Growth and Characterizations of Organic NLO Imidazolium L-Tartrate (IMLT) Single Crystal
Abstract
Good quality single crystals of organic imidazolium L-Tartrate (IMLT) are grown up from aqueous solution by slow solvent evaporation technique. Various structural parameters and monoclinic crystal structure have been confirmed using powder X-ray diffraction method. The presence of various functional groups has been identified by ATR-FTIR. UV-Vis-NIR spectroscopy has shown more than 60% of optical transparency with the lower UV cutoff at 245nm. The optical band gap value of the material is evaluated to be 4.8 eV. Other optical parameters such as refractive index, optical and electrical conductivity, Urbach energy, extinction coefficient, and optical and electrical susceptibility have been evaluated from transmission spectrum data. The above essential parameters manifest appropriate usage of IMLT as an NLO material. The thermogravimetric analysis indicates high thermal stability of material up to 214°C. Apart from that, the dielectric study at various temperatures confirms decrement of dielectric constant and dielectric loss at higher frequencies. The efficiency of Second Harmonic Generation (SHG) is found to be 3.5 times that of the KDP crystals. A range of analysis suggests suitability and potentiality of IMLT crystal for various optoelectronic applications.
1. Introduction
Though the inorganic materials are enjoying their dominance in application of NLO devices, the organic NLO materials are gradually increasing their share in device applications. The organic material crystals with good molecular hyperpolarizability, quadratic nonlinear optical properties, laser damage threshold, molecular nonlinearities, and high structural diversities are preferred for the NLO device applications [1–3]. In recent years, development of various organic donor-ᴨ-acceptor (D-ᴨ-A) systems has drawn remarkable attention due to their highly polarizable structure and efficient intramolecular charge transfer (CT) properties [4]. Against inorganic material, organic materials have a conveniently polarizable push–pull system which enables their usage for two-photon absorbing contrivance, optoelectronic devices, optical data storage mechanism, and organic LED and photovoltaic cells [5–7].
Imidazole has amphoteric property and high polarizability that is expected to contribute adequately to the optical nonlinearity with other moieties. By adding the conjugated bonds or substituting donors and acceptors, the optical nonlinearity can be increased. In the case of synthesis of the titled compound, the Imidazole donates pair of electrons to L-Tartaric acid. Tartaric acid is a reasonably resilient diprotic chiral α- hydroxyl acid having pka1=2.93 and pka2=4.23. Hence it is potentially adept to form 1:1 and 1:2 proton transfer salts with most of Lewis bases [8], although it is possible to form 1:1 hydrogen tartrate salts using stoichiometric control and large number of them have been studied [9]. The ferroelectricity study of bis(imidazolium) L-Tartrate [10], structural, thermal expansion, laser damage threshold (LDT) and SHG efficiency [11], growth with SR method along with dielectric study [12], and quantum mechanical calculation using DFT [13] of imidazolium L-Tartrate has been reported. In the present work an organic NLO crystal of imidazolium L-Tartrate (IMLT) has been grown and characterized by Powder XRD, FTIR, UV-Vis spectroscopy, Thermogravimetry, and linear optical and SHG studies. Some essential dielectric and optical parameters have been reported furthermore. This collective study with mentioned imperative parameters will facilitate investigating the potential of IMLT single crystal as NLO material and its other applications.
2. Experimental
2.1. Synthesis and Crystal Growth
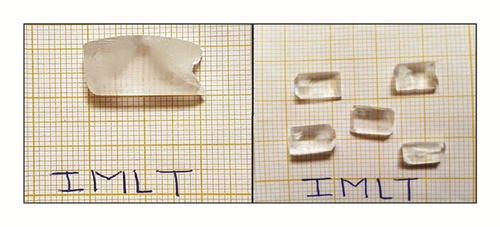
IMLT has been synthesized through mixing AR mark Imidazole (purity ≥ 99%) and L-Tartaric acid powder (purity ≥ 99.5%) in equimolar ratio (1:1) by dissolving in double distilled water. To obtain a homogeneous solution, mixture was stirred for about 100 min at constant temperature of 40°C using magnetic stirrer. The mixture was filtered with Whatman filter paper no 41 and kept in a glass beaker. The beaker was sealed with perforated polythene cover and kept back at constant temperature of 30°C in a dust free environment. The synthesized solution was purified by successive recrystallization process. After 15 days, transparent, good quality, rectangular crystals were recovered having a maximum size of 20mm x 10mm x 5mm as shown in Figure 1.
