Application of a New Generalized Fractional Derivative and Rank of Control Measures on Cholera Transmission Dynamics
Abstract
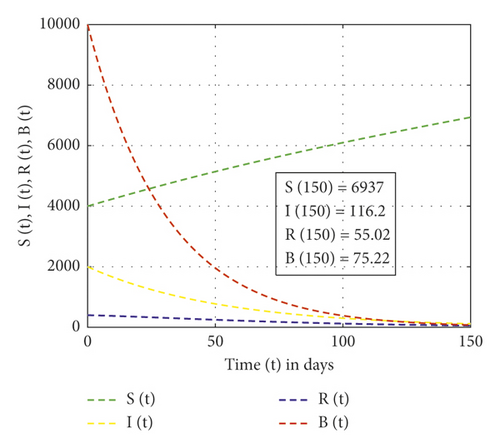
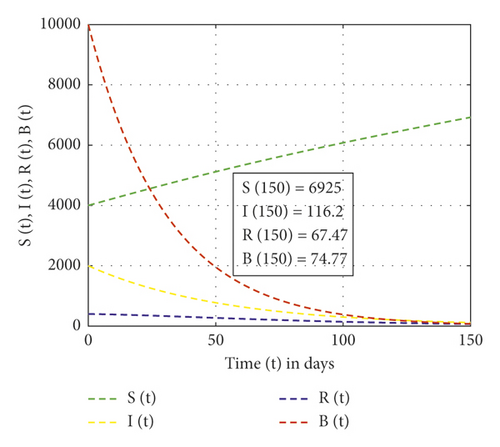
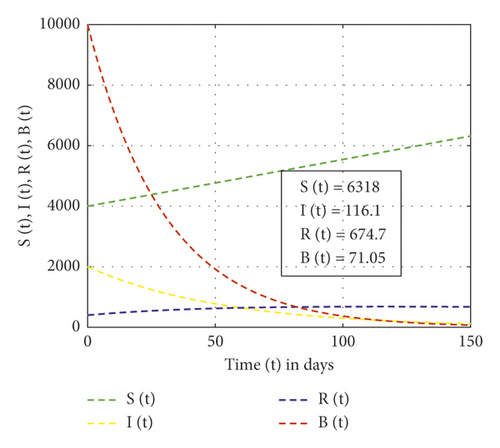
In this study, the mathematical model of the cholera epidemic is formulated and analyzed to show the impact of Vibrio cholerae in reserved freshwater. Moreover, the results obtained from applying the new fractional derivative method show that, as the order of the fractional derivative increases, cholera-preventing behaviors also increase. Also, the finding of our study shows that the dynamics of Vibrio cholerae can be controlled if continuous treatment is applied in reserved freshwater used for drinking purposes so that the intrinsic growth rate of Vibrio cholerae in water is less than the natural death of Vibrio cholerae. We have applied the stability theory of differential equations and proved that the disease-free equilibrium is asymptotically stable if R0 < 1, and the intrinsic growth rate of the Vibrio cholerae bacterium population is less than its natural death rate. The center manifold theory is applied to show the existence of forward bifurcation at the point R0 = 1 and the local stability of endemic equilibrium if R0 > 1. Furthermore, the performed numerical simulation results show that, as the rank of control measures applied increases from no control, weak control, and strong control measures, the recovered individuals are 55.02, 67.47, and 674.7, respectively. Numerical simulations are plotted using MATLAB software package.
1. Introduction
Cholera is a waterborne acute gastrointestinal infection characterized by diarrhea and vomiting, which kills within an hour if not treated [1]. Cholera is caused by drinking water or eating food contaminated by a bacterium called Vibrio cholerae (V. cholerae) [2, 3]. Most of the diseases are controlled by public health organizations including cholera which is characterized with severe vomiting and diarrhea [4]. The incubation period of cholera is less than five days [5]. However, cholera outbreak occurs twice in endemic areas, and it becomes the major issue in developing countries. Cholera infection can be transmitted from human to human or human to environment through effective contacts with bacterium V. cholerae [6].
