Traditional Medicinal Uses, Phytoconstituents, Bioactivities, and Toxicities of Erythrina abyssinica Lam. ex DC. (Fabaceae): A Systematic Review
Abstract
Background. Many studies have been undertaken on the medicinal values of Erythrina abyssinica Lam. ex DC. (Fabaceae). The details, however, are highly fragmented in different journals, libraries, and other publication media. This study was therefore conducted to provide a comprehensive report on its ethnobotany, ethnomedicinal uses, phytochemicals, and the available pharmacological evidence supporting its efficacy and safety in traditional medicine. Method. We collected data using a PROSPERO registered systematic review protocol on the ethnobotany, phytochemistry, and ethnopharmacology of Erythrina abyssinica from 132 reports that were retrieved from electronic databases. Documented local names, morphology, growth habit and habitat, ethnomedicinal and nonmedicinal uses, diseases treated, parts used, method of preparation and administration, extraction and chemical identity of isolated compounds, and efficacy and toxicity of extracts and isolated compounds were captured. Numerical data were summarized into means, percentages, and frequencies and presented as graphs and tables. Results. Erythrina abyssinica is harvested by traditional herbal medicine practitioners in East, Central, and South African communities to prepare herbal remedies for various human and livestock ailments. These include bacterial and fungal infections, tuberculosis, malaria, HIV/AIDS, diarrhea, cancer, meningitis, inflammatory diseases, urinary tract infections, wounds, diabetes mellitus, and skin and soft tissue injuries. Different extracts and phytochemicals from parts of E. abyssinica have been scientifically proven to possess anti-inflammatory, antibacterial, antioxidant, antiplasmodial, antiproliferative, antifungal, antimycobacterial, antidiarrheal, anti-HIV 1, antidiabetic, and antiobesity activities. This versatile pharmacological activity is due to the abundant flavonoids, alkaloids, and terpenoids present in its different parts. Conclusion. Erythrina abyssinica is an important ethnomedicinal plant in Africa harboring useful pharmacologically active phytochemicals against various diseases with significant efficacies and minimal toxicity to mammalian cells. Therefore, this plant should be conserved and its potential to provide novel molecules against diseases be explored further. Clinical trials that evaluate the efficacy and safety of extracts and isolated compounds from E. abyssinica are recommended.
1. Introduction
Erythrina abyssinica Lam. ex DC. (Fabaceae) is an important medicinal plant as evidenced by the existence of its names in various local languages and high frequency of citation in ethnobotanical surveys [1–4]. The genus Erythrina derives from the Greek word “erythros,” translated to mean red (a reflection of the showy red flowers of its various species). The epithet ‘‘abyssinica’’ means ‘‘from Ethiopia’’ [5]. The Erythrina genus houses at least 120 species distributed mainly in tropical and subtropical zones [6]. Plants in this genus are usually referred to as “coral trees” due to their red flowers and branches that resemble the shape of sea coral [7]. Erythrina abyssinica is a deciduous leguminous tree native to East Africa but also found in Central and South Africa [8, 9]. Tropical Asia and Central America have E. abyssinica as an exotic species. The common English names of E. abyssinica are coral tree, Uganda coral, kaffir boom, erythrina, flame tree, red-hot-poker tree, and lucky-bean tree [10]. Some of the local names used across indigenous communities are summarized in Table 1.
| Folk name (local language) | Country | Authors |
|---|---|---|
| Ejjirikiti (Luganda), Murinzi, Kiko Omoko/Echuko (Rutoro, Rukonzo), Oluo (Lugbara), Kisoro, Lochoro, Oding, Loting (Acholi), Kikiri (Kwamba), Engosorot (Ateso), Olawu (Madi), Koli (Jopadhola), Owila kot (Lango), Muyirikiti, Ekilama (Lusoga), Cheroguru, Muragolo (Lugishu), Mutembetembe (Lugwe), Bwiko (Lukiga), Kaborte (Sebei), Kiko, Muko (Lunyangkore, Lutoro), Mudongodongo, Mukobe (Lunyuli) | Uganda | [2, 3, 10–15] |
| Omotembe (Kisii), Muhuti (Kikuyu), Ekirikiti or Ol-Goroshe (Maasai), Muuti (Meru), Kivuti or Muvuti (Kamba), Mulungu (Taita), Mwamba ngoma, Mbamba ngoma, Muhuti, Mjafari or Mwamba (Kiswahili), Kumurembei (Luhya) | Kenya | [10, 16–19] |
| Qanqari (Iraqw), Mriri (Chagga), Muhemi (Hehe), and Muungu (Pare), Kisebhe (Rungwe) | Tanzania | [20–22] |
| Kuara, Korra, Korch (Amharic) | Ethiopia | [10] |
| Umuko (Lunyarwanda) | Rwanda | [23–26] |
| Dus (Arabic), Hab al Arous | Sudan, South Sudan | [10, 27, 28] |
| Chisunga (Lunda) | Democratic Republic of Congo | [10] |
| Mulunku (Chokwe) | Angola | [4] |
| Mulunguti, Mwale (Nyanja) | Mozambique, Zimbabwe, Zambia, Malawi | [10] |
| Mulunguti (Bemba, Tongan) | Zambia, Mozambique, Zimbabwe | [5, 10] |
| Mutiti (Shona) | Zimbabwe | [5] |
| Suwawue, Soaueh (Tigrigna) | Eritrea, Ethiopia | [10, 29] |
Medicinal plants have been a veritable source of cure for a number of human and livestock diseases, and thus, they are widely used in many communities. This is because plants house abundant secondary metabolites (phytochemicals) with potential pharmacological activities. These include flavonoids, alkaloids, terpenoids, phenols, chalcones, quinones, aromatic hydrocarbons, chromones, and coumarins. It is these phytochemicals that are locally extracted in herbal preparations and used as remedies for the management of several diseases. The World Health Organization (WHO) estimated that 80% of the world’s population especially in low- and middle-income countries rely on herbal medicines for primary health care [30]. The use of herbal medicines in the management of several ailments among people continues to gain momentum due to their availability, affordability, perceived effectiveness, and cultural acceptability across ethnic backgrounds [31].
Globally, there has been an increase in natural product research in the last two decades [30, 32]. This has been partly in response to the increasing antimicrobial resistance, emergence of new diseases, and decrease in the chemical diversity of natural product libraries [30, 32–36]. It has also been so in an effort to continue the search for more effective, safer, and cheaper therapeutic agents for existing diseases, to substitute expensive prescription drugs [37–40]. Erythrina abyssinica is among those revered plants [40, 41] that has been widely researched [3]. However, the information on it is highly fragmented in different journals, books, university libraries, and other publication media platforms. This review was therefore undertaken to compile a comprehensive document that describes the ethnobotany, phytochemistry, and ethnopharmacology of E. abyssinica so as to generate integrated and sufficient scientific evidence to support its medicinal use. The study further emphasizes the importance of conserving this medicinal plant amidst the growing destruction of natural resources for settlement, industrialization, construction, and energy production [27, 42–47].
2. Methods
2.1. Protocol Registration and Reporting
The protocol used in this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) and can be accessed from their website (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020187081) with the registration number CRD42020187081. The Preferred Reporting Items for the Systematic Reviews and Meta-Analyses (PRISMA) guidelines [48] have been used in the reporting of this study (Figure 1).
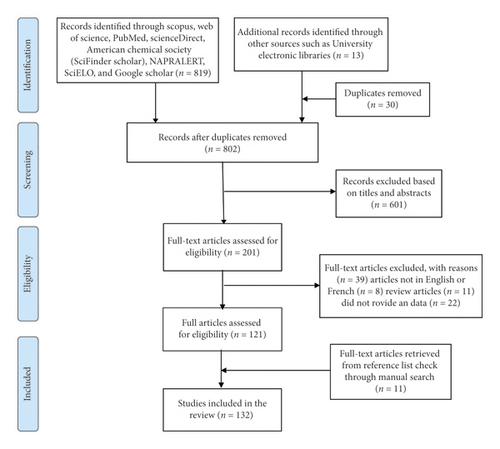
2.2. Literature Search
Electronic data on ethnobotany, phytochemistry, efficacy, and toxicity of E. abyssinica were retrieved from electronic databases such as Scopus, Web of Science Core Collection, PubMed, American Chemical Society, ScienceDirect, Scientific Electronic Library Online (SciELO), Google Scholar, and NAPRALERT (a comprehensive natural products database with ethnomedical and pharmacological information of extracts and isolated compounds). Sets of keywords such as “ethnobotany,” “traditional medicine,” “ethnobotany,” “alternative medicine,” “ethnopharmacology,” “phytochemistry,” “extraction,” “isolation,” “efficacy,” “safety,” “toxicity,” “phytochemicals,” “structural elucidation,” and clinical study were combined with “Erythrina abyssinica.” The retrieved articles were downloaded and stored in EndNote X9 (Thomson Reuters, San Francisco, CA, USA) by three independent authors (SBO, TO, and YG). Duplicate articles were then removed from the file. Further, manual search from the reference lists of screened eligible articles and deposited electronic copies of dissertations and theses in University online libraries were done. The authors continuously received notifications of any new “similar reports” meeting the search criteria from ScienceDirect, Scopus, and Google Scholar.
2.3. Screening
Retrieved articles were first screened based on the titles and abstracts for relevance to the study by three independent reviewers (MPO, SM, and YG). Articles that reported on other species of Erythrina but not abyssinica and also abyssinica but not of genus Erythrina were also excluded. For example, we excluded articles on Entada abyssinica, Erythrina variageta, Erythrina suberosa, Albuca abyssinica, Dregea abyssinica, Harrisonia abyssinica, and Wahlenbergia abyssinica although they appeared in the search results. During the screening, every time a disagreement occurred it was resolved through a discussion between the reviewers and/or by the principal investigator (SBO). The eligible articles were then assessed further for inclusion in the study using the inclusion/exclusion criteria.
2.4. Inclusion and Exclusion Criteria
Full-text articles that at least reported on ethnobotany, ethnopharmacology, and phytochemistry of Erythrina abyssinica written in English or French but translated to English and published in peer-reviewed journals, reports, books, theses, and dissertations dated until January 2021 were considered. All publishing years were included without any geographical restrictions. Articles that reported data not relevant to the study, reviews, and not written in English or French were excluded from the study.
2.5. Data Extraction
A data collection tool was designed in Microsoft Excel (Microsoft Corporation, USA) to capture data on different aspects of E. abyssinica. Three reviewers independently extracted relevant data from the included articles regarding the ethnobotany, ethnopharmacology, and phytochemistry of E. abyssinica. For ethnobotanical data, the diseases or ailments managed, parts used, and mode of preparation and administration were captured. For phytochemistry, the name of isolated pure compounds, chemical class, extraction solvent, and their efficacy and toxicity were captured. For ethnopharmacology, extraction solvent used, bioassay/model used, results of efficacy, and toxicity of extracts were captured. The collected data were checked for completeness and processed independently by two reviewers.
2.6. Data Analysis and Synthesis
Descriptive statistical methods were used to analyse the collected data. Results were expressed as percentages and frequencies and subsequently presented as tables and charts. The analyses were performed using SPSS statistical software (version 20, IBM Inc.).
3. Results and Discussion
3.1. Literature Search and Publications
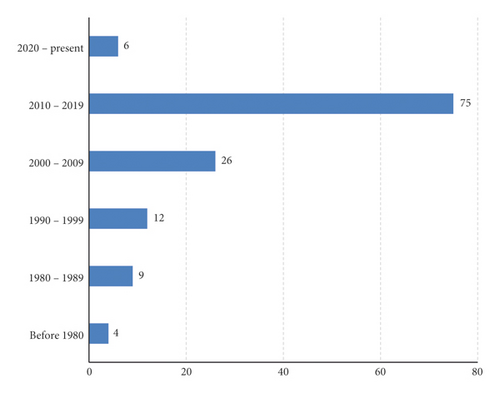
A total of 201 reports were retrieved out of which 132 met the inclusion criteria and were reviewed. Of these, 78 articles reported only on the ethnobotany, 27 articles on pharmacology only, 15 articles on both pharmacology and phytochemistry, 5 articles on phytochemistry only, and 3 articles on both ethnobotany and pharmacology while 4 articles on ethnobotany, pharmacology, and phytochemistry. Most of the articles (56.8%) were published in the 2010–2019 decade, indicating a lot of research is being done as compared to the preceding decades (Figure 2). This could be due to the (1) growing need for more effective and less toxic medicinal products of plant origin, (2) emerging antimicrobial resistance that has rendered most chemotherapeutic agents less effective, (3) new disease outbreaks like Ebola, and (4) increase in noncommunicable diseases such as cancers, hypertension, diabetes mellitus, and sexual dysfunction that require readily available, affordable, effective, and safe therapies.