2.2. Characterization Techniques
The crystal structure was determined by Powder XRD technique through PANalytical system employing Cu Kα radiation. The results were analyzed with the help of Powder X software. For FTIR study, the material was kept in Bruker Optics Attenuated Total Reflection (ATR) setup in range of 450 cm−1 to 4000 cm−1. The UV-Vis-NIR transmission spectrum was recorded for 2.5 mm thick crystalline sample within range of 200 to 1400 nm with a slit width of 5nm and scan speed of 240 nm/min using Perkin Elmer Lambda Spectrophotometer. The scanning range covered near-ultraviolet (200-400 nm), visible (400-800 nm), and NIR (800-1200 nm) regions. Thermal analysis of the material has been carried out from room temperature to 800°C using Perkin Elmer thermal analyzer setup at heating rate of 10°C/minute in N2 atmosphere. The dielectric study was carried from 10 Hz to 10 MHz at different temperatures on pelletized crystals of the known dimensions using PSM-1735 impedance analyzer. The SHG efficiency was evaluated using Kurtz and Perry powder method for Nd:YAG laser of 1064 nm and the result was compared with the standard KDP crystal [14].
3. Results and Discussions
3.1. Powder XRD
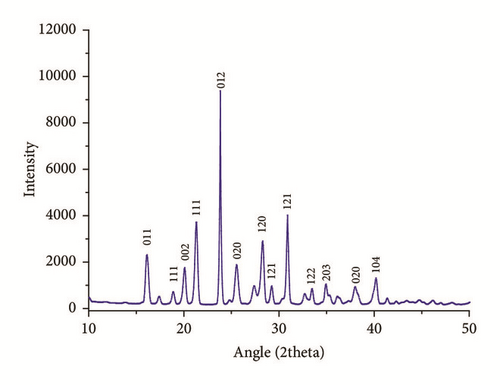
The observed diffraction pattern is shown in Figure 2 and cell parameters of IMLT crystals were identified and given in Table 1. The unit cell parameters are found in very good agreement with previously reported values [11, 13]. The crystallite size has been obtained by Scherrer equation, L = Kλ/Wcosθ, where k = 0.94, λ = 1.54178 Å, and W is full width at half-maxima. The crystalline size allied with strongest peak (012) is obtained as 59.51nm.
3.2. ATR Spectral Analysis
The subsistence of various functional groups has been recognized using FTIR-ATR spectral response. The observed bands arise due to vibrations of Imidazole cations, tartrate anions, and hydrogen bonds. Figure 3 exhibits FTIR spectrum of IMLT.
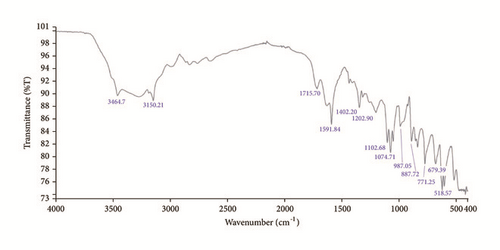
The FTIR spectrum of IMLT shows peak referring towards the O-H stretching at 3464.7cm−1. Aromatic stretching due to Imidazole is also observed at 3150.21cm−1. The intensity peak at 1715.7 cm−1 of carboxyl group (C=O) band is attributed to L-Tartaric acid. The sharp absorption peak at 1591.84 cm−1 is observed to be attributed to C=C aromatic ring vibrations. The peaks correspond to C-O bond of carboxylic acid observed at 1202.90 cm−1 and 1102.68 cm−1. Absorption peaks at 887.72 cm−1, 771.25 cm−1, and 679.39 cm−1 are corresponding to Imidazole aromatic ring.
