Medicinal Plants in the Prevention and Treatment of Colon Cancer
Abstract
The standard treatment for cancer is generally based on using cytotoxic drugs, radiotherapy, chemotherapy, and surgery. However, the use of traditional treatments has received attention in recent years. The aim of the present work was to provide an overview of medicinal plants effective on colon cancer with special emphasis on bioactive components and underlying mechanisms of action. Various literature databases, including Web of Science, PubMed, and Scopus, were used and English language articles were considered. Based on literature search, 172 experimental studies and 71 clinical cases on 190 plants were included. The results indicate that grape, soybean, green tea, garlic, olive, and pomegranate are the most effective plants against colon cancer. In these studies, fruits, seeds, leaves, and plant roots were used for in vitro and in vivo models. Various anticolon cancer mechanisms of these medicinal plants include induction of superoxide dismutase, reduction of DNA oxidation, induction of apoptosis by inducing a cell cycle arrest in S phase, reducing the expression of PI3K, P-Akt protein, and MMP as well; reduction of antiapoptotic Bcl-2 and Bcl-xL proteins, and decrease of proliferating cell nuclear antigen (PCNA), cyclin A, cyclin D1, cyclin B1 and cyclin E. Plant compounds also increase both the expression of the cell cycle inhibitors p53, p21, and p27, and the BAD, Bax, caspase 3, caspase 7, caspase 8, and caspase 9 proteins levels. In fact, purification of herbal compounds and demonstration of their efficacy in appropriate in vivo models, as well as clinical studies, may lead to alternative and effective ways of controlling and treating colon cancer.
1. Introduction
An uncontrolled growth of the body’s cells can lead to cancer. Cancer of the large intestine (colon) is one of the main cause of death due to cancer. While the numbers for colon cancer are somewhat equal in women (47,820) and men (47,700), it will be diagnosed in (16,190) men (23,720) more than women. Multiple factors are involved in the development of colorectal cancer, such as lack of physical activity [1], excessive alcohol consumption [2], old age [3], family history [4], high-fat diets with no fiber and red meat, diabetes [5], and inflammatory bowel diseases, including ulcerative colitis and Crohn’s disease [6].
Prevention of colorectal cancer usually depends on screening methods to diagnose adenomatous polyps which are precursor lesions to colon cancer [7]. The standard treatment for cancer is generally based on using cytotoxic drugs, radiotherapy, chemotherapy, and surgery [8]. Apart from these treatments, antiangiogenic agents are also used for the treatment and control of cancer progression [9].
Colon cancer has several stages: 0, I, II, III, and IV. Treatment for stages 0 to III typically involves surgery, while for stage IV and the recurrent colon cancer both surgery and chemotherapy are the options [10]. Depending on the cancer stage and the patient characteristics, several chemotherapeutic drugs and diets have been recommended for the management of colorectal cancer. Drugs such as 5-fluorouracil (5-FU), at the base of the neoadjuvant therapies folfox and folfiri, are used together with bevacizumab, panitumumab, or cetuximab [7].
Chemotherapy works on active cells (live cells), such as cancerous ones, which grow and divide more rapidly than other cells. But some healthy cells are active too, including blood, gastrointestinal tract, and hair follicle ones. Side effects of chemotherapy occur when healthy cells are damaged. Among these side effects, fatigue, headache, muscle pain, stomach pain, diarrhea and vomiting, sore throat, blood abnormalities, constipation, damage to the nervous system, memory problems, loss of appetite, and hair loss can be mentioned [11].
Throughout the world, early diagnosis and treatment of cancer usually increase the individual’s chances of survival. But in developing countries, access to effective and modern diagnostic methods and facilities is usually limited for most people, especially in rural areas [12]. Accordingly, the World Health Organization (WHO) has estimated that about 80% of the world population use traditional treatments [13]. One of these treatments is phytotherapy, also known as phytomedicine, namely, the use of plants or a mixture of plant extracts for the treatment of diseases. The use of medicinal plants can restore the body’s ability to protect, regulate, and heal itself, promoting a physical, mental, and emotional well-being [14–16]. Various studies have shown the therapeutic effects of plants on fertility and infertility [17], hormonal disorders, hyperlipidemia [18], liver diseases [19], anemia [20], renal diseases [21], and neurological and psychiatric diseases [22]. Therefore, due to all the positive effects showed by medicinal plants, their potential use in cancer prevention and therapy has been widely suggested [23–25].
Since the current treatments usually have side effects, plants and their extracts can be useful in the treatment of colon cancer with fewer side effects. The aims of this review are to present and analyse the evidence of medicinal plants effective on colon cancer, to investigate and identify the most important compounds present in these plant extracts, and to decipher underlying molecular mechanisms of action.
2. Literature Search Methodology
This is a narrative review of all research (English full text or abstract) studies conducted on effective medicinal plants in the treatment or prevention of colon cancer throughout the world. Keywords, including colon cancer, extract, herbs, plant extracts, and plants, were searched separately or combined in various literature databases, such as Web of Science, PubMed, and Scopus. Only English language articles published until July 2018 were considered.
In the current narrative review, studies (published papers) were accepted on the basis of inclusion and exclusion criteria. The inclusion criterion was English language studies, which demonstrated an effective use of whole plants or herbal ingredients, as well as studies which included standard laboratory tests. In vivo and in vitro studies that were published as original articles or short communications were also included. The exclusion criteria included irrelevancy of the studies to the subject matter, not sufficient data in the study, studies on mushrooms or algae, and the lack of access to the full text. Reviews, case reports/case series, and letters to editors were also excluded but used to find appropriate primary literature.
The abstracts of the studies were reviewed independently by two reviewers (authors of this study) on the basis of the inclusion and exclusion criteria. In case of any inconsistency, both authors reviewed the results together and solved the discrepancy. Data extracted from various articles were included in the study and entered into a check list after the quality was confirmed. This check list included some information: authors’ name, year of publication, experimental model, type of extract and its concentration or dose, main components, and mechanisms of action (if reported).