3.2. Taxonomy, Morphology, Distribution, and Propagation
Erythrina abyssinica belongs to the kingdom Plantae, phylum Spermatophyta, subphylum Magnoliophyta (flowering plants), class Magnoliopsida (dicotyledons), order Fabales, family Fabaceae (legumes), subfamily Papilionoideae, genus Erythrina (L.), and species abyssinica (Lam ex. DC.). The frequently encountered synonyms of this species include E. kassneri Baker f., Corallodendron suberifera (Welw. ex Baker) Kuntze, E. bequaerti De Wild., E. tomentosa R. Br., Chirocalyx abyssinicus (Lam.) Hochst., and C. tomentosus Hochst. [3].
Erythrina abyssinica grows as a multibranched deciduous tree or shrub up to a height of 12–15 m tall usually with a rounded spreading crown (Figure 3). The branches have a corky thick deeply fissured bark with prickles (4–8 mm long). The leaves are trifoliate alternately arranged with long (6–20 cm) petiole. The leaflets can be ovate, cordate, and almost circular, rounded at the base and obtuse or notched at the apex, with network venation, dense hair usually at the abaxial surface, and prickles [49, 50]. The inflorescence is raceme, dense, pyramidal, and either terminal or axial with a long peduncle (up to 20 cm) and caducous bracts. Flowers are bisexual and papilionaceous having densely hairy, cylindrical, split at one side calyx, brightly coloured (orange to red) corolla with free keel petals, 10 fused and one free stamen, one carpel with a superior cylindrical oblong ovary, long style, and flat stigma head [51]. The fruits are linear-oblong pods, brown to black in colour, usually hairy, dehisce at two values to release ellipsoid, long (6–12 mm), and bright red seeds [52]. The tree is anchored firmly in the ground by a deep root system [13, 20].


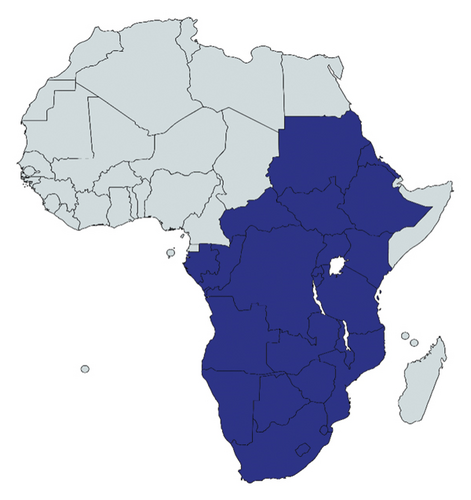
Erythrina abyssinica can be propagated either using seeds, wildings [40], or cuttings, but the former has comparatively lower germination rates of 10–30% with propagation restricted to rainy seasons [3, 11, 53]. It grows naturally in woodland and wooded grasslands (savannah woodlands, grasslands, and scrublands, secondary scrub vegetation, regions with 500–2000 mm annual rainfall and optimal temperatures of 15–25°C) [11, 54–57]. Thus, it is widespread from Sudan, South Sudan, Uganda, Kenya, Rwanda, Burundi, Democratic Republic of Congo, Congo (Brazzaville), Tanzania to Ethiopia, Eritrea, Angola, Namibia, Botswana, Central African Republic, Swaziland, Lesotho, Gabon, Zambia, Zimbabwe, and Mozambique (Figure 4) [3, 10, 11, 53]. It has also been introduced as an ornamental in Mauritius and various places in Tropical Asia and Central America, including Afghanistan, Bangladesh, Bhutan, India, Nepal, Pakistan, and Sri Lanka [10, 53]. In South Sudan for instance, the tree grows at up to 2000 m altitude while in Tanzania, they are found at up to 2300 m. The tree naturally grows on loamy to clay soils, with preference for deep well-drained soils on plateaus and slopes with a pH of 3.5–5.5. The tree is termite- and fire-resistant primarily due to its deep root system but cannot tolerate frost, explaining its limited distribution in cold regions [11, 53].
3.3. Ecological, Traditional, and Medicinal Uses
Erythrina abyssinica being a legume is well known for fixing nitrogen into the soil and thus enhances soil fertility. Because of this, it plays an important role in phytorestoration and forest regeneration in polluted soils [64–66]. Its flowers also secrete nectar that is fed on by pollinating insects especially bees hence being important in both horticulture and apiculture [67]. Although this plant usually grows naturally in the wild, some communities cultivate it in their homesteads as an ornamental plant, for live fencing purposes due to its brightly coloured flowers and prickles, a material for dye, and craft materials such as curios and necklaces (from seeds) [9, 20, 68, 69]. The stem of this plant is also harvested to obtain timber and charcoal for furniture and energy purposes, respectively [20]. In livestock farming, the plant leaves are used as fodder for animals [5, 70, 71].
The stem bark, seeds, roots, root bark, leaves, and flowers of E. abyssinica and the whole plant either in combination or singly are used to prepare herbal remedies for various human ailments (Table 2). However, the stem bark and roots are the most commonly used parts in the preparation of herbal remedies. Even in efficacy, toxicity, and phytochemical studies, the stem bark and roots were the most investigated. This could probably be due to high yield associated with them because of their high potential in concentrating and storing phytochemicals. The seeds were indicated to be poisonous when crushed [11]. The commonest methods of preparation and administration of herbal medicines from this plant are boiling (decoctions) and then drinking, cold infusions (taken orally), pounding dried samples into powder and then licking, pounding fresh samples into a paste and applying topically, squeezing fresh samples and mixing with bathing water, or direct chewing of the different parts (Table 2).
| No. | Disease/ailments treated | Parts used | Method of preparation and administration | Country | Authors |
|---|---|---|---|---|---|
| 1 | Malaria, fevers | R, SB, L, F | Boiled and taken orally | Uganda, Kenya, Tanzania, Ethiopia, Eritrea, DR Congo, Sudan, Rwanda | [9, 13, 18, 21, 24, 28, 58, 72–82] |
| 2 | Inflammatory disorders, eye problems, and pain | SB, R, Sd | Boiled and taken orally; powdered, mixed with petroleum jelly, and smeared on the wound/swollen part. For eye problems, it is applied as liniment | Uganda, Tanzania, Kenya, South Sudan | [13, 19, 20, 27, 72, 83–88] |
| 3 | Bacterial and fungal infections | SB, L, F, WP | Decoction taken orally; powdered and licked; sliced bark chewed; cold infusion taken orally | Uganda, Kenya, Burundi | [13, 72, 89–91] |
| 4 | Skin and soft tissue infections, leprosy, and wounds | SB, F, L | Boiled in petroleum jelly and smeared at the tissue, herbal bath of infected skin part | Uganda, Kenya, Zimbabwe, Rwanda | [20, 24, 72, 81, 87, 92–95] |
| 5 | Tuberculosis (cough) | SB, R, L, F | Decoction taken orally; powdered and licked | Uganda, Kenya, Tanzania, Burundi, Zimbabwe | [31, 61, 72, 73, 95–99] |
| 6 | Cancer | SB, L, F | Boiled and taken orally | Uganda, Kenya | [39, 72, 100] |
| 7 | HIV/AIDS | SB, R, L | Decoction taken orally | Uganda, Kenya, Tanzania | [2, 39, 72, 98, 101–103] |
| 8 | Infertility, birth control, pregnancy related conditions | SB, R | Decoction, squeezing, chewing, taken orally | Uganda, Kenya | [31, 72, 73, 104–106] |
| 9 | Blood disorders (anaemia and jaundice) | R, SB, L, F | Boiled and taken orally | Uganda, Kenya, Tanzania | [27, 31, 72, 84, 107–109] |
| 10 | Venereal diseases | SB, L, F, RB | Boiled and taken orally | Uganda, Kenya, Zimbabwe, Rwanda | [19, 20, 63, 72, 87, 92, 100, 105, 110–112] |
| 11 | Diabetes mellitus | SB, L | Boiled and taken orally | Uganda | [72, 113, 114] |
| 12 | Hepatitis, measles, scabies, herpes, mumps, liver diseases | SB, R, L | Decoction and cold infusions taken. Dried leaf ash is mixed with oil or butter and applied externally to treat scabies | Rwanda, Kenya, Uganda, Tanzania | [22, 23, 101, 115] |
| 13 | Pneumonia | SB | Boiled in water and taken orally | Kenya | [92, 100] |
| 14 | Convulsions and CNS disorders | SB | Decoction, pound, and add salt | Uganda | [31] |
| 15 | Gastrointestinal disorders (diarrhea, stomach ache, vomiting, constipation, ulcers, dysentery, colic) | SB, R, L | Boiled, honey added, and taken orally. Decoction taken, or pounded, salt added, and taken. Root decoction with Rhamnus prinoides roots taken for colic. Decoction of young roots taken for constipation in children | Uganda, Kenya, Tanzania, Eritrea, Angola, Rwanda | [4, 19, 26, 29, 31, 87, 92, 101, 106, 107, 116–118] |
| 16 | Helminthiasis | SB | Decoction taken orally | Uganda, Kenya, Tanzania | [87, 105, 119, 120] |
| 17 | Snake bites/antidote for poisoning | R, SB, RB | Sap used/pounded and applied at the bite. Boiled and taken orally | Uganda, Kenya, Tanzania | [15, 16, 19, 109, 121, 122] |
- Parts used: L: leaves, R: roots, RB: root bark, Sd: seeds, SB: stem bark, F: flowers, and WP: whole plant.
Among the frequently reported ailments for which herbal medicines containing E. abyssinica are used include bacterial and fungal infections, malaria, leprosy, tuberculosis (cough), inflammatory diseases, HIV/AIDS, cancer, and metabolic disorders such as diabetes mellitus, obesity, and anaemia. Other conditions treated using this plant include snake bites, antagonizing poisons, venereal diseases (sexually transmitted diseases, e.g., gonorrhea, syphilis, and urinary tract infections including schistosomiasis), soft tissue and skin infections, diarrhea, infertility and pregnancy-related conditions, pneumonia, epilepsy, central nervous system- (CNS-) related disorders, vomiting, hepatitis, and helminthiasis. In ethnoveterinary medicine, extracts of E. abyssinica are used in the management of poultry and livestock diseases such as new castle disease, anaplasmosis, and helminthosis [43, 89, 119, 123, 124].
3.4. Phytochemical Profile of E. abyssinica
3.4.1. Preliminary Phytochemical Analyses
Qualitative phytochemical screening of medicinal plants is an essential step to their detailed phytochemical and pharmacological investigation [125]. Preliminary phytochemical screening of different solvent extracts of E. abyssinica indicated the presence of tannins, saponins, alkaloids, and flavonoids as the main therapeutic secondary metabolites (Table 3).
| Secondary metabolites | Parts used | Solvent used | Yield (%) | Authors |
|---|---|---|---|---|
| Tannins, saponins, alkaloids, and flavonoids | Bark | Hexane | 2.0 | [60] |
| Alkaloids, terpenoids, saponins, tannins, and flavones | Root bark | Methanol (crude) | Not reported | [126] |
| Alkaloids, saponins, cardiac glycosides, coumarins, and anthraquinone derivatives | Roots | Methanol | 23.6 | [127] |
| Alkaloids, flavonoids, tannins, and cardiac glycosides | Stem | Water | 0.34 (alkaloidal and flavonoid content) | [128] |
| Alkaloids, flavonoids, terpenoids, and saponins | Stem bark | Methanol | 4.82 | [62] |
3.4.2. Structural Elucidation
Like in many natural product research studies, chromatography has been used in the isolation of compounds from crude extracts of E. abyssinica. The most widely used techniques included high-performance liquid chromatography (HPLC), gas chromatography (GC), high-performance thin-layer chromatography (HPTLC), and ultraperformance liquid chromatography (UPLC) [129]. Spectroscopic techniques such as mass spectrometry (MS), ultraviolet (UV) spectrophotometry, one-dimensional nuclear magnetic resonance (1D-NMR) spectroscopy, and its complementary techniques (heteronuclear multiple bond correlation (HMBC) spectroscopy, heteronuclear multiple quantum coherence (HMQC) spectroscopy, nuclear overhauser effect spectroscopy (NOESY), and circular dichroism (CD) spectroscopy) have been used to elucidate chemical structures of the isolated compounds [130]. Chromatography-spectroscopy hyphenated techniques have become more commonly used in recent decades due to the increased efficiency, sensitivity, and detection limits [1]. These include LC-MS, GC-MS, UPLC-MS, HPTLC-UV, HPLC-photodiode array detection, LC-NMR-MS, GC-NMR-MS, and high-resolution electron spray ionization (ESI)-MS [130].
