspase-3 was inhibited by all the compounds found in T. sarmentosa extract (Figure 4(a)). In addition, we also analyzed the effects of these compounds on the H2O2-induced intracellular ROS production. As shown in Figure 4(b), all the compounds present in the T. sarmentosa extract significantly inhibited the H2O2-induced ROS production.
4. Discussion
T. sarmentosa is widely distributed and is found in Taiwan, Vietnam, Malaysia, the Philippines, Indonesia, and many other regions. T. sarmentosa is also widely used as a medicinal material. For example, the stems and leaves are mashed and taken orally with wine to promote blood circulation and treat old and new injuries. The medical efficacy of this plant extract has consistently been reported; however, few studies have explored its potential efficacy because the use of traditional Chinese medicine is not often investigated in many scientific studies.
In this study, we confirmed the antioxidant effect of T. sarmentosa on cardiomyocytes, but it only inhibited H2O2-induced cell death at higher doses. Although T. sarmentosa extract has been used as a traditional Chinese medicine in humans for years, no studies have reported its concentration in human blood after consumption; thus, researchers have not determined whether the dose exerts a cardioprotective antioxidant effect. However, T. sarmentosa was orally administered at doses of 2,000 mg/kg bodyweight in in vivo studies and at doses of 10–100 mg/mL in cell-based experiments [15]. Therefore, the concentration of the T. sarmentosa extract tested in this study should be within a reasonable range. Magnolol is the most popular Chinese medicine used to protect the myocardium and possesses antioxidant activity. The oral dose of Magnolia officinalis extract in live animals ranges from 0.48 to 2.5 g/kg/day [22, 23], which was not significantly different from the T. sarmentosa dose. In addition, the half-life of magnolol in vivo and the organs to which the drug is distributed after injection have been reported in a review [24]. Therefore, compared with magnolol, the cardioprotective effects of T. sarmentosa still need to be verified in additional experiments.
The efficacy of T. sarmentosa is related to its individual components. The individual compounds present in the T. sarmentosa extract used in this study, including caffeic acid, rosmarinic acid, and salvianolic acid, have a benzene ring structure, which has antioxidant properties [25]. In addition, the various compounds present in T. sarmentosa mentioned above have been reported to possess antioxidant properties [26–32]. Although different plants exert effects similar to those of traditional Chinese medicines, many differences in their internal components have been noted, and these differences also result in their different medicinal purposes. For example, many components of T. sarmentosa are similar to those of Salvia officinalis [33], but significant differences were observed after a further analysis of the components [34]; thus, the two plants have different therapeutic purposes. Salvia officinalis is mainly used for relieving anxiety, while T. sarmentosa is mainly used for detoxification and to exert the antioxidant effect.
Many studies have reviewed the antioxidant, anti-inflammatory, and antiangiogenic properties of caffeic acid that exert important antiatherosclerotic effects and protect tissues from the ischemia/reperfusion injury and cell dysfunction caused by different physical and chemical agents [35, 36]. Rosmarinic acid has been shown to protect against inflammation and myocardial cell apoptosis during myocardial ischemia/reperfusion injury by activating peroxisome proliferator-activated receptor γ and inhibiting the production of inflammatory cytokines, such as IL-6, TNF-α, and C-reactive protein [37]. In addition, rosmarinic acid has been shown to improve cardiac dysfunction and mitochondrial damage in mice with diabetic cardiomyopathy by activating the SIRT1/PGC-1α signaling pathway [38]. The pathway by which salvianolic acid A exerts its protective effects was similar to that of rosmarinic acid. Arsenic trioxide induced the production of ROS and decreased the expression level of PGC-1α in cardiomyocytes. The addition of salvianolic acid A could restore the above phenomena to reduce cardiac mitochondrial damage and improve the cardiotoxicity induced by arsenic trioxide [39]. The protective potential of compounds found in the T. sarmentosa extract is closely related to the benzene ring structure of these compounds.
In addition to traditional Chinese medicine, coenzyme Q10 (CoQ10) is widely used in the market as a health food. CoQ10 is a coenzyme that is indispensable in the human body. CoQ10 exists in cells and is mainly distributed in the heart, kidneys, liver, and muscles. CoQ10 functions to stimulate mitochondrial energy production. In addition, CoQ10 functions as an antioxidant in the mitochondria, is involved in the energy production process, and reduces free radicals. Damage to mitochondria maintains the integrity and stability of cells, slows the oxidation of bad cholesterol, delays aging, and activates the immune system [40]. The effective dose of CoQ10 for the prevention of cardiovascular disease is approximately 100–300 mg [41–43]. However, due to the regulatory limitations of many local laws and regulations, high doses of CoQ10 are not available to many people, and thus, its efficacy is controversial. From the perspective of the structures of the compounds, CoQ10 and T. sarmentosa extracts contain similar antioxidant components that have benzene ring structures and unsaturated hydrocarbon chains, indicating the potential use of T. sarmentosa as a health food for heart protection.
5. Conclusions
In summary, the T. sarmentosa extract and several compounds present in the T. sarmentosa extract inhibit the H2O2-induced death of H9c2 cardiomyocytes. Furthermore, the T. sarmentosa extract and several of its compounds inhibit the H2O2-induced expression of cell death-related caspases and production of ROS. Therefore, the T. sarmentosa extract may have potential for use as a cardioprotective health food.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was supported by a grant (TCRD-TPE-110-13) from the Taipei Tzu Chi Hospital through the Buddhist Tzu Chi Medical Foundation, Taipei, Taiwan. The authors thank the Core Laboratory of the Buddhist Tzu Chi General Hospital for providing support.
Open Research
Data Availability
The data used to support the results of this study are included within the article.