3. Results
3.1. Medicinal Plants and Colon Cancer
Overall, 1,150 articles were collected in the first step and unrelated articles were excluded later on according to title and abstract evaluation. Moreover, articles that did not have complete data along with congress and conference proceedings were excluded. Accordingly, a total of 1,012 articles were excluded in this step. Finally, 190 articles fulfilled the criteria and were included in this review. These papers were published within 2000-2017. A total of 190 plants were included in this study. Based on literature search, 172 experimental studies and 71 clinical cases were included.
Overall, results indicate that grape, soybean, green tea, garlic, olive, and pomegranate are the most effective plants against colon cancer. In these studies, fruits, seeds, leaves, and plant roots were used for in vitro and in vivo studies.
3.1.1. In Vitro Studies
Out of 172 studies, 75 were carried out on HT-29, 60 on HCT116, and 24 on Caco-2 cells (Table 1). On HT-29 cells, both Allium sativum root extracts and Camellia sinensis leaf extracts induced cell apoptosis by two different mechanisms, respectively. In fact, the former showed inhibition of the PI3K/Akt pathway, upregulation of PTEN, and downregulation of Akt and p-Akt expression, while the latter was involved in attenuation of COX-2 expression and modulation of NFκB, AP-1, CREB, and/or NF-IL-6. Moreover, an antiproliferative activity has also been detected in Olea europaea fruit extracts, which increased caspase 3-like activity and were involved in the production of superoxide anions in mitochondria. An antiproliferative activity, by means of a blockage in the G2/M phase, has also been reported in Caco-2 cells by Vitis vinifera fruit extracts. Concerning HCT116 cells, several plants, such as American ginseng and Hibiscus cannabinus, induced cell cycle arrest in different checkpoints.
| Scientific name | Parts used | Cell line | Conc. | Type of extract | Important compounds | Cellular effect | Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| Vitis vinifera | Fruit | HCT116 | NM | Lyophilized | Hydroxycinnamic acids, proanthocyanidins, stilbenoids | Increase of dihydroceramides, sphingolipid mediators involved in cell cycle arrest, and reduction of the proliferation rate |
|
[26, 27] |
| Fruit | Caco-2 | 365 mg/g | Methanolic | Catechin, epicatechin, quercetin, gallic acid | Antiproliferative activity and direct initiation of cell death | Blockage in the G2/M phase | [28, 29] | |
| Seed | Caco-2 | 10–25 μg/mL | Aqueous | Procyanidins |
|
Reduced MPO (myeloperoxidase) activity | [29] | |
| Skin | NM | 7.5, 30, 60 μg/mL | Methanolic | 4 ′-Geranyloxyferulic acid | NM | NM | [30] | |
| Seed | Colon cancer stem cells | 6.25, 12.5, 25 μg/mL | NM | (+)-catechin, (−)-epicatechin | NM |
|
[31] | |
| Allium sativum | Root | HT-29 | 20, 50, 100 mg/mL | Ethanolic | NM | Induction of apoptosis and cell cycle arrest |
|
[32] |
| Glycine max | Seed | Caco-2, SW620, HT-29 | 12.5 μg/mL | Aqueous | Anthoxanthin | Cell death and significant reduction of cell density | Enhancement of Rab6 protein levels | [33] |
| Seed | HT-29 | 240, 600 ppm | Crude | Saponin | Suppression of PKC activation and increase of alkaline phosphatase activity | [33] | ||
| Seed | HT-29 | NM | Crude | Saponin | NM |
|
[34] | |
| Camellia sinensis | Leaf | HT-29 | 0, 10, 30, 50 μM | Aqueous | Catechin, epigallocatechin gallate | 1.9-fold increase in tumor cell apoptosis and a 3-fold increase in endothelial cell apoptosis |
|
[35] |
| Leaf | Caco-2, HT-29 | 300 μM | Aqueous | Theaflavins (TF-2T, F-3, TF-1) | Human colon cancer cell apoptosis induction | Modulation of NFκB, AP-1, CREB, and/or IL-6 | [36] | |
| Leaf | HT-29 |
|
Hot water extract | Flavan-3-ol (catechin & tannin) & polyphenols (teadenol B) | Inhibition of proliferation of HT-29 cells | Increased expression levels of caspases 3/7, 8, and 9 | [35] | |
| Olea europaea | Fruit | HT-29 |
|
Methanolic and chloroform | Maslinic acid, oleanolic acid | Antiproliferative activity |
|
[37] |
| Fruit, leaf | SW480 and HT-29 | 100–400 m/z | Methanolic & hexane | Oleic acid, linoleic acid, gamma-linolenic acid, lignans, flavonoids, secoiridoids | Reduced cell growth in both cell lines |
|
[38] | |
| Fruit | Caco-2 | 50 μM | Aqueous | Phenolic compounds, authentic hydroxyl tyrosol (HT) | Reduced proliferation of Caco-2 cells | Reduction of the methylation levels of CNR1 promoter | [39] | |
| Fruit | HT115 | 25 μg/mL | Hydroethanolic | Phenolic compounds (p-hydroxyphenyl ethanol, pinoresinol & dihydroxyphenyl ethanol) | NM | Inhibition by reduced expression of a range of α5 & β1 | [40] | |