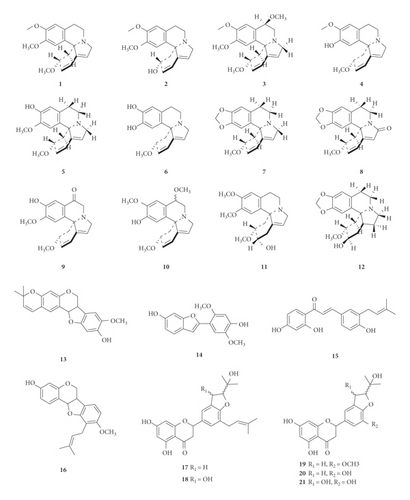
A total of 122 phytochemicals which are primarily alkaloids, flavonoids, and triterpenoids have been isolated from E. abyssinica (Figure 5; Table 4). Some of the isolated compounds are specific to E. abyssinica while others have been reported to be present in other species of the genus Erythrina [149]. Because genus Erythrina belongs to the family Fabaceae, its members have a rich diversity of secondary metabolites (phytochemicals) amongst themselves due to possession of various biosynthetic pathways [150]. However, some species share common phytochemicals, and hence, these act as biomarkers for nutraceutical, pharmacological, and toxicological potentials in the food and drug industries [130, 151].
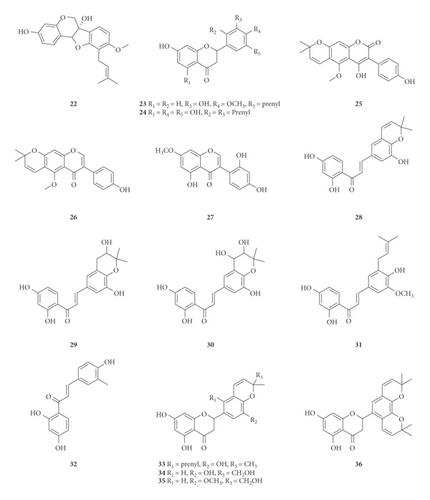
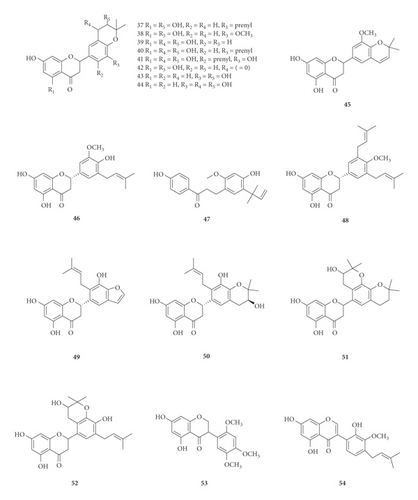
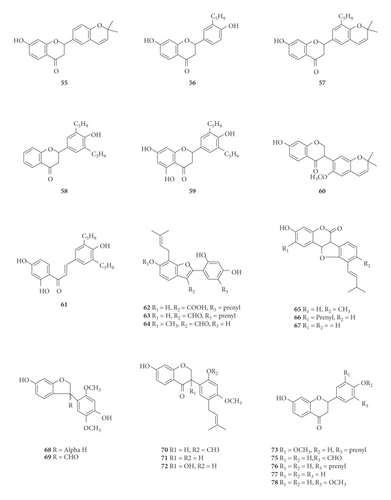
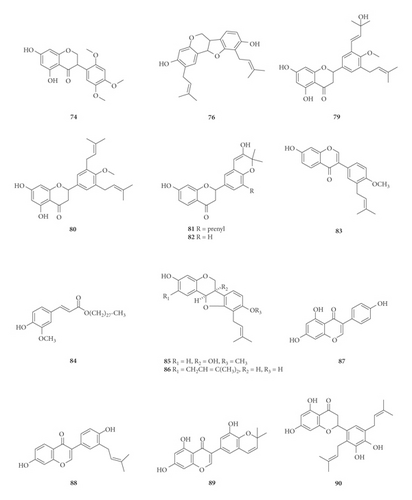
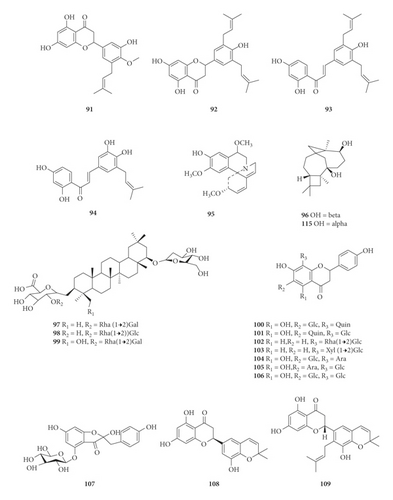
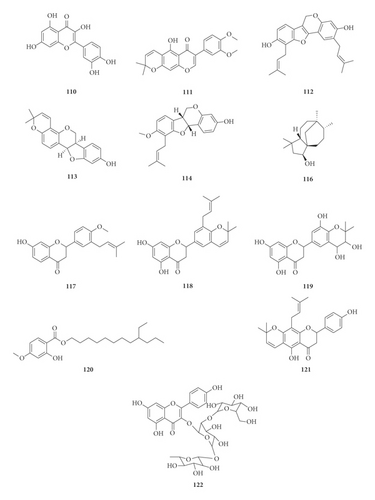
| Name of the compound identified | Chemical class | Part used | Solvent used | Techniques used | Bioactivity tested | Result | Authors |
|---|---|---|---|---|---|---|---|
| (+)-Erysotrine (1) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erythravine (2) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erythristemine (3) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erysovine (4) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erysodine (5) | Alkaloid | Sd | Chloroform, ethanol | NMR | Curare-like activity | Strong activity | [131, 132] |
| (+)-Erysopine (6) | Alkaloid | Sd | Chloroform, ethanol | NMR | Curare-like activity | Strong activity | [131, 132] |
| (+)-Erythraline (7) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-8-Oxoerythraline (8) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-11-Oxoerysodine (9) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-11-Methoxyerysovine (10) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erythratidine (11) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| (+)-Erythratine (12) | Alkaloid | NS | NS | NMR | Not tested | Not applicable | [131] |
| 8-Methoxyneorautenol (13) | Pterocarpan | RB | Acetone | HRMS, NMR, HMBC | Radical scavenging properties | Moderately active | [133] |
| Eryvarin L (14) | Benzofuran | Rt | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Good antioxidant activity | [134] |
| Licoagrochalcone A (15) | Chalcone | Tw | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Good radical scavenging activity | [134] |
| 3-Hydroxy-9-methoxy-10-(3,3-dimethylallyl) pterocarpene (16) | Pterocarpan | RB | Acetone | HRMS, NMR, HMBC | Radical scavenging properties | Very active | [133] |
| (2S)-5,7-Dihydroxy-3′-prenyl-2″ξ-(4″-hydroxyisopropyl) dihydrofurano[1″,3″ : 4′,5′] flavanone (17) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| (2S)-5,7-Dihydroxy-3′-prenyl-2″ξ-(4″-hydroxy-isopropyl)-3″-hydroxy-dihydrofurano[1″,3″:4′,5′]flavanone, and (2S)-5,7,3′-trihydroxy-2′-prenyl-2″ξ-(4″-hydroxyisopropyl)-3″-hydroxy-dihydrofurano[1″,3″: 4′,5′] flavanone (18) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| (2S)-5,7-Dihydroxy-3′-methoxy-2″ξ-(4″-hydroxyisopropyl) dihydrofurano[1″,3″:4′, 5′]flavanone (19) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| (2S)-5,7,3′-Trihydroxy-2″ξ-(4″-hydroxyisopropyl) dihydrofurano[1″,3″ : 4′,5′] flavanone (20) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| (2S)-5,7,3′-Trihydroxy-2″ξ-(4″-hydroxyisopropyl)-3″-hydroxy-dihydrofurano[1″,3″:4′,5′] flavanone (21) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| Erythrabyssin I (22) | Pterocarpan | Rt | Methanol | UV, NMR, HPLC | Antimicrobial activity | Moderate antiyeast and antifungal activities | [136] |
| Erylatissin C (23) | Flavanone | SB | Methanol | UV, HPLC, NMR, HMQC, HMBC | PTP 1B inhibitory activity | No activity | [135] |
| Abyssinin III (24) | Flavanone | SB | Methanol | HPLC, NMR, HREI-MS, HMQC, HMBC, NOESY | Not tested | Not applicable | [82] |
| Indicanine B (25) | Coumarin | RB | DCM: MeOH | FTIR, UV, EI-MS, NMR | Antimicrobial activity | Active | [137] |
| Indicanine C (26) | Isoflavone | RB | DCM: MeOH | FTIR, UV, EI-MS, NMR | Antimicrobial activity | Not active | [137] |
| Cajanin (27) | Isoflavone | RB | DCM: MeOH | FTIR, UV, EI-MS, NMR | Antimicrobial activity | Not active | [137] |
| Abyssinone A (28) | Chalcone | SB | Methanol | UV, CD, NMR, HRMS | Not tested | Not applicable | [138] |
| Abyssinone B (29) | Chalcone | SB | Methanol | UV, CD, NMR, HRMS | Not tested | Not applicable | [138] |
| Abyssinone C (30) | Chalcone | SB | Methanol | UV, CD, NMR, HRMS | Not tested | Not applicable | [138] |
| Abyssinone D (31) | Chalcone | SB | Methanol | UV, CD, NMR, HRMS | Not tested | Not applicable | [138] |
| 3-Methylbutein (32) | Chalcone | Rt | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Good bioactivities | [134] |
| 2(S)-5,5′,7-Trihydroxy-2′-prenyl-(2″,2″-dimethylpyrano)-(5″,6′′:3′,4′)flavanone (33) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| i2(S)-5,5′,7-Trihydroxy-[2′′-(5″- hydroxy)-methylpyrano]-(5″,6′′:3′,4′)flavanone (34) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| 2(S)-5,7-Dihydroxy-3′-methoxy-[2′′-(5″-hydroxy)-methylpyrano]-(5″,6′′:3′,4′)flavanone (35) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| 2(S)-5,7-Dihydroxy-[(5″,6′′:3′,4′)-(2″,2″-dimethylpyrano)-(5‴,6‴:5′,6′)]-(2‴,2‴-dimethylpyrano)flavanone (36) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| 2(S)-5,7-Dihydroxy-5′-prenyl-[2″,2′′-(3″-hydroxy)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (37) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| 2(S)-5,7-Dihydroxy-5′-methoxy-[2″,2′′-(3″-hydroxy)-dimethyl-pyrano]-(5″,6′′:3′,4′)flavanone (38) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| 2(S)-5,7-Dihydroxy- [2″,2′′-(3″,4″-dihydroxy)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (39) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| 2(S)-5,7-Dihydroxy-5′-prenyl-[2″,2′′-(3″,4″-dihydroxy)-dimethylpyrano)]-(5″,6′′:3′,4′)flavanone (40) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| 2(S)-5,6′,7-Trihydroxy-5′-prenyl-[2″,2′′-(3″,4″-dihydroxy)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (41) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| 2(S)-5,5′,7-Trihydroxy-[2″,2′′-(4″-chromanone)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (42) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| 2(S)-5′,7-Dihydroxy-[2″,2′′-(3″-hydroxy)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (43) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| 2(S)-5′,7-Dihydroxy-[2″,2′′-(3″,4″-dihydroxy)-dimethylpyrano]-(5″,6′′:3′,4′)flavanone (44) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138] |
| Abyssinin I (45) | Flavanone | SB | Methanol | HPLC, NMR, HREI-MS, HMQC, HMBC, NOESY | Not tested | Not applicable | [82] |
| Abyssinin II (46) | Flavanone | SB | Methanol | HPLC, NMR, HREI-MS, HMQC, HMBC, NOESY | Not tested | Not applicable | [82] |
| Licochalcone A (47) | Chalcone | Rt | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Abyssinone V 4′-methyl ether (48) | Flavanone | SB | Methanol | UV, HPLC HREIMS, NMR, HMQC, HMBC, NOESY | Not tested | Not applicable | [82] |
| Abyssinoflavanone IV (49) | Prenylated flavanone | SB | Methanol | UV, NMR, CD, HREI-MS, HPLC, HMQC, HMBC, NOESY | Not tested | Not applicable | [82, 138] |
| Abyssinoflavanone V (50) | Prenylated flavanone | SB | Methanol | UV, NMR, CD, HREI-MS, HPLC, HMQC, HMBC, NOESY | Not tested | Not applicable | [82, 138, 139] |
| Abyssinoflavanone VI (51) | Prenylated flavanone | SB | Methanol | UV, NMR, CD, HREI-MS, HPLC, HMQC, HMBC, NOESY | Not tested | Not applicable | [82, 138–140] |
| Sigmoidin D (52) | Flavanone | Rt, SB | Chloroform: methanol (1 : 1), methanol | UV, NMR, CD, EI-MS, HRMS, HMBC | Antimicrobial and antioxidant activities, PTP 1B inhibitory activity | Weak antimicrobial and antioxidant activities, no activity | [82, 134, 138] |
| 5,7-Dihydroxy-2′,4′,5′-trimethoxyisoflavanone (53) | Isoflavanone | Rt | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| 5-Prenylpratensein (54) | Isoflavone | Rt | Chloroform: methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Abyssinone I (55) | Flavanone | RB | 80% aqueous MeOH, ether | UV, HPLC | Antimicrobial activity | Moderate activity | [136, 139, 141] |
| Abyssinone II (56) | Flavanone | RB | 80% aqueous MeOH | UV, HPLC | Antimicrobial and PTP1B inhibitory activities | Moderate and no activity | [136, 141] |
| Ether | |||||||
| Ethyl acetate | |||||||
| Abyssinone III (57) | Flavanone | RB | Ethyl acetate | HPLC, IR, UV, MS, CD, NMR | PTP1B inhibitory and antifungal activities | Weak activity | [136, 142] |
| Ether | |||||||
| Abyssinone IV (58) | Flavanone | RB | 80% aqueous MeOH | UV, NMR, HMBC, EI-MS, HPLC | Antimicrobial and antioxidant activities | Moderate activity | [134, 136, 141] |
| Chloroform : methanol (1 : 1) | |||||||
| Abyssinone V (59) | Flavanone | Rt, SB | Chloroform : methanol (1 : 1) | UV, NMR, HMBC, HREI-MS, CD, HPLC, NOESY | Antimicrobial, antiplasmodial, antioxidant. and PTP1B inhibitory activities | Weak activity | [82, 134, 136, 141–143] |
| Methanol | |||||||