Different mathematical models have been carried out to describe the transmission dynamics of cholera infection [7, 8]. Codeco developed the first basic mathematical model for cholera infection. Furthermore, a new fractional derivative was developed by Hattaf [9] and applied to analyze the memory effect on the HIV-infected population. However, the new fractional derivative has not been applied to study the cases in the cholera infection.
In this study, we modified the model developed in [5], we focus on the logistic growth model of Vibrio cholerae concentration in reserved freshwater used for drinking purposes, and the contribution of infected individuals to the environment is assumed to be properly managed. That is, all waste disposals from cholera-infected patients are properly managed, and the contributions of humans to the environment are restricted. Hence, we developed an optimal control mathematical model that takes into consideration the loss of immunity after cholera infection recovery. Our model considers the reimmunization system for those individuals that lose immunity. Furthermore, we have applied a new fractional derivative and analyzed the relationship between the order of the derivative and the extinction status of cholera infection.
2. Mathematical Model Developments
In this study, we have developed the deterministic mathematical model of cholera with optimal control strategies. We have divided the total population as (i) susceptible (S): these are individuals free of the infection but can be infected with the infection if exposed to the infectious people or environment contaminated with the cholera-causing bacterium; (ii) infected (I): these are individuals that get infected with cholera and can transmit infection to others with possible contacts that cause infection; (iii) recovered (R): these are individuals recovered from the cholera infection with treatment. The recovered individuals who lose immunity become susceptible; (iv) concentration of the Vibrio cholerae bacterium B(t): this covers an environment contaminated with Vibrio cholerae that cause the cholera epidemic.
- (i)
The total population is considered to be nonconstant
- (ii)
All humans die due to cholera at the disease-induced death rate of φ
- (iii)
All waste products contain Vibrio cholerae which are assumed to be managed, and there is no contribution of the infected person to reserved freshwater
- (iv)
All human populations are assumed to die at the natural death rate μ
- (v)
All new infected human individuals are recruited to the susceptible at the recruitment rate λ
- (vi)
Treatment is performed in reserved freshwater
- (vii)
Cholera-infected individuals recover at a recovery rate of η
- (viii)
Cholera infection-recovered individuals lose immunity at the rate γ
- (ix)
Cholera infection is transmitted from human to human at the rate β
- (x)
V. cholerae is ingested from the environment at the ingestion rate βc
- (xi)
The saturated incidence rate is used in the transmission of cholera infection from the environment to humans
- (xii)
The concentration of V. cholerae in water that causes 50% chance of cholera infection is denoted by κ
- (xiii)
The carrying capacity of V. cholerae is K
- (xiv)
V. cholerae die at the rate of ν
- (xv)
Cholera infection transmission preventive control effort is u1
- (xvi)
Immunity loss control effort is u2
- (xvii)
V. cholerae clearing control effort is u3
In general, the stated assumptions can be described by the flow diagram.
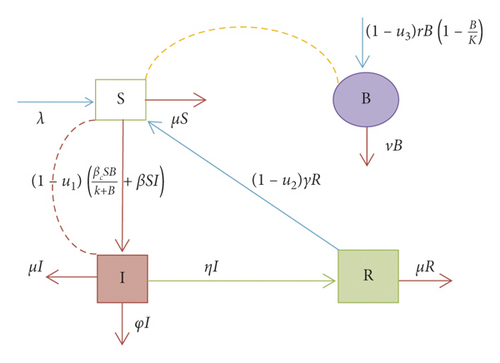
3. Mathematical Analysis of Only Cholera Model without Optimal Control
3.1. Invariant Regions
Theorem 1. Solutions of model (1) are invariant in the region such that
Proof. As in [10], to prove this theorem, we add the first three equations of model (1) so that
Applying a definite integral over time interval [0, t], the preceding inequality gives
Here, as time t gets larger and larger, Nh approaches to least upper bound λ/μ. Also, Nh ≤ λ/μ , for all time t. Similarly, from the last equation of model (2), we obtain B ≤ K for all time t. Therefore, the invariant region is given by
3.2. Positivity Property of Solutions
Theorem 2. For given positive initial conditions, all possible solutions of model (1) are positive in the invariant such that
Proof. To prove the positivity property, we apply the methods employed in [11]. Consider the first equation of model (1):
Rearranging the preceding equation, we get
Also, we have
Applying integration on the preceding equation over time interval [0, t], we get
Hence,
Since the expressions
3.3. Disease-Free Equilibrium (DFE)
3.4. Endemic Equilibrium (EE)
3.5. Reproduction Number
3.6. Local Stability of Disease-Free Equilibrium
Theorem 3. The disease-free equilibrium (E0) of model (2) is locally asymptotically stable for the reproduction number less than one and intrinsic growth rate (r) of bacteria less than the natural death rate. On the contrary, E0 is unstable for the reproduction number greater than one.