| Olive mill wastewater | HT-29, HCT116, CT26 | NM | Methanolic | Hydroxytyrosol |
|
|
[41] | |
| Fruit | Caco-2 | 0-2,000 μg/mL | Ethanolic | Tyrosol, hydroxytyrosol, oleuropein, rutin, quercetin and glucoside forms of luteolin and apigenin | NM | (i) Induction of the cell cycle arrest in S-phase | [42] | |
| Punica granatum | Juice | HT-29 | 50 mg/L | Aqueous | Ellagitannins, punicalagin | Inhibition of cancer cell proliferation |
|
[43] |
| Seed | LS174 | 63.2 μg/mL | Supercritical fluid | Punicic acid, γ-tocopherol, α-tocopherol | Cytotoxic activity |
|
[44] | |
| Glycyrrhiza glabra | Root | HT-29 | 12.2 and 31 μg/mL | Ethanolic | Licochalcone | NM | Increase of the protein levels of proapoptotic Bax | [37] |
| Opuntia ficus-indica | Fruit | Caco-2 | 115 μM | Aqueous | Betalain pigment indicaxanthin | Apoptosis of proliferating cells |
|
[38] |
| Fruit | HT-29 & Caco-2 & NIH 3 T3 (as control) | Against HT-29 (4.9 μg/mL) against Caco-2 (8.2 μg/mL) | Alkaline hydrolysis with NaOH | Isorhamnetin glycosides (IG5 and IG6)-phenol | Cell death through apoptosis and necrosis | Increased activity of caspase 3/7 | [45] | |
| Piper betle | Leaf | HT-29 and HCT116 | 200.0 μg/mL | Aqueous | Hydroxychavicol | Antioxidant capacity and induction of a greater apoptotic effect |
|
[41, 46] |
| Fragaria×ananassa | Fruit | HT-29 | 0.025, 0.05, 0.25, 0.5% | Ethanolic | Ascorbate, ellagic acid | Decreased proliferation of HT-29 cells | Increase in the levels of 8OHA and decrease in the levels of 8OHG | [40] |
| Sasa quelpaertensis | Leaf | HT-29 HCT116 | 0, 100, 200, 300 mg/L | Ethanolic | p-Coumaric acid, tricin | Inhibited colony formation | Nonadherent sphere formation suppressed CD133+ & CD44+ population | [41] |
| Salvia chinensis | Stem | HCT116, COLO 205 | 10, 20, 40,60, 80, 100 mg/L | Polyphenolic | Terpenoids, phenolic acid, flavonoids, dibenzylcyclooctadiene | Apoptosis & loss of mitochondrial membrane | Induced G0/G1 cell cycle | [42] |
| Rubus idaeus L. | Fruit | HT-29, HT-115, Caco-2 | 3.125, 6.25, 12.5, 25, 50 mg/L | Acetate | Polyphenol, anthocyanin, ellagitannin | NM | Decreased population of cells in G1 phase | [47] |
| LoVo | 50 μL | Aqueous | NM | Inhibited proliferation of LoVo | Suppression of the NFκB pathway | [48] | ||
| Curcuma longa | Root | HT-29, HCT15, DLD1, HCT116 |
|
Ethanolic | Curcumin (diferuloylmethane) | Inhibited formation of HCT116 spheroids | NM | [49] |
| Eleutherococcus senticosus | Root | HCT116 | 12.5, 25, 50, 100 | Methanolic | Eleutherosides, triterpenoid saponins, glycans | NM | Activation of natural killer cells and thus enhancement of immune function | [50] |
| Tabernaemontana divaricata L. | Leaf | HT-29, HCT15 | 10, 30, 100 mg/L | Ethyl acetate, chloroform, methanolic | Alkaloids | NM | Inhibited the unwinding of supercoiled DNA | [45] |
| Millingtonia hortensis | Root, flower, leaf | RKO | 50, 100, 200, 400, 800 mg/L | Aqueous | Phenylethanoid glycoside, squalene, salidroside, 2-phenyl rutinoside | Apoptosis induction |
|
[46] |
| Powder | RKO | 200, 400, 800 μg/mL | Aqueous | Water soluble compounds | Antiproliferative effect | NM | [51] | |
| Thai purple rice | Seed | Caco-2, Cat. No. HTB-37 | 16.11 μg/mL | Methanol acidified | Cyanidin-3-glucoside and peonidin-3-glucoside, anthocyanins, phenolic compounds |
|
NM | [52] |
| Annona muricata | Leaf | HCT116, HT-29 | 11.43 ± 1.87 μg/ml and 8.98 ± 1.24 μg/ml | Ethanolic | Alkaloids, acetogenins, essential oils | Block of the migration and invasion of HT-29 and HCT116 cells |
|
[53] |
| NM | HT-29, HCT116 | <4, <20 μg/mL | EtOAc | Annopentocin A, annopentocin B, annopentocin C, cis- and trans-annomuricin-D-ones, annomuricin E | NM | Suppression of ATP production and NADH oxidase in cancer cells | [54] | |
| Pistacia lentiscus L. var. chia | Leaf | HCT116 | NM | Ethanolic | Resin, known as Chios mastic gum (CMG) | Causes several morphological changes typical of apoptosis in cell organelles |
|
[55] |
| Resin | HCT116 | 100 μg/mL | Hexane | Caryophyllene | Induction of the anoikis form of apoptosis in human colon cancer HCT116 cells |
|
[56] | |
| American ginseng (Panax quinquefolius) | Biological constituents | HCT116 | 0-2.0 mg/mL | Aqueous | Ginseng (GE) or its ginsenoside (GF) and polysaccharide (PS) | Proliferation was inhibited by GE, GF, and PS in wild-type and p21 cells |
|
[57] |
| Purple-fleshed potatoes | Fruit | Colon cancer stem cells | 5.0 μg/mL | Ethanol, methanol, ethyl acetate | Anthocyanin, β-catenin, cytochrome c | Critical regulator of CSC proliferation and its downstream proteins (c-Myc and cyclin D1) and elevated Bax and cytochrome c |