| Ether | |||||||
| Ethyl acetate | |||||||
| Abyssinone VI (60) | Isoflavone | NS | Ether | UV, HPLC | Antifungal activity | Not reported | [136] |
| Abyssinone VII (61) | Chalcone | Rt | Chloroform : methanol (1 : 1), ether | UV, NMR, EI-MS, HMBC, HPLC | Antimicrobial and antioxidant activities | Good activity | [134, 136] |
| Erythribyssin N (62) | Benzofuran | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Marked stimulation | [144] |
| Sigmoidin K (63) | Benzofuran | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Marked stimulation | [144] |
| Isosojagol (64) | Benzofuran | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Less stimulation | [144] |
| Erythribyssin F (65) | Coumestan | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Marked stimulation | [144] |
| Eryvarin Q (66) | Coumestan | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Less stimulation | [144] |
| Erypoegin F (67) | Coumestan | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Marked stimulation | [144] |
| Erythribyssin H (68) | Benzofuran | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Less stimulation | [144] |
| Eryvarin R (69) | Benzofuran | SB | Methanol | HPLC, IR, UV, MS, NMR | AMPK activity | Less stimulation | [144] |
| Erythribyssin E (70) | Isoflavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Strong activity | [142] |
| Prostratol C (71) | Isoflavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Strong activity | [142] |
| Erythribyssin J (72) | Isoflavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Strong activity | [142] |
| 5-Deoxyabyssinin II (73) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Strong activity | [142] |
| 7-Demethylrobustigenin (74) | Isoflavone | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Erythribyssin K (75) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | No activity | [142] |
| Erythrabyssin II (76) | Pterocarpan | Rt | Chloroform : methanol (1 : 1), methanol | UV, NMR, HPLC | Antimicrobial (antibacterial) and radical scavenging properties | Good radical scavenging, antiyeast and antifungal activities | [134, 136] |
| Liquiritigenin (77) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | No activity | [142] |
| Liquiritigenin-50-O-methyl ether (78) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | No activity | [142] |
| Burttinone (79) | Flavone | SB | Methanol | UV, NMR, CD, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| Burttinonedehydrate (80) | Flavone | SB | Methanol | UV, NMR, CD, HRMS | PTP 1B inhibitory activity | Good activity | [138] |
| Erythribyssin G (81) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Weak activity | [142] |
| Erythribyssin I (82) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | No activity | [142] |
| 7-Hydroxy-4′-methoxy-3-prenylisoflavone (83) | Isoflavone | SB | Methanol | UV, FTIR, TLC, NMR, HMBC | Antimicrobial and antiplasmodial activities | Moderately active | [145] |
| Octacosyl-E-ferulate (erythrinasinate A) (84) | Coumaric acid | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134,145] |
| Erythrabyssin I (85) | Pterocarpan | NS | Ether, 80% MeOH | UV, NMR, EI-MS, HMBC, HPLC | Antifungal activity | Good activity | [134, 136, 141] |
| Erythrabyssin II (86) | Pterocarpan | Rt | Chloroform : MeOH (1 : 1), 80% MeOH | UV, NMR, EI-MS, HMBC, HPLC | Antimicrobial and antioxidant activities | Moderate activity | [134, 136, 141] |
| Genistein (87) | Isoflavone | Rt, Tw | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Neobavaisoflavone (88) | Flavanone | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Semilicoisoflavone B (89) | Isoflavone | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Sigmoidin A (90) | Flavanone | SB | Methanol | UV, HPLC HREI-MS, HMQC, HMBC, NOESY NMR | Antilipase activity | Exhibited antilipase activity | [82, 146] |
| Sigmoidin B (91) | Flavanone | Rt | Chloroform : methanol (1 : 1) | UV, NMR, HREI-MS, HMBC, NOESY | Antimicrobial and antioxidant activities | Good activities | [82, 134] |
| Sigmoidin B 4′-(methyl ether) (92) | Flavanone | SB | Methanol | UV, HPLC HREI-MS, HMQC, HMBC, NOESY NMR | Not tested | Not applicable | [82] |
| Phaseolin (93) | Chalcone | NS | Ether | UV, NMR, HMBC, EI-MS, HPLC | Antimicrobial activity | Good activity (antiyeast and antifungal activities) | [134, 136, 141] |
| Phaseollidin (94) | Chalcone | Rt | Chloroform : methanol (1 : 1), ether | UV, NMR, HMBC, EI-MS, HPLC | Antimicrobial and antioxidant activities | Weak activity | [134, 136] |
| Erythrartine/11-methoxyerysodine (95) | Alkaloid | Sd | Chloroform | TLC, MS, UV, NMR, | Anti-HIV-1 and cytotoxicity | Weak activity | [59] |
| Caryolane-1,9-diol (96) | Sesquiterpene | Rt | Chloroform : methanol (1 : 1) | UV, NMR, HMBC, EI-MS, HPLC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Abyssaponin A (97) | Triterpenoid | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Strong activity | [147] |
| Abyssaponin B (98) | Triterpenoid | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Strong activity | [147] |
| Soyasapogenol B (99) | Triterpenoid | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Strong activity | [147] |
| Abyssinoside A (100) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Moderate activity | [147] |
| Abyssinoside B (101) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Moderate activity | [147] |
| Abyssinoside C (102) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Weak activity | [147] |
| Abyssinoside D (103) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Anticancer activity | Moderate activity | [147] |
| Schaftoside (104) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Not tested | Not applicable | [147] |
| Isoschaftoside (105) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Not tested | Not applicable | [147] |
| Vicenin-2 (106) | Flavanone | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Not tested | Not applicable | [147] |
| Hovetrichoside C (107) | Aurananol | SB | Ethanol | NMR, UV, HRESI-TOFMS, ESI-MS/MS | Not tested | Not applicable | [147] |
| Sigmoidin C (108) | Flavanone | Rt | Chloroform : methanol (1 : 1), methanol | UV, NMR, HREI-MS, HMBC, NOESY, HPLC | Antimicrobial and antioxidant activities | Weak activity | [82,134] |
| Sigmoidin F (109) | Flavanone | Rt, SB | Chloroform : methanol (1 : 1), methanol | UV, NMR, HREI-MS, HMBC, HPLC, NOESY | Antimicrobial and antioxidant activities | Weak activity | [82, 134] |
| Quercetin (110) | Flavone | RB | Acetone | HRMS, NMR, HMBC | Radical scavenging properties | Moderately active | [133] |
| 5,4′-di-O-Methylalpinumisoflavone (111) | Isoflavone | RB | DCM: MeOH | FTIR, UV, EI-MS, NMR | Antimicrobial activity | Not active | [137] |
| Erycristagallin (112) | Pterocarpan | RB | Acetone | HRMS, NMR, HMBC | Radical scavenging properties | Moderately active | [133] |
| Shinpterocarpin (113) | Pterocarpan | RB | Acetone | HRMS, NMR, HMBC | Radical scavenging properties | Moderately active | [133] |
| Sandwicensin (114) | Pterocarpan | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| 3,6-Caryolanediol (115) | Sesquiterpenes | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| Clovane-2,9-diol (116) | Sesquiterpenes | Rt | Chloroform : methanol (1 : 1) | UV, NMR, EI-MS, HMBC | Antimicrobial and antioxidant activities | Weak activity | [134] |
| 7-Hydroxy-2-[4-methoxy-3-(3-methylbut-2-enyl) phenyl] chroman-4-one (117) | Flavanone | RB | Ethyl acetate | IR, UV, MS, CD, NMR | PTP 1B inhibitory activity | Strong activity | [142] |
| Sigmoidin E (118) | Flavanone | Rt, SB | Chloroform : methanol (1 : 1), methanol | UV, NMR, CD, HREI-MS, HPLC, HMQC, HMBC, NOESY | Antimicrobial, antioxidant and PTP 1B inhibitory activities | Weak antimicrobial and antioxidant activities, no activity | [82, 134, 138, 139] |
| Sigmoidin G (119) | Flavanone | SB | Methanol | UV, CD, NMR, HRMS | PTP 1B inhibitory activity | No activity | [138, 139] |
| 9-Ethyldodecyl-4-methoxybenzoate (120) | Benzoate ester | SB | Methanol | TLC, NMR | Antibacterial and termicidal activity | Moderate antibacterial activity | [62] |
| Lupinifolin (121) | Flavonoid | SB | Methanol | TLC, NMR | Antibacterial and termicidal activity | Moderate antibacterial activity | [62] |
| Kaempferol 3-O-(2-O-b-D-glucopyranosyl-6-O-a-L-rhamnopyranosyl-b-D-glucopyranoside (122 | Flavonol | Fl | Methanol (acidified) | NMR, DQF-COSY | Not tested | Not applicable | [148] |
- NS: not specified; Fl: flowers, Sd: seeds; SB: stem bark, Rt: roots; RB: root bark; Tw: twig; FTIR: Fourier transform infrared spectroscopy; ESI-MS/MS: electron spray ionization tandem mass spectrometry; HRESI-TOFMS: high-resolution electron spray ionization time-of-flight mass spectrometry; HMBC: heteronuclear multiple bond correlation spectroscopy; HMQC: heteronuclear multiple quantum coherence spectroscopy; CD: circular dichroism spectroscopy; HRMS: high-resolution mass spectrometry; NOESY: nuclear overhauser effect spectroscopy; DQF-COSY: double quantum filtered correlation spectroscopy; UV: ultraviolet-visible spectroscopy; MS: mass spectrometry; NMR: nuclear magnetic resonance; TLC: thin-layer chromatography; AMPK: adenosine monophosphate-activated protein kinase.
