[Retracted] Environmental Emission Analysis of Biodiesel with Al2O3 Nanometal Additives as Fuel in a Diesel Engine
Abstract
The exploitation of fossil fuels has fueled the modern world’s development since the industrial revolution. Other energy sources, such as wood, charcoal, and animal power, were displaced by these fuels, which were relatively easy to obtain, had low cost of production, and were easily transportable. The possibility of these fossil reserves being depleted in the medium term, combined with an increase in environmental awareness and the reality of environmental degradation, has changed the situation, reactivating the search for alternative fuels. Biofuels such as bioethanol, biomethanol, and biodiesel are among the alternative fuels gaining popularity due to their environmental benefits. This research investigates the behaviour of a diesel engine that runs on biodiesel (a fuel made from new and unrefined algae oil), ethanol (an essential raw nanomaterial that is readily available in India), and nanometal additives.
1. Introduction
Plant products have long been used at the heart of engines; for example, Rudolf Diesel, the inventor of the compression ignition engine or diesel engine, used peanut oil to power his engine [1]. According to one of his creations at the 1900 Paris exhibition, the origins of the use of nonfossil fuels can be traced back more than a century [2]. Throughout these one hundred years, and especially in the second half of the twentieth century, an endless number of investigations and experiences took place, all under the banner of “cooking oil will be the fuel of the future,” a phrase that has gotten a lot of attention in recent years [3]. Nonetheless, due to world-limited conventional fuel reserves, it is necessary to seek viable energy alternatives in the short and medium term to obtain the necessary energy from renewable sources. Biodiesel, a renewable fuel made from vegetable oils derived from seeds, plants, or oleaginous algae, deserves special attention in this regard because of its properties, which allow it to replace fossil diesel in internal combustion engines without modification [4]. They have properties similar to petroleum-derived diesel fuel and can be mixed in any proportion with conventional diesel. Due to the ability to combine several phases of its production, in this case, the obtaining phase, and the possibility of self-sufficiency in the essential input for obtaining it, oil, this fuel, which can be applied to any sector, can be very feasible, particularly for the agricultural sector. Low-cost alternatives include seed, oil extraction, and biofuel processing [5]. The addition of ethanol and nanoparticles to biodiesel blends is primarily to improve fuel combustion in the cylinder. The complete combustion of the fuel in the cylinder generates a large amount of combustion pressure on the piston’s head. Furthermore, biodiesel is an oxygenated fuel with ethanol additives [6]. It produces more heat energy and aids in complete combustion. With the addition of additives and nanoparticles, the heat release rate of biodiesel fuel increases. Many scientists and researchers have reported the engine’s combustion characteristics when using biodiesel blends with additives and nanoparticles [7]. Researchers have investigated cerium oxide 30 ppm in a neat biodiesel blend and investigated a 5 to 6% increase in brake thermal efficiency, a 3% decrease in BSFC, and a 1% increase in BP. Researchers have used 50 ppm graphene nanoparticles as an additive in Honge oil methyl ester. It was found that the graphene-based additive biodiesel fuel improved brake thermal efficiency, reduced BSFC, and improved brake power compared to conventional biodiesel without additives [8]. Adding ethanol, DEE, methanol, and n-butanol to biodiesel reduces the fuel’s overall calorific value, lowers engine performance, and increases fuel consumption. As a result, adding nanoparticles to the blends is the most innovative way to improve the blends’ properties, thereby improving performance and lowering fuel consumption. Only a few researchers have experimented with nanoparticles containing ethanol as an additive. The high thermal conductivity of graphene improves fuel properties, combustion, and engine performance. There is very little information available about graphene nanoparticles as a biodiesel additive [9]. Cooler combustion, cleaner lubricating oil, lower octane requirements, and lower emissions are all advantages of having a clean combustion chamber. Minor fuel droplets vaporize entirely, leaving no unburned residue. As a result, there is complete combustion, which increases power and mileage. Many experimental studies using a wide range of metal additives to improve fuel properties and engine performance and reduce emissions have been reported. Many experimental studies have been published that use various metal additives to improve fuel properties and engine performance while also lowering emissions [10]. Experiments have shown that bimetallic platinum and cerium diesel fuel-borne catalysts reduce engine emissions and improve the performance of the diesel particulate filter and that particulate matter emissions decrease while NOx emissions increase as oxygenate content in the fuels increases. Because of their large surface area per unit volume, CeO2 nanoparticles may have high catalytic activity, resulting in increased fuel efficiency and lower pollution emissions. ZnO powder has a wide range of applications. With this background in mind, the current research project focuses on biodiesel’s performance, emissions, and physicothermal properties with the addition of a pure Al2O3 nanopowder additive for the fuel using an ultrasonic shaking process [11]. Based on the literature review findings, it is concluded that using diesel-biodiesel with Al2O3 metal oxide nanoparticles as additives to improve performance and reduce exhaust emissions is a viable solution.