|
[58] |
| Phaseolus vulgaris | Leaf | HT-29 | NM | Ethanolic | Polysaccharides, oligosaccharides | Changes in genes involved or linked to cell cycle arrest |
|
[59] |
| Opuntia spp. | Fruit | HT-29 | 5.8 ± 1.0, 7.5 ± 2.0, 12 ± 1% (V/V) | Hydroalcoholic | Betacyanins, flavonoids (isorhamnetin derivatives) and phenolic acids (ferulic acid) | NM | Induced cell cycle arrest at different checkpoints—G1, G2/M, and S | [60] |
| Suillus luteus | NM | HCT15 | 400 μg/mL | Methanolic | Protocatechuic acid, cinnamic acid, α-tocopherol, β-tocopherol, mannitol, trehalose, polyunsaturated fatty acids, monounsaturated fatty acids, saturated fatty acids | (i) Increase in the cellular levels of p-H2A.X, which is suggestive of DNA damage |
|
[61] |
| Poncirus trifoliata | Leaf | HT-29 | 0.63 μM | Aqueous (in acetone) | β-Sitosterol, 2-hydroxy-1,2,3-propanetricarboxylic acid 2-methyl ester | Arrest of cell growth was observed with β-sitosterol | NM | [62] |
| Rosmarinus officinalis L. | Leaf | SW 620, DLD-1 | 0-120 μg/mL | Methanolic | Polyphenols | Antiproliferative effect of 5-FU | Downregulation of TYMS and TK1, enzymes related to 5-FU resistance | [63] |
| Leaf | HT-29 | SC-RE 30 μg/mL and CA 12.5 μg/mL | Ethanolic | Polyphenols (carnosic acid (CA) and carnosol) |
|
Activation of Nrf2 transcription factor and common regulators, such as XBP1 (Xbp1) gene related to the unfolded protein response (UPR) | [64] | |
| NM | HT-29 | 10, 20, 30, 40, 50, 60, 70 μg/mL | NM | Carnosic acid, carnosol, rosmarinic acid, rosmanol | NM | NM | [65] | |
| Leaf | HGUE-C-1, HT-29, and SW480 | 20–40 mg/mL | CO2-supercritical fluid extract | Carnosic acid, carnosol, and betulinic acid | NM |
|
[66] | |
| Glehnia littoralis | Leaf | HT-29 | 50 mg/mL | Methanolic | Bergapten, isoimpinellin, xanthotoxin, imperatorin, panaxydiol, falcarindiol, falcarinol | Induced apoptosis by the decreased expression of the antiapoptotic Bcl-2 mRNA |
|
[67] |
| Verbena officinalis | Leaf | HCT116 | 20 mg/mL | Aqueous | Phenylethanoid glycosides, diacetyl-O-isoverbascoside, diacetyl-O-betonyoside A, and diacetyl-O-betonyoside A |
|
|
[68] |
| Mentha spicata | Leaf | RCM-1 | 12.5 μg/mL | N-Hexane | Acetic acid 3-methylthio propyl ester (AMTP), methyl thio propionic acid ethyl ester (MTPE) | Exhibited antimutagenic activity | Auraptene (7-geranyloxycoumarin) having a monoterpene moiety and β-cryptoxanthin (one of the tetraterpenes) increased antibody production | [69] |
| Euphoria longana Lam. | Seed | SW 480 | 0–100 μg/mL | Ethanolic | Corilagin, gallic acid, ellagic acid |
|
Release and expression of VEGF indicated that all fractions showed the anti-VEGF secretion activity | [70] |
| Sutherlandia frutescens | Flower | Caco-2 | 1/50 dilution of the ethanolic extract | Ethanolic | Amino acids, including L-arginine and L-canavanine, pinitol, flavonoids, and triterpenoid saponins as well as hexadecanoic acid and γ-sitosterol | Disruption of the key molecules in the PI3K pathway thereby inducing apoptosis | Decrease in cell viability and increment in pyknosis as well as loss in cellular membrane integrity | [71] |
| Melissa officinalis | Leaf | HT-29, T84 | 346, 120 μg/mL | Ethanolic | Phenolic acids (rosmarinic acid, coumaric acid, caffeic acid, protocatechuic acid, ferulic acid, chlorogenic acid), flavonoids, sesquiterpenes, monoterpenes, triterpenes |
|
|
[72] |
| Sargassum cristaefolium | Leaf | HT-29 | 500 mg/mL | Ethanolic | Fucoidans |
|
Accumulation of cells in G0/G1 phase | [73] |
| Hedyotis diffusa | NM | HT-29 | 400 mg/mL | Ethanolic and then DMSO | Octadecyl (E)-p-coumarate, P-E-methoxy-cinnamic acid, ferulic acid, scopoletin, succinic acid, aurantiamide acetate, rubiadin | Suppress tumor cell growth and induce the apoptosis of human CRC cells |
|
[74] |
| Zingiber officinale Roscoe | Peel | LoVo | 100 mg/mL | Ethanolic | Linoleic acid methyl ester, α-zingiberene, and zingiberone | Interesting antiproliferative activity against colorectal carcinoma | NM | [75] |
| Scutellaria barbata | Leaf | LoVo | 413.3 mg/L | Methanolic | Scutellarein, scutellarin, carthamidin, isocarthamidin, wogonin | Induce cell death in the human colon cancer cell line | Increase in the sub-G1 phase and inhibition of cell growth | [76] |
| Pistacia atlantica, Pistacia lentiscus | Resin | HCT116 | 100 μg/mL | Hexane extract | Caryophyllene | Induce the anoikis form of apoptosis in human colon cancer HCT116 cells |
|
[56] |
| Citrus reticulata | Peel | SNU-C4 | 100 μg/mL | Methanolic | Limonene, geranial, neral, geranyl acetate, geraniol, β-caryophyllene, nerol, neryl acetate | Induce the apoptosis on SNU-C4, human colon cancer cells | Expression of proapoptotic gene, Bax, and major apoptotic gene, caspase 3 | [77] |