3.3. UV-Visible Studies
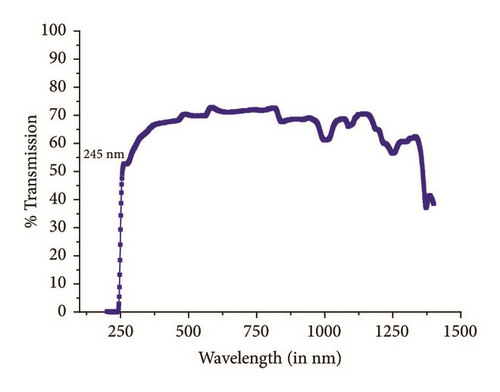
Optical characterization is a vital parameter to be considered for good quality check of crystalline materials. At lower wavelengths, the optical transmission is discontinued in ultraviolet region due to absorption of energy which arises from electronic transition from valance band to unfilled conduction band [15]. Figure 4 displays a plot of %transmittance versus wavelength (nm) of crystal having thickness of 2.5mm. High degree of transparency with no significant absorption is observed within range of 300nm-1400nm. In Figure 4 a strong absorption with a sharp fall of transmittance at 245nm shows lower cutoff wavelength of title compound. This proposes almost homogeneous distribution of energy among all molecules of IMLT crystal, which else will results in a gradual decrease in transmittance from the longer wavelength to 245nm. The undulations witnessed at around 800 nm are due to instrument artefact. Absorption bands observed in the region from 600 nm to 1400 nm are attributed to intramolecular charge transfer transitions among the marginal donor group and acceptor core [16]. Sufficiently low cutoff wavelength and wide transparency window of a crystal suggest good optical quality. The absorption coefficient (α) and the optical parameters, such as refractive index (η), optical band gap (Eg), and extinction coefficient (K), can be determined from the transmission (T) spectrum based on the standard formulas. The crystalline quality has been observed by calculation of Urbach energy value.
A plot of (αhν)1/2 versus hν is shown in Figure 5. To decide the optical band gap value experimentally, m=1/2 is found as the best fitted value for the linearity of the plot and that indicates indirect band gap transition. The traversing point on energy axis indicates the band gap energy value of IMLT crystal. The optical band gap value of IMLT crystal is Eg = 4.80 eV. Band gap energy of crystal conveys the capability of dielectric medium (crystal) to be polarized under the effect of strong radiation. The large band gap of IMLT crystal supports the requirement for the good quality nonlinear optical applications.
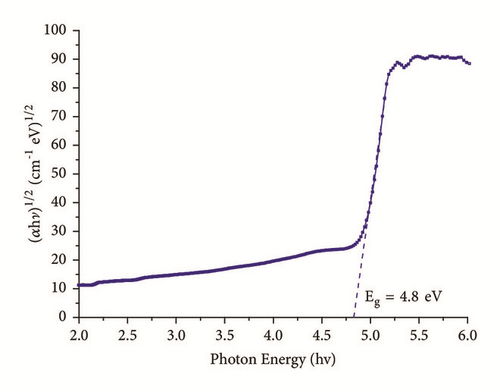
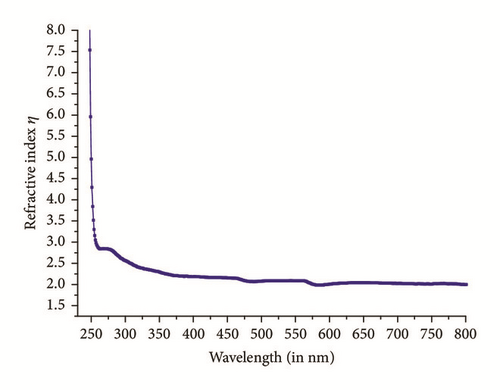
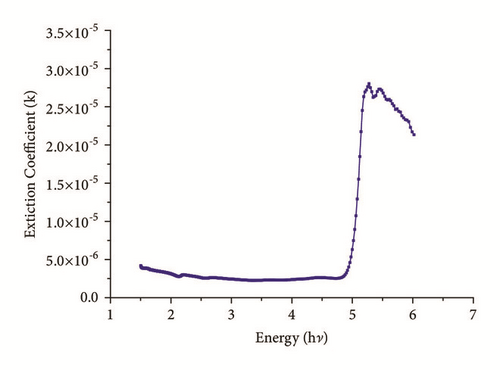
The Urbach energy value was obtained by calculating inverse of the slope observed in linear portion of lower photon energy region as shown in Figure 8. The slope value is found to be 5.1957 and hence the Urbach energy value (Eu) is 0.19 eV. The less value of Urbach energy (Eu) suggests a lesser amount of crystalline defects.