2. Characteristics of Biodiesel Used as Fuel
Biodiesel is a diesel engine fuel that is made from renewable raw materials such as vegetable oils [3]. When a vegetable oil or animal fat undergoes a transesterification reaction, this is what happens. Reacting with alcohol (methanol or ethanol) in the presence of a catalyst produces biodiesel and glycerol (NaOH or KOH). During transesterification, the catalyst and the alcohol combine to form a very strong polar chemical compound that breaks down the triglycerides in glycerin (glycerol) and forms ester chains with alcohol (biodiesel). Unsold and unrefined algae oil extracted from seeds using an endless screw machine were the raw materials used to make biodiesel in this case [12]. The oil was sieved and allowed to settle for 24 hours to remove impurities and suspended solids before drawing clean oil from the container’s top. Due to cost concerns, it was decided to use ethanol, which, despite being rarely recommended, is used in most of the literature reviews [13]. According to some reports, the water content allows for a 96% transesterification reaction with ethanol. When ethanol is used, potassium hydroxide is the most commonly recommended catalyst for initiating and speeding up transesterification [14]. The biodiesel characterization tests were carried out in the Fuels and Lubricants Laboratory of the Division Center, where this type of test is usually carried out. Table 1 summarises the findings of the research. It should be noted that algae oil has a viscosity of 35 cSt, which has been reduced to some extent but not completely. The flashpoint value was higher than average in about 40% of the cases. The rest of the variables investigated are within the biodiesel standard’s range [15].
| Properties | B20 | B20 + Al2O3 |
|---|---|---|
| Viscosity 40°C (cSt) | 12.1 | 12.3 |
| Flash point (°C) | 190 | 210 |
| Flash point (°C) | 134 | 143 |
| Density (g/cm3) | 0.905 | 0.925 |
| Viscosity index | 229 | 234 |
3. Engine Test Setup
The experimental setup consists of a 4-stroke, single-cylinder engine with a variable loading dynamometer. The setup includes the equipment required to measure the crank angle, combustion pressure, airflow, temperature, and load and fuel flow. All of these devices are linked to your computer. The configuration consists of an individual panel box comprising a fuel tank, an airbox, a manometer, fuel, an airflow measurement transmitter, a fuel-measuring unit, an engine indicator, and a process indicator. Rotational speeds are provided to measure the cooling water flow rate, and a rotameter is provided to measure the calorimeter water flow rate. Engine emissions are measured by the type 5 gas analyzer AVL DITEST Gas 1000 BL. An analysis of engine combustion, performance, and emission characteristics is possible through the experimental arrangement. The engine display includes instant pressure measurement, TDC position recording, and CA at the same instant. These measurements are necessary for engine combustion analysis and the calculation of the work indicated. The apparent HRR and the burning process’s extent are the most significantly useful quantities accessible from the engine combustion analysis’s recorded engine data. In optical systems, HR research is complex. Its use as an analytical tool covers a wide range of objectives, including the advancement of modern combustion engines; the investigation of optional fuel combustion; statistical simulation substantiation; and the study of engine geometry, isolation, and modern injection techniques. The explanation of an advanced engine with a measurement system is given here, considering the importance of representing measurements for engine development and research. The investigator must monitor and obtain the most valuable outputs from recorded data to carry out proper experimentation. For mechanical engineers, observing burned-gas pressure is important as the engine starts; the Watt indicator has been installed. While the IC engine becomes the main extensive heat machine, pressure value measurement and old-time measurement are required for its investigations and enhancement and carried out using different indicator mechanisms. Piezoelectric crystals were designed as a pressure-sensing element for phenomena occurring in the engine. At the end of the 1960s, complex analog instruments capable of performing signal processing became available. Such tools have been used initially for specific applications, such as IP assessment, IMEP, and engine misfire and knock investigation. The interface displayed voltage relative to the IMEP throughout a voltmeter or using an electromechanical device that reflected cycles in which misfire or knock occurred. When ADC was introduced into the systems at the beginning of the seventies, flexible and low-complexity investigative sets were developed. The analog signal from the amplifiers was digitally converted and stored in computer memory, allowing additional software manipulation. Therefore, higher storage capacity and suitability were assured in information investigation, retaining an appropriate accuracy level. The block diagram of the experimental installation is shown in Figure 1.