| Echinacea pallida, Echinacea angustifolia, Echinacea purpurea | Root | COLO320 | 150 mg/mL | Hexanic | Caffeic acid derivatives, alkylamides, polyacetylenes, polysaccharides | Induce apoptosis and promote nuclear DNA fragmentation |
|
[78] |
| Nasturtium officinale | Leaf | HT-29 | 50 μL/mL | Methanolic | Phenethyl isothiocyanate, 7-methylsulfinylheptyl, 8-methylsulfinyl | (i) Inhibition of initiation, proliferation, and metastasis |
|
[79] |
| Polysiphonia | NM | SW480, HCT15, HCT116, DLD-1 | 20 and 40 μg/mL | Methanolic | 2,5-Dibromo-3,4-dihydroxybenzyl n-propyl ether | Potentially could be used as a chemopreventive agent against colon cancer |
|
[80] |
| Aristolochia debilis Sieb. et Zucc. | Stem | HT-29 | 200 μg/mL | Methanolic | Aristolochic acid, nitrophenanthrene carboxylic acids | Inhibition of proliferation and induction of apoptosis in HT-29 cells |
|
[81] |
| Myrtaceae | Leaf | HCT116 | 100 μg/mL (in vitro), 200 and 100 μg/disc (in vivo) | Methanolic | Phenols, flavonoid, betulinic acid | Strong inhibition of microvessel outgrowth |
|
[82] |
| Spica prunellae | Leaf | HT-29 | 200 mg/mL (in vitro), 600 mg/mL (in vivo) | Ethanolic | Rosmarinic acid | Inhibits CRC cell growth |
|
[83] |
| Phytolacca americana | Root | HCT116 | 3200 μg/mL | Ethanolic | Jaligonic acids, kaempferol, quercetin, quercetin 3-glucoside, isoquercitrin, ferulic acid | Control of growth and spread of cancer cells | Reduction in the expressions of MYC, PLAU, and TEK | [84] |
| Morus alba | Leaf | HCT15 | 13.8 μg/mL | Methanolic | Epicatechin, myricetin, quercetin hydrate, luteolin, kaempferol, ascorbic acid, gallic acid, pelargonidine, p-coumaric acid | Cytotoxic effect on human colon cancer cells (HCT15) |
|
[85] |
| Rhodiola imbricata | Leaf | HT-29 | 200 μg/mL | Acetone and methanolic | Phenols, tannins, and flavonoids |
|
|
[86] |
| Asiasarum heterotropoides F. | Dried A. radix | HCT116 | 20 mg/mL | Ethanolic | Asarinin and xanthoxylol | Inhibition of the growth of HCT116 cells |
|
[87] |
| Podocarpus elatus | Fruit | HT-29 | 500 mg/mL | Methanolic | Phenolic and anthocyanin | Reduction of proliferation of colon cancer cells |
|
[88] |
| Echinacea purpurea | Flower | Caco-2, HCT116 | 0–2,000 mg/mL | Hydroethanolic | Cichoric acid |
|
|
[89] |
| Root | COLO320 | 150 mg/mL | Hexanic | Caffeic acid derivatives, alkylamides, polyacetylenes, polysaccharides | Induce apoptosis by increasing significantly caspase 3/7 activity and promote nuclear DNA fragmentation |
|
[78] | |
| Hop (Humulus lupulus L.), Franseria artemisioides | Leaf | NM | 100 mg/kg b.w./day | Aqueous | Coumarin, lignans, quinones | 30% reduction of tumor-induced neovascularization | NM | [90] |
| NM | Caco-2 | NM | Ethanolic | Phenolic compounds, flavonoid, diterpenes | Digestive, gastroprotective, antiseptic, anti-inflammatory, and antiproliferative activity | NM | [91] | |
| Fruit | NL-17 | 0, 50, 100, 150 μg/mL | Methanolic | α-Mangostin (xanthone) | NM |
|
[92] | |
| Stem, bark | HT-29 | 50 μg/mL | Chloroform-soluble | β-Mangostin, garcinone D, cratoxyxanthone | Cytotoxic activity against HT-29 human colon cancer | Inhibition of p50 and p65 activation | [93] | |
| Annona squamosa Linn | Leaf | HCT116 | 8.98 μg/mL | Crude, Aq ethyl acetate | Acetogenins (annoreticuin & isoannoreticuin) and alkaloids dopamine, salsolinol, and coclaurine | Inhibition of growth and proliferation of tumor cells |
|
[94] |
| Derris scandens | Stem | HT-29 | 5-15 μg/mL | Ethanolic | Benzyls and isoflavones (genistein, coumarins, scandinone) | Apoptosis and mitotic catastrophe of human colon cancer HT-29 cells |
|
[95] |
| Eupatorium cannabinum | Aerial parts | HT-29 | 25 μg/mL | Ethanolic | Pyrrolizidine alkaloids (senecionine, senkirkine, monocrotaline, echimidine) | Induced alteration of colony morphology |
|
[96] |
| Sorghum bicolor | The dermal layer of stalk | HCT116 & colon cancer stem cells | >16 and 103 μg/mL | Phenolic-rich ethanolic, acetone | Apigeninidin & luteolinidin | Antiproliferative | Target p53-dependent and p53-independent pathways | [97] |
| Dermal and seed head | CCSC | NM | Methanolic | Apigeninidin, luteolinidin, malvidin 3-O-glucoside, apigenin, luteolin, naringenin, naringenin 7-O-glucoside, eriodictyol 5-glucoside, taxifolin, catechins | NM |
|
[98] | |
| Hibiscus cannabinus | Seed | HCT116 | KSE (15.625 μg/mL to 1,000 μg/mL) | Ethanolic | Gallic acid, p-hydroxybenzoic acid, caffeic acid, vanillic acid, syringic acid, and p-coumaric and ferulic acids | Cytotoxic activity against human colon cancer HCT116 cells | Apoptosis via blockade of mid G1-late G1-S transition thereby causing G1 phase cell cycle arrest | [99] |