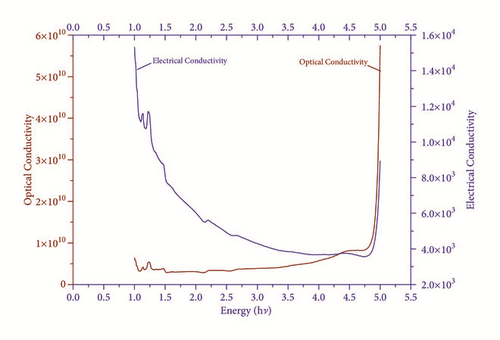
At 1100 nm wavelength the values of real εr and imaginary εi of dielectric constant are 4.8331 and 2.92 × 10−5, respectively.
3.4. Thermal Analysis
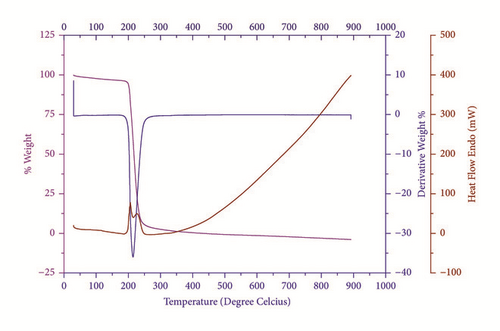
The thermal analysis provides information regarding phase transition, existence of water of crystallization, and different stages of decomposition of the crystal [26]. The IMLT sample of 20.8 mg was analyzed from 25-800°C range in N2 atmosphere (Figure 10). TGA analysis of material revealed that the crystal is stable up to 214.6°C. The weight loss of material is found in single decomposition step in the range of 205°C to 250°C with 88.66% of total weight. The small trace of endothermic peak at 200°C indicates melting followed by decomposition around 220°C. The sharp DTA peak shows good degree of crystallinity and purity of material. The thermal analysis suggests good thermal stability of title compound for any optoelectronic applications below 200°C.
3.5. Dielectric Analysis
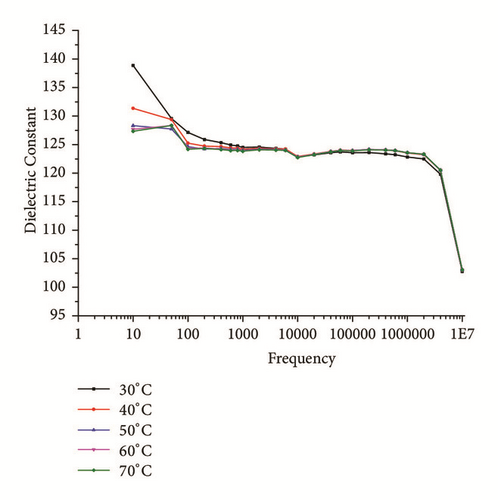
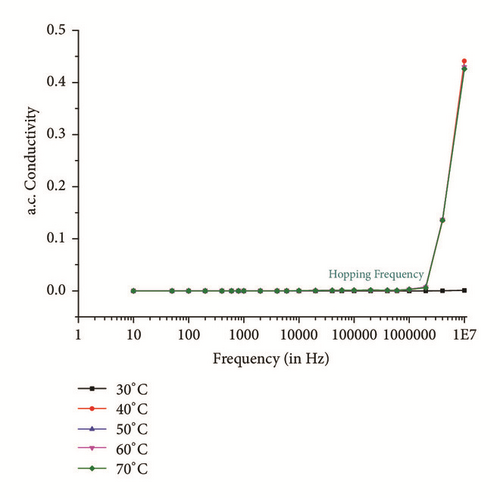
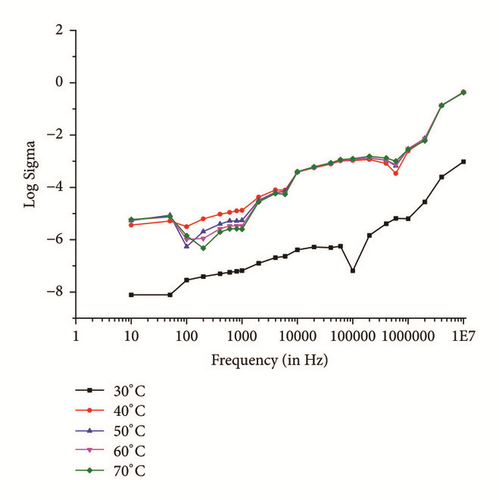
The SHG is proportional to linear susceptibility, and the linear susceptibility depends on polarizability; further, the dielectric constant in frequency range should be studied [27]. Therefore, it is important to study the dielectric behavior with frequency of applied field at different temperatures. Figures 11(a) and 11(b) show the dielectric constant (εr) and dielectric loss (tanδ) with frequency, respectively. From the graphs, it is found that the same decreases with increases in frequency. The higher value of dielectric constant at low frequencies may be attributed to the existence of all of the four polarization mechanisms, namely, electronic (within atoms), ionic (between molecules), interfacial, and orientational polarization, and its lower value at higher frequency may suggest the loss of implication of these polarization mechanisms gradually. The dielectric loss at 30°C temperature and lower frequencies is little high (Figure 11(b)) maybe due to contribution of electronic polarization, as frequency increases contribution of other polarization mechanisms which are prominent with increase in frequency and temperature. Almost similar behavior is observed from 10 KHz in all other ranges of temperature. The lower value of dielectric loss at higher frequencies at different temperature divulges the good optical quality of the crystal with fewer defects, which is the desirable property for NLO applications [28].