Proof. To approve the local stability of E0, we compute the eigenvalues of the Jacobian matrix obtained from model (2) and evaluated at E0. Hence, it follows that
The computed eigenvalues of the preceding matrix are given by
From the stability principle on the eigenvalues computed from the Jacobian matrix evaluated at E0, we conclude that the disease-free equilibrium (E0) is locally asymptotically stable if R0 < 1 and r < ν and unstable if either R0 > 1 or r > ν.
3.7. Global Stability of Disease-Free Equilibrium
-
H1: for dX/dt = H(X, 0), X0 is the globally asymptotically stable equilibrium
-
H2: for (X, Y) ∈ Ω
Here, P = DYG(X, 0) satisfy the condition of the Metzler matrix, and Ω is a region of feasible solutions. Now, we state the following theorem.
Theorem 4. The disease-free equilibrium of the cholera dynamic model is globally asymptotically stable if R0 < 1 and r < ν if conditions H1 and H2 are satisfied and unstable whenever R0 > 1.
Proof. From model (2), we have
Solving H(X, 0) = 0, we obtain S = λ/μ. Hence, X0 = (λ/μ, 0).
Here, X0 is the globally stable equilibrium of equation
From the infected compartments of model (2), we obtain
At the disease-free equilibrium, the preceding equation reduces to the following form:
Now, it follows that the formulated model satisfied the hypothesis conditions required as , where . Therefore, E0 is globally asymptotically stable if R0 < 1.
3.8. Bifurcation Analysis
Theorem 5. The endemic equilibrium (E1) of model (2) exhibits backward bifurcation at R0 = 1.
Proof. Let β∗ be a bifurcation parameter corresponding to parameter β and obtained by setting R0 = 1 and solving for β∗. Therefore, the bifurcation parameter (β∗) is given by
To determine types of bifurcation, we construct Jacobian matrix J(E0, β∗) from model (2) and compute the eigenvalues of J(E0, β∗). Thus, we have
The computed eigenvalues of the preceding matrix are given by
Here, all eigenvalues are negative except λ2 which is simply 0 with condition r < ν. Therefore, according to Akanni et al. [14], model (2) exhibits bifurcation at bifurcation parameter β∗. To determine the type of bifurcation, we compute left eigenvector and right eigenvector that satisfy the conditions v · J(E0, β∗) = 0, J(E0, β∗) · w = 0, and v · w = 1. Hence, the computed left eigenvalues from equation v · J(E0, β∗) = 0 are given by
And the computed right eigenvalues from equation J(E0, β∗) · w = 0 are given by
Also, from the condition v · w = 1, we have
Let us choose v2 = w2 = 1; we compute the following second-order partial derivatives of functions evaluated at the disease-free equilibrium and bifurcation parameter.
Now, set and
Next, the bifurcation coefficients a and b can be computed as follows:
Hence, according to Akanni et al. [14], the model shows supercritical (forward) bifurcation at R0 = 1.
3.9. Stability of Endemic Equilibrium (E1)
Theorem 6. Endemic equilibrium is locally asymptotically stable if R0 > 1.
Proof. It follows from the center manifold theory, a model that shows forward bifurcation has endemic equilibrium that exhibits locally asymptotic stability if R0 > 1 [14].
Theorem 7. Endemic equilibrium is globally asymptotically stable if R0 > 1.
Proof. It follows from the center manifold theory, a model that shows forward bifurcation has endemic equilibrium that exhibits globally asymptotic stability if R0 > 1 [14].