| Salix aegyptiaca L. | Bark | HCT116 & HT-29 | 300 μg/mL | Ethanolic | Catechin, salicin, catechol and smaller amounts of gallic acid, epigallocatechin gallate (EGCG), quercetin, coumaric acid, rutin, syringic acid, and vanillin | Anticarcinogenic effects in colon cancer cells | Apoptosis via inhibition of phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinase signaling pathways | [100] |
| Rubus coreanum | Fruit | HT-29 | 400 μg/mL | Aqueous | Polyphenols, gallic acid, sanguine | Induction of apoptosis |
|
[101] |
| Codonopsis lanceolata | Root | HT-29 | 200 μg/mL | N-Butanol fraction | Tannins, saponins, polyphenolics, alkaloids | Apoptosis in human colon tumor HT-29 cells |
|
[102] |
| Gleditsia sinensis | Thorn | HCT116 | 800 μg/mL | Aqueous | Flavonoid, lupine acid, ellagic acid glycosides |
|
Inhibition of proliferation of colon cancer cells | [90] |
| Thorn | HCT116 | 600 μg/mL | Ethanolic | NM | Inhibitory effect on proliferation of human colon cancer HCT116 cells |
|
[91] | |
| Ligustrum lucidum | Fruit | DLD-1 | 50 μg/mL | Aqueous | Oleanolic acid, ursolic acid | Inhibited proliferation | (i) Reduction of Tbx3 rescued the dysregulated P14ARF-P53 signaling | [94] |
| Zingiber officinale | Rhizome | HCT116 | 5 μM | Ethanolic | 6-Paradol, 6- and 10-dehydrogingerdione, 6- and 10-gingerdione, 4-, 6-, 8-, and 10-gingerdiol, 6-methylgingerdiol, zingerone, 6-hydroxyshogaol, 6-, 8-, 10-dehydroshogaol, diarylheptanoids | Inhibitory effects on the proliferation of human colon cancer cells |
|
[103] |
| Grifola frondosa | Fruit | HT-29 | 10 ng/mL | Aqueous | Phenolic compounds (pyrogallol, caffeic acid, myricetin, protocatechuic acid) | Inhibition of TNBS-induced rat colitis | Induced cell cycle progression in G0/G1 phase | [104] |
| Cucumaria frondosa | The enzymatically hydrolyzed epithelium of the edible | HCT116 | <150 μg/mL | Hydroalcoholic | Monosulphated triterpenoid glycoside frondoside A, the disulphated glycoside frondoside B, the trisulphated glycoside frondoside C | Inhibition of human colon cancer cell growth |
|
[105] |
| Rolandra fruticosa | Leaf & twigs | HT-29 | 10 and 5 mg/kg/day | Methanolic | Sesquiterpene lactone (13-acetoxyrolandrolide) | Antiproliferative effect against human colon cancer cells | Inhibition of the NFκB pathway, NFκB subunit p65 (RelA), upstream mediators IKKβ and oncogenic K-ras | [106] |
| Cydonia oblonga Miller | Leaf & Fruit | Caco-2 | 250–500 μg/mL | Methanolic | Phenolic compound (flavonol and flavone heterosides, 5-O-caffeoylquinic acid) | Antiproliferative effect against human kidney and colon cancer cells |
|
[107] |
| Morchella esculenta | Fruits | HT-29 | 820 mg/mL | Methylene chloride | Steroids (mainly ergosterol derivatives) & polysaccharides & galactomannan | Antioxidant activity in HT-29 colon cancer cells | Inhibition of NF-B activation in the NF-B assay | [108] |
| Sedum kamtschaticum | Aerial part | HT-29 | 0–0.5 mg/mL | Methanolic | Buddlejasaponin IV | Induced apoptosis in HT-29 human colon cancer cells | Induction of apoptosis via mitochondrial pathway by downregulation of Bcl-2 protein levels, caspase 3 activation, and subsequent PARP cleavage | [109] |
| Ginseng and Glycyrrhiza glabra | Leaf | HT-29 | 500 μL | Aqueous | Uracil, adenine, adenosine, Li-glycyrrhetinic acid, quiritin | NM | Antiproliferative effect determination of the protein levels of p21, cyclin D1, PCNA, and cdk-2, which are the key regulators for cell cycle progression | [110] |
| Orostachys japonicus | Leaf & stem | HT-29 | 2 mg/mL | Aqueous | Flavonoids, triterpenoids, 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, polysaccharide | Antiproliferation in HT-29 colon cancer cells | Inhibited proliferation at G2 point of the cell cycle and apoptosis via tumor suppressor protein p53 | [111] |
| Ginkgo biloba | Fruit & leaf | HT-29 | 20–320mg/L | Aqueous | Terpene lactones and flavonoid glycosides |
|
Increase in caspase 3 activities and elevation in p53 MRN reduction in Bcl-2 mRNA | [112] |
| Oryza sativa | Seed | HT-29, SW 480, HCEC | 100 μg/mL | Ethyl acetate | Phenolic compound (tricin, ferulic acid, caffeic acid, and methoxycinnamic acid) | Inhibition of the human colon cancer cell growth |
|
[113] |
| Cnidium officinale Makino | Root | HT-29 | 305.024/mL | Ethanolic | Osthole, auraptenol, imperatorin | Inhibited proliferation of human colon cancer cells (HT-29) | Inhibition of the cellular proliferation via G0/G1 phase arrest of the cell cycle and induced apoptosis | [114] |