| Temperature | A | S |
|---|---|---|
| (°C) | (S m−1rad−n) | |
| 30 | 8.43646 x10−10 | 0.93224 |
| 40 | 1.96309 x 10−09 | 0.80073 |
| 50 | 4.67563 x 10−08 | 0.83174 |
| 60 | 3.12306 x 10−08 | 0.82525 |
| 70 | 6.22558 x 10−07 | 0.83315 |
3.6. NLO Study
The Second Harmonic Generation (SHG) efficiency of fundamental laser pulse has been assessed using the Kurtz and Perry powder method [14]. A Q-switched beam of Nd:YAG laser, having wavelength of 1064 nm with input beam energy of 0.70 joule and pulse width of 6 ns with repetition rate of 10 Hz, was used. Powder sample of grown IMLT crystal was packed and exposed to laser radiation. A green light flash emission was observed, which indicates NLO behavior of the material. From Table 3 it can be seen that IMLT has a Second Harmonic Generation (SHG) conversion efficiency of 3.5 times that of the standard KDP crystals. The zigzag chain structure of the title compound supports higher SHG. The Π- electron system of the Imidazole ring improves the SHG efficiency of the IMLT crystal associated with L-Tartaric acid, whose NLO conversion efficiency is remarkable with the KDP.
| Sample Name | Input Energy | Output Energy |
|---|---|---|
| (millijoule) | (joule) | |
| KDP | 0.70 | 31.3 |
| IMLT | 0.70 | 8.94 |
4. Conclusion
Single crystals of IMLT were successfully grown by slow evaporation solution growth technique at constant temperature of 30°C. The X-ray diffraction analysis confirmed monoclinic crystal structure and lattice parameters. Existence of functional groups is confirmed using FTIR-ATR analyses. UV spectral analysis revealed lower UV cutoff at 245nm with wide transparency window. Calculation of optical band gap (Eg), refractive index, absorption coefficient, extinction coefficient, optical and electrical conductivity, and real and imaginary values of dielectric constants from UV spectrum proposes suitability of material for optoelectronic application. TG/DTA suggests thermal stability of material up to 200°C. Dielectric measurements were carried out using dielectric constant and loss at different frequencies and temperature. The ac conductivity variation with frequency obeys the Jonscher’s power law. The 3.5-time greater efficiency of second order NLO and then that of the standard KDP crystal make it promising NLO material.
Disclosure
The authors are affiliated with Pandit Deendayal Petroleum University, and the corresponding author is permanent employee of L D College of Engineering, Director of Technical Education, and Government of Gujarat. The research has been done with existing facilities and research support provided by them. There is no specific funding provided exclusively for this work.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors are grateful to the authorities of PDPU and MNIT Jaipur for extending thermal analysis facilities; to Dr. V. Raghvan, Abdur Rahman Institute, for SHG measurement; and especially to Dr. Girish Joshi and his research scholar Aarthishree for dielectric measurement.
Open Research
Data Availability
The XRD, FTIR, UV-Visible, and dielectric data used to support the findings of this study are available from the corresponding author upon request.