(1) Alkaloids. In the present study, we retrieved thirteen alkaloids (1–12 and 95) that have been isolated from E. abyssinica (Table 4, Figure 5). The Erythrina alkaloids have a tetracyclic carbon skeleton with three rings (A, B, and C) common to all the alkaloids and the fourth ring (D) which varies among the different alkaloids [1, 152]. Lactonic alkaloids contain ring D as an unsaturated δ-lactone, dienoid alkaloids possess a benzenoid ring D (with two double bonds at C-1 and C-2, and C-6 and C-7), and alkenoid alkaloid possess a benzenoid ring D with a double bond between C-1 and C-6. Aromatic alkaloids and those containing a double bond at C-16 undergo stereoisomerism to give rise to other alkaloid derivatives [152].
- (i)
Benzofurans. Benzofurans are heterocyclic compounds consisting of benzene and furan rings fused together. Five benzofurans (65–69) have been isolated from the stem bark of E. abyssinica [144].
- (ii)
Chalcones. Chalcones, also known as chalconoids or benzyl acetophenones, are α, β-unsaturated ketones made up of two aromatic rings (designated as rings A and B) with diverse substituents. They possess conjugated double bonds and a completely delocalized π-electron system on both benzene rings. Chalcones have been widely known in medicinal chemistry as potential templates for the synthesis of therapeutic agents [153]. In this study, seven chalcones (15, 28–32, and 47) were retrieved to have been isolated from the roots and stem bark of E. abyssinica.
- (iii)
Coumestans. Coumestans are oxidized derivatives of pterocarpans consisting of a benzoxole fused to a chromen-2-one to form 1-benzoxolo[3,2-c]chromen-6-one. They are responsible for the phytoestrogenic activity of most medicinal plants of the family Fabaceae [154]. Two coumestans, erythribyssin N (62) and isosojagol (64), were isolated from the stem bark of E. abyssinica.
- (iv)
Isoflavones and Flavanones. Isoflavones are a large group of flavonoids possessing a 3-phenylchroman skeleton that is biosynthetically obtained by rearrangement of the 2-phenylchroman flavonoid system. They are naturally occurring exclusively in the family Fabaceae (Leguminosae). Differences among isoflavones arise from the presence of extra heterocyclic rings, different oxidation states in this skeleton, and the number of substituents on the isoflavone moiety [155]. On the other hand, flavanones have the basic 2,3-dihydroflavone structure. They are distinguished from the rest of the flavonoid class by the lack of a double bond between C-2 and C-3 and the presence of a chiral center at C-2 position. Members differ from one another in the position and/or the number of the constituent methoxy and hydroxyl substituents [156]. Unlike isoflavones, flavanones are naturally occurring in members of family Fabaceae, Compositae, and Rutaceae. A total of six isoflavones (25–27, 83, 110, and 111) and 72 flavanones (14, 17–22, 24, 33–46, 48–61, 63, 70–75, 77–82, 84, 87–92, 100–103, 108, 109, 118–119, and 121–122) have been isolated from E. abyssinica root bark, stem bark, and roots.
- (v)
Pterocarpans. Pterocarpans are structural analogs to isoflavonoids with a benzofurochromene skeleton. They can also be derived from coumestans through reduction reactions. They have two asymmetric centers at C-6a and C-11a and may exist as cis or trans isomers. The presence of pterocarpans has been attributed to their synthesis by members of the family Fabaceae in response to infections by microorganisms as defense molecules [157]. Eleven pterocarpans (13, 16, 23, 76, 85, 86, 93, 94, and 112–114) were isolated from the roots and root bark of E. abyssinica [133, 134, 136, 141].
(3) Terpenoids (Sesquiterpenes and Triterpenoids). Sesquiterpenes are terpenoids with fifteen carbons (C15) consisting of three isoprene units. They are the dominant constituents of essential oils and other pharmacologically active oxygenated hydrocarbons occurring in higher plants. They naturally exist as hydrocarbons or oxygenated derivatives of hydrocarbons such as carbonyl compounds, alcohols, lactones, and carboxylic acids [158]. Three sesquiterpenes, 3,6-caryolanediol (115) and clovane-2,9-diol (116) along with caryolane-1,9-diol (96), were isolated from E. abyssinica roots [134]. On the other hand, two new triterpenoids, abyssaponin A (97) and abyssaponin B (97) along with a triterpenoid saponin, soyasapogenol B (99), were isolated from E. abyssinica stem bark [147].
3.5. Pharmacology of E. abyssinica and Isolated Compounds
In this section, we report investigations which evaluated the pharmacological potential of both extracts and isolated pure compounds from E. abyssinica. Indeed, phytochemicals in this species possess antibacterial, antifungal, antiviral, anticancer, antioxidant, anti-inflammatory, antimycobacterial, anti-HIV/AIDS, antiplasmodial, antihelmintic, antiobesity, antipyretic, antidiabetic, antianemic, and hepatoprotective bioactivities (Tables 4 and 5).
| Activity | Model used | Plant part | Extract/compound | Bioassay | Results | Author(s) |
|---|---|---|---|---|---|---|
| Antioxidant | In vitro | Ethanol, methanol | Leaves, root bark | DPPH radical scavenging assay | Extract showed dose-dependent DPPH radical scavenging activity that was comparable to that of ascorbic acid at all doses (10–320 μg/mL) | [127,159] |
| Antioxidant | In vitro |
|
Stem bark | DPPH radical scavenging assay | After 1 h, the DPPH radical scavenging activity was as follows: abyssinone VII: IC50 = 25 μg/mL, sigmoidin B: IC50 = 18.5 μg/mL, eryvarin L: IC50 = 29 μg/mL, and 3-methylbutein: IC50 = 37 μg/mL, ascorbic acid: IC50 = 18 μg/mL, gallic acid: IC50 = 4 μg/mL. and quercetin: IC50 = 7 μg/mL | [134] |
| Antioxidant | In vitro | Acetone | Root bark | DPPH radical scavenging assay | After 30 minutes, the DPPH radical scavenging activity was as follows: crude extract: IC50 = 7.7 μM, abyssinone IV: 32.4 μM, abyssinone V: 30.1 μM, abyssinin III: 21.7 μM, erycristagallin: IC50 = 8.2 μM, 3-hydroxy-9-methoxy-10-(3,3-dimethylallyl) pterocarpene: IC50 = 10.8 μM, and quercetin: IC50 = 5.4 μM | [133] |
| Anti-inflammatory | In vivo | Root bark | Methanol | Chronic trypanosomiasis-induced neuroinflammation mouse model | The aqueous extract-treated group (50 mg/kg) had lower astrocyte reactivity (34,545 astrocytes/mm3) than the untreated group (69,886 astrocytes/mm3). Also, they had a reduced degree of neuroinflammation (1.2) compared to the untreated group (2.8). The extract was thought to reduce the infiltration of inflammatory cells into the cerebrum. | [50] |
| Water | The methanol extract did not have a significant effect on the modulation of neuroinflammation | |||||
| Antihyperglycemic | In vivo | Root bark | Water | Oral glucose tolerance assay using male guinea pigs (Cavia porcellus) | 38% inhibition factor against hyperglycemia at a dose of 500 mg/kg (6 mg/kg glibenclamide = 49.6%) | [114] |
| Antihyperglycemic | In vivo | Leaf | Ethanol | Oral glucose tolerance assay using male | After 4 hours of hyperglycemia induction, the extract significantly and dose dependently reduced the mean blood glucose; 100 mg/kg = 25%, 200 mg/kg = 46.4%, 400 mg/kg = 60.7%, and 5 mg/kg glibenclamide = 35.7% | [159] |
| Wistar albino rats | ||||||
| Anticancer | In vitro | Stem bark | Ethanol | MTT and protein tyrosine phosphatase (PTP1B) inhibitory assay | Compounds exhibited PTP1B inhibitory activity with IC50 values ranging from 4.2 to 19.3 μM and strong cytotoxic activity with IC50 values from 5.6 to 28.0 μM | [160] |
| After 72 hours of exposure; MCF7: IC50 = 19.4 μM, MCF/AMR: IC50 = 12.0 μM, MCF/ADR: IC50 = 16.1 μM, MDA-MB-231: IC50 = 28.0 μM, and PTB1B: IC50 = < 30 μM. | ||||||
| Anticancer | In vitro | Seeds | Chloroform | Sulforhodamine B assay using HeLa, Hep-G2, HEP-2, HCT116, MCF-7, and HFB4 cells | The crude alkaloidal fraction showed cytotoxic activity against the tumor cells with IC50 values of 13.8, 10.1, 8.16, 13.9, 11.4, and 12.2 μg/mL against HeLa, Hep-G2, HEP-2, HCT116, MCF-7, and HFB4 cells, respectively. | [59] |
| After 72 hours of exposure, the IC50 of isolated compounds on Hep-G2 and HEP-2 cells were as follows, respectively: erythraline: IC50 = 21.60 and 15.8 μg/mL, erysodine: IC50 = 19.90 and 11.8 μg/mL | ||||||
| Erysotrine: IC50 = 21.60 and 15.8 μg/mL, 8-oxoerythraline: IC50 = 18.50 and 3.89 μg/mL, 11-methoxyerysodine: IC50 = 11.50 and 11.4 μg/mL | ||||||
| Antianaemic | In vivo | Stem bark | Water extract | Phenyl hydrazine anaemia-induced mouse model | Improved haematinic activity in a dose-dependent manner. Extracts increased the red blood cell differentials in anaemic rats at increasing doses of 50, 100, and 350 mg/kg | [128] |
| Antiobesity | In vitro | Stem bark | Sigmoidin A | Pancreatic lipase and alpha-glucosidase inhibition assay | IC50 = 4.5 and 62.5 μM for pancreatic lipase and alpha glucosidase inhibition, respectively (orlistat = 0.3 μM, acarbose = 190.6 μM) | [146] |
| Antipyretic and estrogenomimetic | In vivo | Stem bark | Methanol | Smart button data loggers using ovariectomized rats using | At a dose of 200 mg/kg, the extract reduced the average number of hot flushes (171 in treated vs. 264 in the untreated group). The treated group also had shorter durations of hot flushes (683 minutes) compared to the untreated (1935 minutes) | [161] |
| Hepatoprotective | In vivo | Stem bark | Water | Nonalcoholic fatty liver disease model using rats to evaluate the fasting blood glucose, insulin tolerance, hepatic triglycerides, serum biochemistry, and liver histology | The extract had significant effects on fasting blood glucose as well as hepatic indices including liver weights, hepatic triglycerides, liver weight-body weight ratio, serum AST, serum ALT levels, serum triglycerides, serum total cholesterol, and serum LDL-cholesterol; however, the extracts showed no significant effects on HDL-cholesterol. At high doses (400 mg/kg), the extract protected the liver against inflammation, steatosis, and hepatic ballooning | [162] |
| Wound healing | In vivo | Leaf and stem bark | Methanol | Wound incision assay | 82.1 and 88.7% wound area healed after 15 days for the stem bark and leaf extract, respectively, at a dose of 10% w/w | [94] |
| The mean skin protein was 32.5 and 35.5% for the stem bark and leaf, respectively (oxytetracycline = 40.5%). | ||||||
| Although the leaf extract had better healing properties than the bark, there was no significant difference between both extracts and the negative control | ||||||
| Antiplasmodial | In vivo | Stem and root bark | Acetone | 4-day ANKA suppressive bioassay using P. berghei | % chemosuppression: root (49.7%), stem (44.2%), and chloroquine (97.3%) | [163] |
| Antiplasmodial | In vitro | Leaves | n-Hexane | Nonradioactive antiplasmodial 3H hypoxanthine inhibition assay using P. falciparum multidrug-resistant Indochicha I (W2) and chloroquine-sensitive Sierra Leone I (D6) | After 24 hours, n-hexane extract: IC50 = 0.0 μg/mL, DCM extract: IC50 = 190.1 μg/mL, methanol extract: IC50 = 348.2 μg/mL, mefloquine: IC50 = 19.2 μg/mL. | [145] |
| Dichloromethane (DCM) | ||||||
| Methanol | ||||||