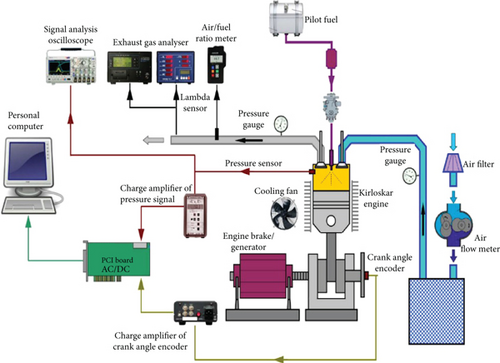
4. Speed Characteristics
A Kirloskar four-stroke engine with prechamber injection is connected to a load-transmitting cup dynamometer on the test bench. The technical characteristics of the engine and the dynamometer are shown in Table 2.
| Engine type | Make Kirloskar 1-cylinder, 4-stroke, water-cooled, diesel (computerized), modified-to-VCR engine |
|---|---|
| Model | TV1 |
| Bore diameter | 87.50 mm |
| Stroke | 110.00 mm |
| Combustion principle | Compression ignition |
| Engine capacity | 661 cc |
| Peak pressure | 77.5 kg/cm2 |
| Power | 3.5 kW at 1500 rpm |
| Dynamometer | Eddy current dynamometer cooled by water includes a loading unit |
| Airbox | Fabricated by MS including an orifice meter and U-tube manometer |
| Fuel tank | Maximum capacity of 15 liters including metering column |
| Piezoelectric sensor | Range 345 bar, including no noise cable |
| Encoder | 1-degree resolution, 5500 rpm speed including TDC pulse |
| Data acquisition system | NI USB-6210, 250 kb/s, 16 bit |
| Digital voltmeter | 0-200 mV range mounted on panel |
| Temperature detector | RTD-type, K-type thermocouple, and PT100 |
| Load indicator | 0-50 kg range, digital type |
| Load sensor | Strain gauge with 0-50 kg range |
| Direction of rotation | Clockwise (looking from flywheel end side) |
| Idle speed range | 750 rpm to 2000 rpm |
| Min. running speed | 1200 rpm |
| Fuel timing for the engine | 23° BTDC |
| Valve clearance | At inlet: 0.18 mm |
| Valve clearance | At exhaust: 0.20 mm |
| Bumping clearance | 0.046 inches to 0.052 inches |
| BMEP at 1500 rpm | 6.35 kg/cm2 |
| Lubricating oil pump | Gear type |
| Lub. oil pump | 6.50 l/min |
| Sump capacity | 2.70 liters |
| Lub. oil consumption | 1.5% normally exceed fuel |
| Connecting rod length | 234 mm |
| Overall dimensions | 617 × 504 × 877 mm |
| Weight | 160 kg |
| Software | “EnginesoftLV” engine performance analysis |
4.1. Injection Advance Angle
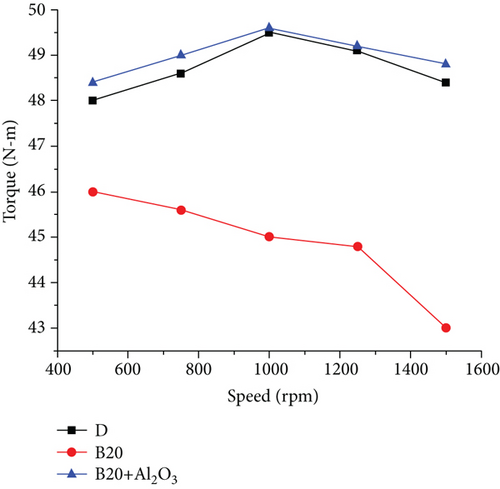
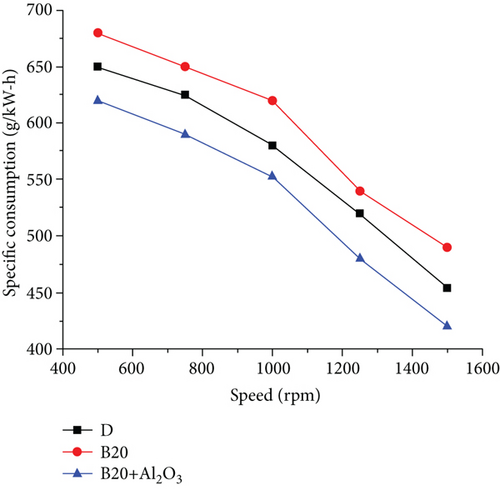
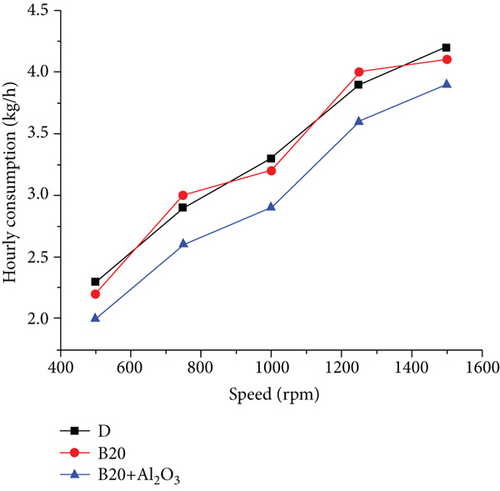