| Cnidium officinale Makino | Root | HT-29 | 0.1-5 mg/mL | Aqueous | N-(3-(Aminomethyl)benzyl)acetamidine | Inhibited the invasiveness of cytokine-treated HT-29 cells through the Matrigel-coated membrane in a concentration-dependent manner |
|
[115] |
| Long pepper (PLX) | Fruit | HT-29 and HCT116 | 0.10 mg/mL | Ethanolic | Piperidine alkaloids, piperamides, piperlongumine |
|
Induced whole cell ROS production | [116] |
| Achyranthes aspera | Root | COLO 205 | 50-100 and 150-200 μg/mL |
|
Phenolic compounds |
|
|
[117] |
| Thymus vulgaris | Leaf | HCT116 | 0.2, 0.4, 0.6, 0.8 mg/mL | Carvacrol and thymol | Inhibited proliferation, adhesion, migration, and invasion of cancer cells | [118] | ||
| Dictyopteris undulata | NM | SW480 | 40 μg/mL | Ethanolic | Cyclozonarone benzoquinone | NM | Induced apoptosis by reducing Bcl-2 levels, upregulating Bax, and disrupting the mitochondrial membrane potential, leading to the activation of caspases 3 and 9 | [119] |
| Dendrobium microspermae | NM | HCT116 | 0.25, 0.5, 1.0 mg/mL | Methanolic | NM | NM | Upregulation of Bax and caspases 9 and 3 and downregulation of Bcl-2 expression of genes | [120] |
| Cannabis sativa | Dry flower & leaf | DLD-1 and HCT116 | 0.3–5 μM | Methanolic | Cannabidiol, phytocannabinoids | Reduced cell proliferation in a CB1-sensitive |
|
[121] |
| Phoenix dactylifera L. | Fruit | Caco-2 | 0.2 mg/mL | Aqueous | Phenolic acids (gallic, protocatechuic, hydroxybenzoic, vanillic, isovanillic, syringic, caffeic, ferulic, sinapic, p-coumaric, isoferulic), flavonoid glycosides (quercetin, luteolin, apigenin, and kaempferol), and anthocyanidins | Increasing beneficial bacterial growth and inhibition of proliferation of colon cancer cells | NM | [122] |
| Melia toosendan | Fruit | SW480, CT26 | 0, 10, 20, 30, 40, 50 μg/mL | Ethanolic | Triterpenoids, flavonoids, polysaccharide, limonoids | NM |
|
[123] |
| Crocus sativus L. | Flower | HCT116 | 0.25, 0.5, 1, 2, 4 μg/mL | Ethanolic | Carotenoid, pigment, crocin, crocetin | Induced DNA damage and apoptosis |
|
[124] |
| Tepals and leaf | Caco-2 | 0.42 mg/mL | NM | Polyphenols, glycosides of kaempferol, luteolin, and quercetin | Proliferation of Caco-2 cells was greatly inhibited | NM | [125] | |
| Luffa echinata | Fruit | HT-29 | 50, 100, and 200 μg/mL | Methanolic | Amariin, echinatin, saponins, hentriacontane, gypsogenin, cucurbitacin B, datiscacin, 2-O-β-D-glucopyranosyl cucurbitacin B, and 2-O-β-D-glucopyranosyl cucurbitacin S | Increase in the population of apoptotic cells |
|
[126] |
| Vitis aestivalis hybrid | Fruits (wine) | CCD-18Co | 25, 50, 100 μg/mL | NM | Polyphenolics | NM |
|
[127] |
| Xylopia aethiopica | Dried fruit | HCT116 | 0, 5, 10, 15, 20, 25, 30 μg/mL | Ethanolic | Ent-15-oxokaur-16-en-19-oic acid (EOKA) | NM | (i) Induced DNA damage, cell cycle arrest in G1 phase, and apoptotic cell death | [128] |
| Sorghum | Grain | ER-β; nonmalignant young adult mouse colonocytes | 1, 5, 10, 100 μg/mL | Aqueous | Flavones (luteolin and apigenin), 3-deoxyanthocyanins naringenin (eriodictyol and naringenin) | Reduced cell growth via apoptosis | Increased caspase 3 activity | [129] |
| NM | HT-29, HCT116 | 0.9-2.0 mg/mL | Hydroethanolic | Procyanidin B1, delphinidin-3-O-glucoside, tannin, cyanidin-3-O glucoside |
|
|
[130] | |
| Panax notoginseng (Burk.) F.H. Chen | Root | LoVo and Caco-2 | 0, 100, 250, and 500 μg/mL | Alcoholic | Saponin, ginsenoside | NM | Delay in progression of the G0/G1, S, or G2/M cell cycle phases | [131] |
| Brassica oleracea L. var. italica | Broccoli florets | HCT116 | 0, 1, 2.5, 5, 10 μg/mL | Ethanolic | Glucoiberin, 3 hydroxy,4(α-L-rhamnopyranosyloxy), benzyl glucosinolate 4-vinyl-3-pyrazolidinone 4-(methyl sulphinyl), butyl thiourea, β-thioglucoside N-hydroxysulphates | NM | NM | [132] |
| Cistanche deserticola | Dried stem | SW480 |
|
Aqueous | Polysaccharides, phenylethanoid glycosides |
|
Decreased frequency of hyperplasia and Helicobacter hepaticus infection of the intestine | [133] |
| Chaenomeles japonica | Fruit | Caco-2 and HT-29 | 10, 25, 50, 75, 100, 125, 150 μM CE | NM | Procyanidins | NM | NM | [134] |
| Prunus mume | Fruit | SW480, COLO, and WiDr | 150, 300, and 600 μg/mL | Hydrophobic | Triterpenoid saponins | NM |
|
[135] |
| Solanum lyratum | NM | COLO 205 | 50, 100, 200, 300, 400 μg/mL | EtOH | β-Lycotetraosyl | Induced S phase arrest and apoptosis |
|
[136] |
| Onopordum cynarocephalum | Aerial parts | HCT116, HT-29 |
|
Aqueous | Flavonoids, lignans, and sesquiterpene lactones | NM |
|
[137] |
| Eleutherine palmifolia | Bulbs | SW480 | 2.5, 5, 10 μg/mL | MeOH | Eleutherin, isoeleutherin | NM |
|
[138] |