| Antiplasmodial | In vitro | Stem | Ethyl acetate extract | Nonradioactive antiplasmodial 3H hypoxanthine inhibition assay using P. falciparum multidrug-resistant Indochicha I (W2) and chloroquine-sensitive Sierra Leone I (D6) | After 24 hours, ethyl acetate extract: D6: IC50 = 7.9 μg/mL, W2: IC50 = 5.3 μg/mL, chalcones: IC50 ranged from 10 to 16 μM, flavanones: IC50 ranged from 4.9 to 13.6 μM, isoflavonoids: IC50 ranged from 18.2 to 24.9 μM, chloroquine: IC50 ranged from 0.009 to 0.08 μM, and quinine: IC50 ranged from 0.04 to 0.21 μM | [49] |
| Isolated compounds (chalcones, flavanones, isoflavonoids) | ||||||
| Antiplasmodial | In vivo | Stem and root bark | Methanol | Four-day ANKA suppressive bioassay using P. berghei and P. falciparum |
|
[28, 164] |
| Antiviral | In vitro | Seeds and stem | Chloroform, ethanol | MTT assay using HIV-1-infected MT-4 cells | Stem alkaloidal fraction: IC50 = 53 μM, efavirenz: IC50 = 45 μM | [59, 112] |
| Stem had antiviral activity (reduction factors of the viral titer of 104) against polio, Semliki forest, and herpes viruses | ||||||
| Antimycobacterial | In vitro | Stem bark | Methanol | Microdilution assay against Mycobacterium tuberculosis, Mycobacterium kansasii, Mycobacterium fortuitum, and Mycobacterium smegmatis | At a dose of 2 mg/mL, the extract completely inhibited the growth of all Mycobacterial strains (0 GU). However, at 1 mg/mL, there was significant growth of Mycobacterium tuberculosis (19741 GU), Mycobacterium kansasii (724 GU), Mycobacterium fortuitum (174 GU), and Mycobacterium smegmatis (4915 GU) | [165] |
| Antimycobacterial | In vitro | Root bark | Methanol | Microdilution assay against pan-sensitive strain (H37Rv), rifampicin-resistant strain (TMC-331), Mycobacterium avium | Antimycobacterial activity of extract against H37Rv: MIC = 0.39 mg/mL, TMC-331: MIC = 2.35 mg/mL, Mycobacterium avium: MIC = 0.39 mg/mL. The MICs of isoniazid were 0.25 μg/mL and 9.38 μg/mL for H37Rv and TMC-331, respectively | [126] |
| Antimycobacterial | In vitro | Stem bark | Methanol | Microdilution assay against M. tuberculosis | Percentage inhibition of colony formation of different combinations: 0.06 μg/mL ethanol extract with 0.01 μg/mL rifampicin and isoniazid = 99.2%, 0.06 μg/mL methanol extract with 0.01 μg/mL rifampicin and isoniazid = 99% and 0.01 μg/mL rifampicin and isoniazid = 86.2% | [166] |
| Ethanol | ||||||
| Antihelmintic | In vitro | Stem bark | Ethanol | Worm motility assessment assay on Ascaridia galli | After 24 hours of exposure, at 50 mg/kg of extracts, average number of worms immobilized out of 10: leaf = 9.46, stem = 7.17, root = 7.92, piperazine = 10 | [124] |
| Root bark | ||||||
| Leaves | ||||||
| Antihelmintic | In vitro | Leaves | Ethanol | Worm motility assessment assay on Ascaridia galli | At 5% concentration of extracts, average number of worms immobilized out of 10 at different times: 12 h = 5, 24 h = 6, 36 h = 9, 48 h = 10 | [120] |
| Antibacterial | In vitro | Stem and root barks, whole plant, leaves | Ethanol, methanol, chloroform, water | Microbroth dilution assay against S. aureus E. coli, | Ethanolic extracts inactive against E. coli, S. typhi, and P. aeruginosa. Extracts exhibited different antibacterial activities against S. aureus depending on the part of the plant and also the location from where they were harvested. In Mbarara, the root extract was more active (MIC 31.3 mg/mL) than the stem extract (MIC = 3.5 mg/mL). On the other hand, the root extract of Bushenyi was more active (31.3 mg/mL) than that of Ntungamo (4.7 mg/mL). | [19, 26, 91, 123, 127] |
| S. typhi, Bacillus cereus, and P. aeruginosa | Methanolic extract showed better antibacterial activity (6.0 mm inhibition diameter, MIC = 0.23 mg/mL) against S. aureus than the positive reference controls: ampicillin (4.0 mm) and amoxicillin (5.0 mm) | |||||
| In vitro antidiarrheal activity | Chloroform extract of the whole plant had bioactivity against S. aureus, with 7.45 mm inhibition zone diameter | |||||
| Methanolic extract of root bark showed bioactivity against S. aureus, B. cereus, and P. aeruginosa with MIC and MBC of 3.125, 50.00, and 125.00, and 6.25, 100.00, and 250.00 mg/mL, respectively. Aqueous extract of root bark showed bioactivity against S. aureus, B. cereus, E. coli, and P. aeruginosa with MIC and MBC of 3.125, 12.50, 250.00, and 125.00, and 3.125, 25.00, 250.00 and 250.00 mg/mL, respectively. | ||||||
| Leaf powder exhibited potential antidiarrheal activity in mice. | ||||||
| Antibacterial | In vitro | Stem and root bark | Methanol | Microbroth dilution assay against Bacillus cereus, E. coli, Micrococcus luteus, and P. aeruginosa | The extracts were not active on all the bacterial strains | [100] |
| Antibacterial and antifungal | In vitro | Root bark | Erythrabyssins I and II | Microbroth dilution assay against E. coli, S. aureus, Bacillus subtilis, Saccharomyces cerevisiae, Penicillium crustosum, P. aeruginosa, Candida utilis, Mucor mucedo, Cryptococcus neoformans, and Candida albicans | E. coli and P. aeruginosa: MIC values of all compounds were greater than 100 μg/mL; | [60, 141] |
| Abyssinones I, II, III, IV, V, VI | S. aureus: with exception of abysssinone II and VI, all the other compounds had MIC values below 100 μg/mL. | |||||
| Phaseolin | Bacillus subtilis: with exception of abyssinones II and VI, all the other compounds had MIC values below 100 μg/mL. | |||||
| Phaseollidin, extract | Penicillium crustosum: MIC values of all compounds were greater than 100 μg/mL. | |||||
| S. cerevisiae and C. utilis: with exception of erythrabyssin I and phaseolin, all the other compounds had MIC values above 100 μg/mL. | ||||||
| M. mucedo: with exception of erythrabyssin I, abyssinones I and II, Phaseolin, all the other compounds had MIC values greater than 100 μg/mL. | ||||||
| Extract had effective MICs at 25% (w/v) and 12.5% (w/v) with moderate fungal growth observed at 6.25% (w/v) against C. neoformans and C. albicans | ||||||
| Antibacterial and antifungal | In vitro | Stem bark | Hexane, dichloromethane, methanol | Microbroth dilution assay against E. coli, S. aureus, methicillin-resistant S. aureus (MRSA), P. aeruginosa, Klebsiella pneumoniae, Microsporum gypseum, Trichophyton mentagrophytes, C. albicans, Cryptococcus neoformans | Extracts not active on E. coli, weak activity against P. aeruginosa and K. pneumoniae (MIC greater than 50 mg/mL). The methanol extract more active on MRSA (MIC = 6.25 mg/mL) and DCM on S. aureus (MIC = 25.0 mg/mL). Hexane extracts were the least active on all strains. | [62, 167] |
| All extracts had good activity against M. gypseum (MIC less than 12.5 mg/mL) but weak activity against C. albicans and C. neoformans (MIC greater than 100 mg/mL). The hexane extract was active on T. mentagrophytes (MIC = 25.0 mg/mL). | ||||||
| Lupinifolin and 9-ethyldodecyl 2-hydroxy-4-methoxybenzoate from methanolic extract had zone of inhibition of 9.0 mm each against B. subtilis and E. coli, respectively. The compounds and crude extract inhibited Fusarium spp., Trichophyton spp., and Penicillium spp. with inhibition zones of 9.0–18.0 mm. |
- MIC: minimum inhibitory concentration; IC50: inhibitory concentration; GU: growth units.
3.5.1. Anti-Inflammatory Activity
The aqueous root bark of E. abyssinica at doses less than 100 mg/kg showed considerable in vivo anti-inflammatory activity against Trypanosoma brucei-induced inflammation in mice [50]. The extract-treated group had a lower number of astrocyte reactivity and reduced perivascular cuffing than the nontreated mice. It was suggested that the extracts reduced the infiltration of the inflammatory cells into the cerebrum of the brain. The anti-inflammatory activity was attributed to the alkaloids and flavonoids present in the extracts although the pure compounds responsible were not identified [50]. Interestingly, other crude extracts and pure compounds isolated from members of the genus Erythrina have been validated to possess good anti-inflammatory activities via different mechanisms. For example, the ethyl acetate and ethanol extracts of E. latissimi, E. caffra, and E. lysistemon showed good anti-inflammatory activity through reduction in the synthesis of prostaglandins as a result of inhibition of cyclooxygenase activity [168]. Erycristagallin isolated from E. mildbraedii inhibited leukotriene synthesis via the 5-lipoxygenase pathway, thereby demonstrating in vitro anti-inflammatory activity (IC50 = 23.4 μM) in polymorphonuclear leukocytes [169]. Three flavonoids (abyssinone V, erycrystagallin, and 4′-hydroxy-6,3′,5′-triprenylisoflavonone) isolated from the methanolic stem bark extract E. variegate had strong phospholipase A2 (PLA2) inhibitory activity with IC50 values of 6, 3, and 10 μM, respectively [170]. This implied that these compounds can significantly reduce the synthesis of arachidonic acid and consequently diminish the synthesis of prostaglandins and leukotrienes. Two prenylated flavanones (sigmoidin A and sigmoidin B) isolated from E. sigmoidea were reported to selectively inhibit 5-lipoxygenase but had no effect on cyclooxygenase-1 activity. Sigmoidin A had a dose-response inhibitory potency (IC50 = 31 mM). In the PLA2-induced mouse paw oedema assay, only the sigmoidin B inhibited oedema formation with a percentage inhibition of 59% compared to cyproheptadine (positive control) which had 74% after 60 minutes. In the TPA test, both compounds reduced the induced oedema by 89% and 83%, respectively. It was suggested that the compounds had different mechanisms of action depending on whether one or two prenyl groups were present in ring B of the flavonoid [83]. Since these same compounds have been isolated from E. abyssinica, it is highly probable that the reported anti-inflammatory activity of this plant is due to one or a combination of these mechanisms.
3.5.2. Antioxidant Activity
The in vitro 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay has been widely used to evaluate the antioxidant activity of various phytochemicals and extracts. The ethanolic extract of E. abyssinica (10–320 μg/mL) showed dose-dependent DPPH radical scavenging that was comparable to that of ascorbic acid (a known antioxidant) [159]. Abyssinone VII, sigmoidin B, eryvarin L, and 3-methylbutein isolated from the stem bark and root wood of E. abyssinica showed considerable DPPH radical scavenging potency (IC50 = 12–52 μg/mL) although the standard antioxidants (ascorbic acid, gallic acid, and quercetin) had superior activity (IC50 = 4–18 μg/mL) [134]. The acetone crude extract of the root bark of E. abyssinica (IC50 = 7.7 μg/mL) and two isolated pterocarpenes, erycristagallin (IC50 = 8.2 μg/mL) and 3-hydroxy-9-methoxy-10-(3,3-dimethylallyl) pterocarpene (IC50 = 10.8 μg/mL), showed DPPH radical scavenging activity in a dose-dependent manner similar to that of quercetin (IC50 = 5.4 μg/mL) [133]. The radical scavenging activity of these compounds is due to their free phenolic groups which can donate electrons to the radicals [171]. For flavonoids, the O-dihydroxyl structure in the B ring, the 2,3-double bond in conjunction with the 4-oxo function in the C ring, and the 3- and 5-hydroxyl groups with hydrogen bonding to the keto group are responsible for the antioxidant activity. In pterocarpans, the 3,3-dimethylallyl groups enhance the radical scavenging activities and also increase the lipophilicity of these compounds making them better antioxidants than polar flavonoids [133].