Biodiesel fuel increased engine temperature above typical working values in the first stage of the experiment, reaching 98°C. This is due to the fact that, under the same conditions, the combustion delay time for gasoline increases when compared to diesel [16]. This is due to gasoline’s higher flashpoint. The injection angle was increased from 10 to 20°, and the temperatures returned to normal, ranging from 50 to 72°. The atmospheric conditions in which the experiment was carried out were as follows: atmospheric pressure, 1,017 bar; dry bulb temperature, 30°C; wet-bulb temperature, 26°C; relative humidity, 75%. The torque curves in the upper part of Figure 2 correspond to torque values with differences of 5 to 12%. The smallest differences occur at the lowest rotational speeds, and as the motor revolutions increase, the differences become larger [17]. The specific fuel consumption is shown in Figure 3. It can be seen that for low revolutions, these curves almost overlap, resulting in similar consumptions per unit of power [18]. The differences are more pronounced in the middle zone of rotation speed, increasing proportionally with the motor rotation speed. These are observed slopes and similar behaviour characteristics, except that diesel is slightly higher than biodiesel, and the point where they differ in their highest value, about 10%, is at 1460 rpm. The behaviour of hourly consumption (Figure 4) is depicted by the last pair of curves at the bottom of the graph. As the engine revolutions increase, these curves trend upward [19]. It should be noted that the hourly consumption of biodiesel is approximately 12% higher than that of diesel. The behaviour of engine parameters when using diesel and biodiesel is at a rotational speed of 1450 rpm, the point at which the differences are most significant [20]. The torque, which is 9.45% lower when using biodiesel, is represented by the first pair of bars from the left to the right, followed by the bars of effective power. When using biodiesel, this decreases by 9.76%. The third set of bars shows the consumption schedule, which shows that biodiesel consumption is 11.95% higher than diesel [21]. The fourth set of bars, which represents specific consumption, shows that biodiesel consumption is 24.25% higher than diesel consumption. Finally, the last bars show engine performance, which is 18.99% lower than diesel when using biodiesel [3].