4. Application of a New Generalized Fractional Derivative with the Nonsingular Kernel
Definition 1. (new generalized fractional derivative with nonsingular kernel; see [9]).
Let α ∈ [0,1), β, γ > 0, and f ∈ H1(a, b). In the sense of Caputo, the α-order new generalized fractional derivative of function f(t) with respect to the weight function w(t) is defined as
For simplicity, we symbolize by . Assuming that when a cholera infection spreads within a community, the individuals get knowledge about the infection, we replace the classical derivative by . Then, (41) becomes
Applying the Laplace transform to (43), we get
Let aα = N(α) + μ(1 − α). Then,
Therefore,
Applying inverse Laplace, the preceding equation reduces to
Letting w(t) = 1 and applying the integration by parts, the preceding equation reduces to
In the next section, using numerical simulations, we will analyze the impact of the order of the new fractional derivative on the behavior of solutions of the cholera infection model.
5. Numerical Simulations
The parameters presented in Table 1 are used in the simulation of solutions of the SIR-B model and are either assumed logically or taken from the literature.
6. Results and Discussion
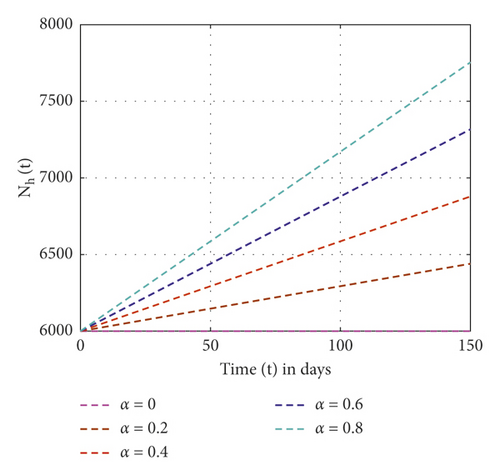
In this study, the three-dimensional dynamical system is formulated and analyzed, considering the interaction of human to human and human to environment in the transmission of the Vibrio cholerae bacterium. The analysis of the SIR-B model of the cholera epidemic shows that we can control cholera more effectively if we apply strong effort on the preventive, sanitation, hygiene, vaccination, and follow-up program. Figure 2 shows that, without control measures, the recovered individuals and others become susceptible. Figure 3 shows that weak control measures do not bring a significant change on the epidemic even though the number of pathogens is reduced. Figure 4 shows that the high contribution of control measures helps in the reduction of the Vibrio cholerae bacterium in freshwater but increases recovered individuals. Also, from Figures 2–4, one can observe that, as control measures increase applied as no control, weak control, and strong control, the recovered individuals increase as 55.02, 67.47, and 674.7, respectively. The SIR-B model of cholera exhibits globally asymptotic stability at disease-free equilibrium if R0 > 1 and unstable if r > ν. Figure 5 describes that, as the order of fractional derivatives increases, the total population size approaches to disease-free equilibrium of the cholera model. This shows that, as the human population gets free of cholera, the behaviors of the population change along with the order of the new fractional derivative. Furthermore, the memory effect results we obtained in this study are supported with the study done in [9].
7. Conclusion
The current study pointed out that, as we apply strong control measures at all levels, the concentration of Vibrio cholerae in freshwater will be reduced. Furthermore, the study pointed out that, to significantly reduce the concentration of Vibrio cholerae in reserved freshwater, the intrinsic growth rate of the Vibrio cholerae bacterium must be less than the natural death rate of the Vibrio cholerae bacterium in freshwater. The stability of the disease-free equilibrium SIR-B model is accompanied with the intrinsic growth rate of Vibrio cholerae that needs to be less than its natural death rate and reproduction number less than one. The new fractional derivative results in that the memory effect in the human population has a direct relationship. As the order of the new fractional derivative increases, human memories of cholera infection controlling also increase.
Disclosure
This article is a part of a PhD thesis at Wollega University.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The first author would like to thank Hawassa College of Teacher Education, Ministry of Science and Higher Education, and Wollega University for their support to join the PhD program and continue in the research work.
Open Research
Data Availability
No data were used to support this study.