3.5.3. Anticancer Activity
The chloroform, methanol, and ethyl acetate extracts showed cytotoxic activity against different tumor cells (cervical, liver, laryngeal, colon, and breast) and strongly inhibited protein tyrosine phosphatase (PTP1B) activity with IC50 ranging between 1 and 100 μg/mL. Using the dimethylthiazol-2,5-diphenyl-tetrazolium bromide (MTT) assay, the abyssinones A–D and abyssaponins (A and B) isolated from E. abyssinica stem bark exhibited considerable cytotoxicity against MCF-7 and MDA-MB-231 breast adenocarcinoma cell lines with IC50 ranging between 12.9 and 74 μM as compared to resveratrol (IC50 = 13.9–19.3 μM) [147]. The mechanisms by which these phytochemicals mediated their anticancer activity were however not elucidated. However, related phytochemicals isolated from E. suberosa showed to induce apoptosis through the inhibition of NF-kB factor and via an increase in cytosolic cytochrome C that stimulates caspases 9 and 3 which further activates intrinsic apoptosis pathway [172].
3.5.4. Antidiabetic and Antiobesity Activity
The aqueous extract of this plant showed significant antihyperglycemic activity at a dose of 500 mg/kg in rats using the oral glucose tolerance test (OGTT) with a hyperglycemia inhibition factor of 38.5% as compared to glibenclamide (49.6%). It was suggested that probably the inhibition of the SLGT-1 and GLUT-2 transporters along with α-glucosidase were the possible mechanisms for the antidiabetic activity [114]. In an acute OGTT, the ethanolic extract of E. abyssinica significantly decreased blood glucose levels in both normal and streptozotocin- (STZ-) induced diabetic rats in a dose-dependent manner (100, 200, and 400 mg/kg) when compared with negative (normal saline) and positive control (glibenclamide) [159]. In a subchronic antidiabetic test, daily oral administration of the same doses of extract for six weeks significantly lowered blood glucose levels in STZ-induced diabetic rats in a dose-dependent manner when compared with the diabetic control group. In this study, glibenclamide (5 mg/kg) significantly lowered blood glucose in nondiabetic rats only but not in diabetic rats [159].
Benzofurans, coumestans, and flavanones isolated from the stem bark of E. abyssinica had marked stimulation of the AMP-activated protein kinase (AMPK) activity with varying potencies at 10 μM concentrations with coumestans and benzofurans showing the highest potency. The prenyl groups in coumestans and benzofurans were suggested to be responsible for the enhanced stimulatory activity while their substitution with a methoxy group in the B ring could be responsible for the decreased activation of the AMPK. Activated AMPK plays a critical role in glucose and lipid metabolism such as enhancing insulin sensitivity, stimulating glucose uptake in the muscles, suppressing gluconeogenesis in the liver, increased oxidation of fatty acids oxidation, and diminished fatty acid synthesis. All these mechanisms are responsible for the antidiabetic activity of the isolated phytochemicals [144]. Further, prenylated flavanones from the stem bark of E. abyssinica inhibited protein tyrosine phosphate 1B (PTP1B) activity in an in vitro assay with IC50 values ranging from 15.2 to 19.6 μM compared to RK-682 (positive control, IC50 = 4.7 μM). Since PTP1B regulates the insulin and leptin signaling pathways, its inhibition has been reported to result in hypoglycemic effect, and hence, its inhibitory compounds have a great potential in acting as antidiabetic and antiobesity agents [135, 142, 160]. Sigmoidin A, a flavanone isolated from the stem bark of E. abyssinica showed a considerable in vitro inhibitory activity on pancreatic lipase (IC50 = 4.5 μM) and α-glucosidase enzyme (IC50 = 62.5 μM). Although orlistat (an antiobesity drug) exhibited a superior inhibitory activity against pancreatic lipase (IC50 = 0.3 μM), the observed activity suggested that prenylated flavonoids have potential antilipase activity and hence could be antiobesity agents. Interestingly, its α-glucosidase inhibitory potency was better than that of acarbose (IC50 = 190.6 μM), a standard antidiabetic agent [146].
3.5.5. Antiparasitic Activity
The antiplasmodial activity of E. abyssinica has been evaluated using the nonradioactive antiplasmodial (in vitro) and four-day Plasmodium berghei ANKA suppressive (in vivo) bioassays [163]. The ethyl acetate extracts had strong in vitro antiplasmodial activity against chloroquine-resistant and chloroquine-sensitive Plasmodium strains with IC50 values of 5.3 and 7.9 μg/mL, respectively [49, 163]. Subsequently, isolated chalcones, flavanones, and isoflavonoids had promising antiplasmodial activity against chloroquine-sensitive and chloroquine-resistant P. falciparum strains with IC50 ranging from 4.9 to 24.9 μM although chloroquine still had superior activity [49].
Another earlier in vitro study by Kebenei et al. [143] assessed the possible use of artemisinin in combination with a potential antimalarial drug from ethyl acetate extract of E. abyssinica stem bark reported that abyssinone V isolated from the extract was effective against chloroquine-sensitive (D6) P. falciparum parasites with IC50 value of 3.19 μg/mL. The interaction of artemisinin and abyssinone V analyzed using combination ratios of 10 : 90 to 90 : 10, respectively, against P. falciparum led to the identification of an antimalarial combination therapy of artemisinin and abyssinone V with sum of fraction inhibiting concentration (FIC) of 0.79 at a ratio of 2 : 3, respectively [143].
In an in vivo study, the root extracts of this plant suppressed P. berghei infection by 77%, 71%, and 48% in mice treated at 50, 25, and 12.5 mg/kg, respectively. It was also found out that the mice treated with a higher dose (50 mg/kg) had a significantly longer survival time than those treated with lower doses and even chloroquine [164]. The crude leaf extracts of E. abyssinica had weak activity against P. falciparum chloroquine-sensitive Sierra Leone I (D6) and multidrug-resistant Indochicha I (W2) strains with IC50 ranging from 165 to 468 μg/mL [145]. Conversely, erythinasinate A and 7-hydroxy-4′-methoxy-3-prenylisoflavone isolated from E. abyssinica methanolic leaf extract had moderate antiplasmodial activity against W2 and D6 with IC50 between 120 and 150 μg/mL [145]. Isolated compounds had a much higher antiplasmodial activity than the crude extract. Isolation removes matrix interference and increases the concentration of the active ingredient at the drug target [173]. In another study, the ethyl acetate extract of this plant at 10 μg/mL inhibited the growth of P. falciparum by 83.6% as compared to chloroquine (98.1%) [73]. This antiplasmodial activity was also confirmed in E. burttii, a related species. The acetone root bark extract of E. burttii had good in vitro antiplasmodial activity against the chloroquine-resistant and chloroquine-sensitive P. falciparum strains with IC50 of 1.73 and 0.97 μg/mL, respectively [163]. The methanolic leaf extract of E. abyssinica also exhibited moderate mosquitocidal and larvicidal activities with 65.5% and 65.1% mortality and corresponding IC50 values of 231.90 and 218.90 mg/mL, respectively. However, the activities were lower compared to that of the standard drug temephos (99.90 %) [49, 145].
The antihelmintic activity of E. abyssinica has been validated using the worm motility assessment assay on Ascaridia galli. The ethanolic leaf extract of this plant at increasing doses up to 50 mg/mL had good antihelmintic activity against A. galli comparable to that of piperazine [124]. At 50 mg/mL, the extract immobilized 95% of the worms as compared to 100% of the standard drug. In another study, 5% concentration of the extract killed all the worms after 48 hours [120]. Although the active phytochemicals were not identified, it was suggested that the antihelmintic activity of this plant could be due to tannins and alkaloids present in the crude extracts. This is because tannins are polyphenolic compounds like some synthetic antihelmintic drugs such as oxyclozanide and niclosamide. Therefore, the tannins could in a similar way interfere with energy release in the worms through uncoupled oxidative phosphorylation. But also, the tannins could bind to free proteins in the gastrointestinal tract or glycoprotein on the cuticle of the helminth, thereby impairing food absorption, motility, and reproduction. On the other hand, alkaloids being able to stimulate excitatory cells could cause neurological dysfunction that result in paralysis and consequent death of the parasites [124].
3.5.6. Antibacterial and Antifungal Activities
The antibacterial and antifungal activities of the crude extracts and isolated compounds of E. abyssinica have been widely evaluated using the microbroth dilution assay against various pathogens. The bacteria tested against included Escherichia coli, Staphylococcus aureus, Bacillus subtilis, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhi, and Bacillus cereus while the fungi were Micrococcus luteus, Candida utilis, Candida albicans, Mucor mucedo, Saccharomyces cerevisiae, Penicillium crustosum, Microsporum gypseum, Trichophyton mentagrophytes, and Cryptococcus neoformans. The hexane, dichloromethane, ethyl acetate, methanol, and ethanol extracts of this plant showed antibacterial and antifungal activities with minimum inhibitory concentrations (MICs) between 3 and 10,000 μg/mL against different pathogens. Generally, the extracts had strong activity against Gram-positive bacteria and moderate to weak activity against Gram-negative bacteria [100, 123, 141, 145, 167, 174, 175]. It was suggested that this could be due to the unique cell wall of Gram-negative bacteria which consists of an additional lipopolysaccharide layer and periplasmic space that make it difficult for antibiotics to penetrate into them. The wide variation in the MIC values could be due to the difference in the resistance profiles of the tested microorganisms with those strains that are more resistant having higher values of MIC compared to the sensitive strains. Although standard drugs had superior activity, isolated pure compounds had higher activity (slightly lower MIC values) than the crude extracts. Flavonoids from the stem bark had MIC ranging between 0.3 and 10 μg/mL against B. subtilis, S. aureus, E. coli, and S. cerevisiae as compared to the antibacterial chloramphenicol (MIC = 0.001–0.5 μg/mL) and antifungal miconazole (MIC = 0.005 μg/mL) [134]. Two pterocarpans and eight flavonoids isolated from the root bark had significant activity against S. aureus and B. subtilis with MIC ranging between 6.25 and 50 μg/mL. But moderate activity against many Gram-negative bacterial and fungal strains with MIC greater than 100 μg/mL [141]. Phaseolin and erythrabyssin I showed significant antifungal activity (MIC = 6–50 μg/mL) against S. cerevisiae, C. utilis, R. chinensis, and M. mucedo [136]. In a recent study, Schultz et al. [176] reported that ethyl acetate and ethanolic extracts of E. abyssinica bark did inhibit Enterococcus faecium EU-44 (IC50 = 64 μg/mL and MIC > 256 μg/mL), Staphylococcus aureus UAMS-1 (IC50 = 32 μg/mL and MIC 64 μg/mL), Acinetobacter baumannii CDC-0033 (IC50 = > 256 μg/mL and MIC > 256 μg/mL) but had no activity against Klebsiella pneumoniae CDC-004, Pseudomonas aeruginosa AH-71, and Enterobacter cloacae CDC-0032. Further, the extracts did not exhibit quorum sensing above 40% at 16 μg/mL in a quorum-sensing inhibition plant extract library screen on S. aureus accessory gene regulator I reporter strain [176]. No study reported the mechanism of action of either the extracts or isolated compounds. Therefore, it remains to be determined whether the phytochemicals are microbiostatic or microbicidal.
3.5.7. Antimycobacterial Activity
The crude methanolic root extract of E. abyssinica showed considerable antimycobacterial activity on the rifampicin-resistant (TMC-331) and pan-sensitive (H37Rv) Mycobacterium tuberculosis strain with a MIC of 2.35 mg/mL and 0.39 mg/mL, respectively. The MICs for isoniazid were 9.38 and 0.25 μg/mL for TMC-331 and H37Rv, respectively [126]. In another study using the automated BACTEC Mycobacterial Growth Indicator Tube (MGIT) 960 TB system, the methanolic root bark of this plant inhibited the growth of four Mycobacterial strains (M. tuberculosis, M. smegmatis, M. kansasii, and M. fortuitum) at a concentration of 2 mg/mL. Isoniazid, a standard antitubercular drug had a growth inhibitory concentration of 0.5 mg/mL [177]. In a synergistic interaction study, the methanol and ethanol extracts of E. abyssinica (0.49 μg/mL) when combined with either rifampicin or isoniazid (0.01 μg/mL) had a complete inhibitory effect on the growth of M. tuberculosis (H37Rv). The standard drugs and methanol and ethanol extracts at the same tested concentration had innumerous, 125 and 10 colony-forming units [166]. It was postulated that probably the flavonoids, alkaloids, tannins, and terpenoids present in the extracts interacted with the standard drugs at the drug target levels, hence enhancing the activity of each other. The confirmed synergism could be used to explain the concomitant use of herbal medicines alongside the conventional therapies but also reaffirms the benefit of combination therapy in the management of susceptible and resistant tuberculosis. Despite the widespread use of E. abyssinica in the traditional management of tuberculosis, we did not find any reports on isolation and characterization of compounds from this plant against M. tuberculosis.