4.2. Exhaust Gas Emissions in the External Speed Test
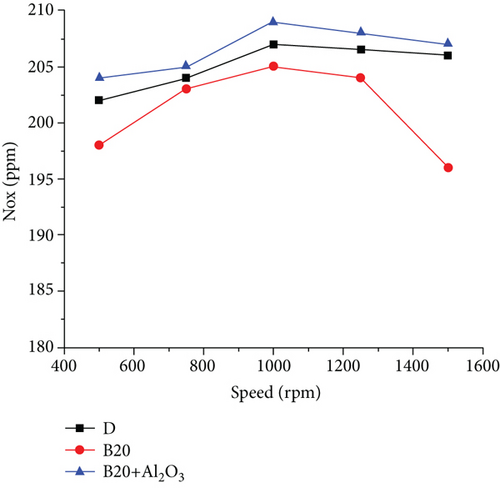
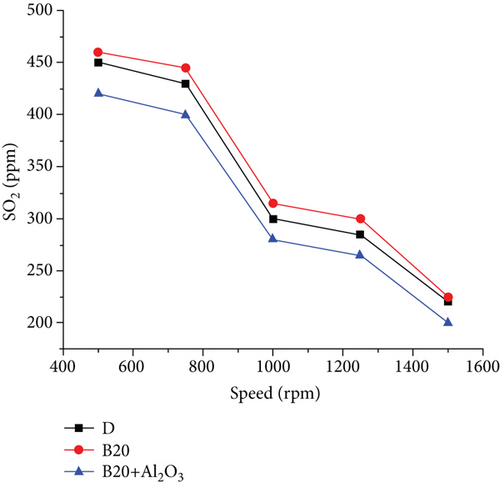
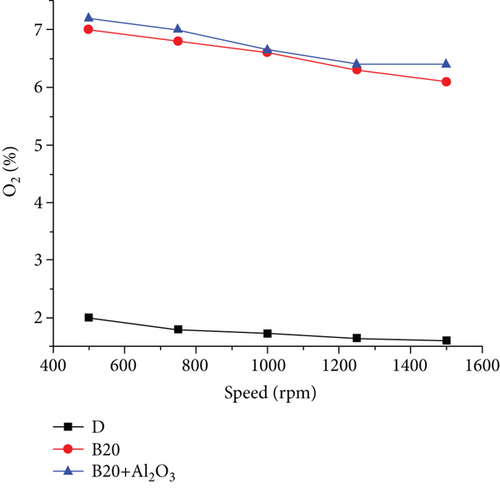
The measurement of gas emissions was carried out for three points, similar for both fuels, to achieve a reference for comparison between them [22]. Figures 5 and 6 show the behaviour of gas emissions, paying particular attention to NOx and SO2 emissions for three different engine rotation speeds. The first component of the exhaust gases to be analyzed is SO2 which, as can be seen in the case of diesel at 1000 rpm, is at a concentration of 420 ppm; at 1250 rpm, it is at a concentration of 368 ppm; and at 1500 rpm it is at a concentration of 240ppm. For those same points using biodiesel, the SO2 concentration was 0 ppm, which shows that the sulfur content of biodiesel is zero [23]. The exhaust gas temperature parameter is higher when using diesel fuel; these curves are similar, with a tendency to increase as the rpm increases. For the case of diesel, the higher temperature is due to the higher caloric power of this fuel compared to biodiesel, and the percentages of temperature increases at each point are 5.4%, 10.8%, and 12.8%, respectively [24]. The maximum temperature values in diesel reach up to 399°C, while they reach up to 353°C when using biodiesel. The nitrogen oxide emissions in the engine are given by the maximum temperature reached in combustion. As in this case, diesel is higher, and then the emission of nitrogen oxides will be higher. These curves have similar behaviour. However, that corresponding to diesel is higher and reaches a maximum value of 215 ppm [25]. In comparison, for biodiesel, the maximum value is 115 ppm, representing an almost double reduction of this pollutant. Figure 7 shows the behaviour of excess air and the remaining free O2 in the exhaust gases [18]. The O2 found present in the exhaust gases using biodiesel is higher than that recorded when using diesel. In biodiesel, a certain amount of oxygen is present, higher than that found in diesel, and participates in the combustion reaction [26]. Part of the oxygen enters with the intake air and leaves without reacting with the exhaust gases. These differences are even more accentuated when working at low rotation speeds, reaching a difference of 65%. Therefore, the excess air for biodiesel is higher than that for diesel, and the minimum point is at approximately 1250 rpm [27].
5. Conclusions
The experiments in the diesel engine using biodiesel made from algae oil and as raw materials are similar to those reported in the literature. The viscosity of the biodiesel used was acceptable for use in the motor used, even though it was still higher than that specified in the ASTM standard. The sulfur dioxide emissions are reduced to zero, and nitrogen oxides are reduced by half when biodiesel is used. Carbon monoxide emissions are also reduced. The differences in engine parameters when using diesel and biodiesel fuels increase as the engine revolutions increase. The torque, power, hourly consumption, and specific consumption differences for the engine are highly recordable.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper. This study was performed as part of the employment of the authors at Ambo University, Ethiopia.
Open Research
Data Availability
The data used to support the findings of this study are included in the article.