3.5.8. Antiviral Activity
The anti-HIV-1 activity of this plant was evaluated using the MTT method. The alkaloidal fraction showed cytotoxicity of HIV-1-infected MT-4 cells with an IC50 of 53 μM compared to efavirenz which had an IC50 of 45 μM. The anti-HIV activity was attributed to the isoquinoline-type alkaloids present in the fraction that inhibit the HIV-1 replication through inhibition of viral entry and reverse transcription processes [59]. The other antiviral activities of this plant have not been validated. However, erysodine, erysotrine, and erythraline isolated from E. crista-galli but also present in E. abyssinica showed significant antiviral activity against tobacco mosaic virus (TMV) with IC50 of 1.48, 1.28, and 1.52 μM, respectively, using the leaf disc method. The positive control ningnanmycin had an IC50 of 0.18 μM [178]. Of great interest was the new alkaloid glycoside, erythraline-11-β-O-glucopyranoside which showed a much superior antiviral activity (IC50 = 0.59 μM) against TMV as compared with its aglycone, erythraline (IC50 = 1.52 μM).
3.5.9. Antianaemic and Hepatoprotective Activity
The haematinic activity of this plant was evaluated in mice using the phenyl hydrazine-induced anaemic mice model. At doses less than 100 mg/kg, the aqueous stem bark extract of E. abyssinica significantly increased the diminished levels of haemoglobin (Hb), red blood cells (RBCs), and packed cell volume (PCV) in mice at the end of four weeks following daily oral administration of the extract. On the other hand, the extract did not have a significant effect on the levels of white blood cells, mean corpuscular volume, mean corpuscular haemoglobin, and other differentials. The observed antianaemic activity was attributed to the flavonoids, alkaloids, and cardiac glycosides present in the aqueous extracts. However, isolation and characterization were not done to identify the pure compounds responsible for this activity.
The hepatoprotective effect of the extract was evaluated using the nonalcoholic fatty liver disease (NAFLD) model on rats fed on high-fat and glucose diet. The water extracts at daily oral doses of 200 and 400 mg/kg for 56 days showed significant inhibitory effects against the development of nonalcoholic fatty liver disease. The extract was hepatoprotective against steatosis, inflammation, and hepatic ballooning. The extracts also significantly altered other hepatic-related biochemical indices as compared to standard drug pioglitazone [162]. This hepatoprotective activity was attributed to the coumestans, benzofurans, and pterocarpans present in the water extracts that regulate the activity of AMP kinases and protein tyrosine phosphatase 1B.
3.5.10. Antipyretic and Estrogenic Activity
The estrogenic activity of this plant was studied using the smart button data loggers’ model in ovariectomized rats. The methanol extract (200 mg/kg) and estrogen (1 mg/kg) reduced the number and frequency of hot flushes (171) as compared to those ovariectomized rats that did not receive the extract (264). Also, the rats treated with extract and estrogen had significantly reduced durations (683 and 869 minutes, respectively) of hot flashes than the untreated rats (1935 minutes). Thus, the methanol extract seemed to offer protection against small temperature rises which trigger hot flashes in the ovariectomized untreated rats. Although the real chemicals in the extract responsible for the antipyretic activity were not identified, it was postulated that the chemicals mimic estrogen by increasing the sweating threshold and thermoneutral zone size [161]. In a related study, the estrogenic activity of the erythroidines isolated from E. poeppigiana was evaluated using various estrogen receptor- (ER-) dependent test systems. These included the receptor binding affinity and cell culture-based ER-dependent reporter gene assays. It was found out that both α-erythroidine and β-erythroidine showed significant binding affinity values for ERα of 0.015 % and 0.005%, respectively, whereas only β-erythroidine bound to ERβ (0.006 %). In reporter gene assays, both erythroidines showed a significant estrogenic stimulation of ER-dependent reporter gene activity in osteosarcoma cells that was detectable at 10 nM in a dose-dependent manner [179]. These erythroidines have also been reported to be present in E. abyssinica and thus could be responsible for the estrogenic activity of this plant.
3.5.11. Anticonvulsant and Anxiolytic Activity
The long-known neuropharmacological activity of this plant was the curariform activity which is largely attributed to alkaloids present in it. Erysodine and erysopine isolated from the seeds of E. abyssinica showed significant curare-like activity both in vitro and in vivo [132]. The other CNS demonstrated activities of compounds present in E. abyssinica include anticonvulsant [180, 181], analgesic [180], and anxiolytic. In another study, erysodine and erysothrine (0, 3, or 10 mg/kg) administered orally exhibited anxiolytic effect in mice with comparable efficacy to diazepam (2 mg/kg) administered intraperitoneally. Using the elevated plus maze (EPM) model, only erysodine (10 mg/kg) increased the percentage of open arm entries and open arm time. In the light-dark transition model (LDTM), both erysothrine and erysodine demonstrated anxiolytic-like activity. However, while erysodine (10 mg/kg) increased both times spent in the illuminated compartment and the number of transitions between compartments, erysothrine (3 mg/kg) increased the number of transitions only. It was further observed that none of the two alkaloids neither altered the locomotory behaviour (i.e., the number of closed arm entries) of the animals in the EPM [182].
3.6. Toxicity Profile of E. abyssinica
Toxicological evaluation of medicinal plants, isolated pure compounds, and corresponding herbal products is one of the key requirements for their approval and licensing as pharmaceutical products by regulatory authorities. This is because apart from possessing pharmacological activity that can be exploited for therapeutic benefits, the same phytochemicals may interact with the same or different receptors and elicit toxicity. Some toxicities may either be dose-dependent or dose-independent. On the other hand, some may be immediate while others delayed. Although no substance can be declared to be completely devoid of toxicity, toxicity tests (acute, subacute, subchronic, and chronic) are used to determine the relative toxicity of potential therapeutic agents.
Despite the huge data regarding the pharmacological potential of E. abyssinica, there is a paucity of data regarding its toxicity. The seeds are traditionally known to be poisonous [11]. In an in vitro acute toxicity assay using the brine shrimp lethality model, the methanolic and ethanolic extracts of E. abyssinica had LC50 ≥ 1000 μg/mL [127] and 997 μg/mL [159], respectively. A related in vitro study using the haemolytic assay reported that the hexane (62.5 μg/mL), dichloromethane (62.5 μg/mL), ethyl acetate (62.5 μg/mL), and methanol (125 μg/mL) extracts of this plant showed low percentage haemolysis (15.5, 9.1, 15.4, and 39.7%) of red blood cells [175]. The higher percentage haemolysis observed with the methanol extract was attributed to the higher concentration of methanol extract. These in vitro results indicated that the extracts were safe within 24 hours of administration.
In a study which determined the in vivo acute toxicity of crude extracts from this plant, it was found out that the median lethal dose (LD50) of leaf and stem bark extracts was above 300 mg/kg body weight. All the mice orally administered with the extracts (100, 200, and 300 mg/kg) survived up to 72 hours and there were no significant behavioural changes between the treatment and control groups [183]. In another study, the methanolic root extract was found to have an oral LD50 of 776.2 mg/kg in mice [126]. As with the previous study, acute toxicity signs became more apparent at the highest doses. But still they were limited to sedation and reduced motor activity. Based on the OECD 2001 guidelines, since the LD50 is greater than 300 mg/kg, it can be inferred that the crude extracts are weakly toxic within 24 hours of a single high dose [184]. It is important to know that the seeds of E. abyssinica contain curare-like alkaloids. Thus, it is believed that, at high doses, these may cause anaesthesia, paralysis, and even death by respiratory failure [185].
In a subacute toxicity evaluation of the extract from this plant, the mice were dosed with 100, 200, and 300 mg/kg of the extract daily for 30 days. There was no significant difference in behaviour and physical and general activity parameters such as water intake, food consumption, and body weight between the treated groups and control group (no extract given) throughout the period of the experiments [183]. However, there were variations in biochemical parameters between the E. abyssinica-treated groups and nontreated group although it was not statistically significant. Particularly the treated group had higher levels of urea and creatinine and lower levels of potassium and sodium. There was also high total and/or conjugated bilirubin associated with E. abyssinica-treated groups. This could probably suggest possible liver insufficiency or interference with bile flow. However, this finding was inconclusive as it could be due to other contributing factors other than the liver. Another study reported that the E. abyssinica (1000 mg/kg) significantly increased the levels of urea and creatinine and level of serum diagnostic enzymes particularly alkaline phosphatase, lactate dehydrogenase, gamma-glutamyltransferase, and alpha-amylase in treated mice after 28 days of daily oral administration [128]. This probably indicated some degree of impairment of renal, liver, and heart functions. Histopathological evaluation of the tissues of the liver revealed necrotic foci, dilated and congested blood vessels, numerous hepatocytes with double nuclei in view, and infiltration of inflammatory cell, while the kidney tissues showed necrotic foci in the papillary region, loss of tubules in necrotic foci, and vacuolated cells in place of original cells. The liver being the primary detoxifying organ of the body while the kidney being the excretory organ are highly susceptible to damage by phytochemicals present in the extracts/herbal medicines.
The haematological parameters were also slightly altered by extract administration, suggesting an effect on the hematopoietic tissue [183]. As with the biochemical parameters, the assays did not conclusively show haemolysis or other blood-related toxicity of the extracts. In contrast, another study found out that the stem extract (1000 mg/kg) did not significantly alter the haematological indices of the treated rats as compared to the nontreated after 28 days of daily oral administration [128]. It can therefore be inferred that extracts of this plant have minimal toxicity effect on the hematopoietic tissue. Since this plant has been reported to have minimal toxicity on the liver, kidney, and hematopoietic tissue, it should be used with caution in traditional medicine. More evidence regarding its chronic toxicity is needed to guarantee its safety especially in the management of chronic conditions.
3.7. Clinical Studies
We did not find any relevant report reporting results of a clinical trial on either a pharmaceutical product or an herbal product from E. abyssinica. This could be probably due to the huge financial requirement to conduct clinical trials but also other challenges surrounding herbal medicine use.
4. Conclusion and Future Perspectives
E. abyssinica has been proven to harbor useful pharmacologically active phytochemicals against various diseases with significant efficacies although with some minimal toxicity profiles. There is therefore a need to generate more toxicological data about this plant and different isolated phytochemicals so as to generate sufficient evidence as regards their safety for human use. Once proven safe, the plant could provide a cheap and sustainable source of novel molecules for the development of new therapeutic agents for human ailments. To the best of our knowledge, we did not find any E. abyssinica-based pharmaceutical products in the literature, different pharmacopoeia, and drug development pipeline. The active phytochemicals identified could therefore be prioritized and/or optimized for further drug development. There is also a need to standardize and promote rational herbal medicine use through encouraging registration and licensing of products with proven efficacy and safety. Clinical studies utilizing extracts and isolated compounds from E. abyssinica are required. Due to its ethnomedicinal purposes, communities should be sensitized and encouraged to conserve this plant species.
Abbreviations
-
- AMPK:
-
- Adenosine monophosphate-activated protein kinase
-
- CNS:
-
- Central nervous system
-
- E. abyssinica:
-
- Erythrina abyssinica Lam. ex. DC.
-
- HIV:
-
- Human immunodeficiency virus
-
- LD50:
-
- Median lethal dose
-
- MIC/IC50:
-
- Minimum inhibitory concentration
-
- PLA2:
-
- Phospholipase A2
-
- WHO:
-
- World Health Organization.
Disclosure
This work was initially presented at Natural Products Research Network for Eastern and Central Africa Uganda Chapter (NAPRECA-U) in its virtual seminar held on 24 September 2020.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors are grateful to the World Bank and the Inter-University Council of East Africa (IUCEA) for the scholarship awarded to SBO, SM, MPO, and TO through the Africa Centre of Excellence II in Phytochemicals, Textiles and Renewable Energy (ACE II PTRE) at Moi University, Kenya, which made this communication possible. Our sincere appreciation goes to the preceding authors for their efforts in sharing their research findings on the medicinal values of E. abyssinica. This research was supported by the International Foundation for Science (IFS), Stockholm, Sweden, and Organisation for the Prohibition of Chemical Weapons (OPCW) through a grant to Samuel Baker Obakiro (Grant no. I-1-F-6451-1).
Open Research
Data Availability
This is a review article and no raw experimental data were collected. All data generated or analyzed during this study are included in this published article.