| Asparagus officinalis | Spears | HCT116 | 76 μg/mL | Acetone | Steroidal saponins (HTSA-1, HTSAP-2, HTSAP-12, HTSAP-6, HTSAP-8) | NM |
|
[139] |
| Phyllanthus emblica L. | Seed, pulp | HCCSCs, HCT116 | 200 μg/mL | Methanolic | Trigonelline, naringin, kaempferol, embinin, catechin, isorhamnetin, quercetin |
|
|
[140] |
| Red grape | NM | HT-29, HCT116 | 0.9-2.0 mg/mL | Hydroethanolic | Delphinidin glycosides, quercetin derivatives, delphinidin-3-O-glucoside (high), cyanidin-3-O-glucoside |
|
|
[130] |
| Black lentil | NM | HT-29, HCT116 | 0.9-2.0 mg/mL | Hydroethanolic | Delphinidin glycosides, procyanidin B1, delphinidin-3-O-glucoside (high), cyanidin-3-O-glucoside |
|
|
[130] |
| Graptopetalum paraguayense | Leaf | Caco-2, BV-2 | 0.2, 0.4, 0.6, 0.8, 1.0 mg/mL | Hydroethanolic | Oxalic acid, hydroxybutanedioic acid, gallic acid, quercetin, chlorogenic acid glucans with fucose, xylose, ribose (GW100) arabino-rhamnogalactans (GW100E) |
|
|
[141] |
| Butea monosperma | Flower | SW480 | 200, 370 μg/mL | Floral | n-Butanol | Significant antiproliferative effect |
|
[142] |
| Rehmannia glutinosa | NM | CT26 | 5, 20, 80 μM | NM | Catalpol | Inhibited proliferation and growth invasion of colon cancer cells |
|
[143] |
| Telectadium dongnaiense | Bark | HCT116 | 1.5, 2.0 μg/mL | MeOH extract | 4-Dicaffeoylquinic acid, quercetin 3-rutinoside, periplocin | NM |
|
[144] |
| Gloriosa superba | Root | SW620 | 30 ng/mL | Protein hydrolysate extract | Protein hydrolysate | NM |
|
[145] |
| Boswellia serrata | Resin | HT-29 | 100, 150 μg | Methanolic | Boswellic acid | Decreased cell viability |
|
[146] |
| Typhonium flagelliforme | Leaf | WiDr | 70 μg/mL | Ethyl acetate | Glycoside flavonoid, isovitexin, alkaloids | NM | Inhibition of COX-2 expression | [28] |
| Diospyros kaki | Fruit | HT-29 | 2,000 μg/mL | Hydroacetone extract | Polyphenol | Impaired cell proliferation and invasion | NM | [147] |
| Carpobrotus edulis | Leaf | HCT116 | 1,000 mg/mL | Hydroethanolic | Gallic acid, quercetin, sinapic acid, ferulic acid, luteolin 7-o-glucoside, hyperoside, isoquercitrin, ellagic acid, isorhamnetin 3-O-rutinoside | Inhibited proliferation |
|
[148] |
| Piper methysticum | Root | HT-29 | 10, 20, 30, 40, 50 μg/mL | Aqueous | 11-Hydroxy-12-methoxydihydrokavain, 11-hydroxy-12-methoxydihydrokavain, prenyl caffeate, pinostrobin chalcone, 11-methoxytetrahydroyangonin, awaine, methysticin, dihydromethysticin, 5,6,7,8-tetrahydroyangonin, kavain, 7,8-dihydrokavain, yangonin, desmethoxyyangonin, flavokawain B | Inhibited the growth | NM | [26] |
| Salvia ballotiflora | Ground aerial parts | CT26 | 6.76 μg/mL | Hexane-washed chloroform extract | 19-Deoxyicetexone, 7,20-dihydroanastomosine, icetexone, 19-deoxyisoicetexone | Cytotoxic activity | NM | [149] |
| Tinospora cordifolia | Stem | HCT116 | 1, 10, 30, 50 μM | Hydroalcoholic | Clerodane furano diterpene glycoside, cordifoliosides A and Β, sitosterol, ecdysterone, 2β,3β:15,16-diepoxy-4α, 6β-dihydroxy-13(16),14-clerodadiene-17,12:18,1-diolide | Induced chromatin condensation and fragmentation of nuclei of few cells |
|
[150] |
| Euterpe oleracea | Fruit | NM | 35 μg/mL | Hydroethanolic | Vanillic acid, orientin, isoorientin | NM |
|
[151] |
| Salvia miltiorrhiza | NM | HCT116 | 7.4 ± 1.0, 4.4 ± 0.5 μg/mL | Ethanolic | Diterpene quinone | NM | Decreased levels of pro-caspases 3 and 9 | [152] |
| Coffea | Bean | HCT116 | 1 mg/mL | Aqueous | Chlorogenic acid complex (CGA7) | NM | (i) DNA fragmentation, PARP-1 cleavage, caspase 9 activation, downregulation of Bcl-2 and upregulation of Bax | [153] |
| Illicium verum | Fruit | HCT116 | 10 mg/mL | Ethanolic | Gallic acid quercetin | Induction of apoptosis and inhibition of key steps of metastasis | NM | [154] |
| Garcinia propinqua Craib | Leaf | HCT116 | NM | CH2Cl2 extract | Benzophenones, xanthones, and caged xanthones | Potent inhibitory cytotoxicities | NM | [155] |
| Stem, bark | HCT116 | 14.23, 23.95 μM | MeOH, CH2Cl2, and EtOAc extract | Xerophenone A, doitunggarcinones A and B, sampsonione, 7β-H-11-benzoyl-5α-ydroxy-6, 10-tetramethyl-1-(3-methyl-2-butenyl)-tetracyclotetradecane-2,12,14-trione, hypersampsone M, assiguxanthone A (cudraxanthone Q), 40 10-O-methylmacluraxanthone (16), 41- and 5-O-methylxanthone V1 | NM | NM | [156] | |
| Malus pumila Miller cv. Annurca | Fruit | Caco-2 | 400 mg/L | Methanolic | Chlorogenic acid, (+)catechin, (–)epicatechin, isoquercetin, rutin, phloridzin, procyanidin B2, phloretin, quercetin | WNT inhibitors and reduced WNT activity elicited by WNT5A | NM | [157] |
| Malus domestica cv. Limoncella | Fruit | Caco-2 | 400 mg/L | Methanolic | Chlorogenic acid, (+)catechin, (–)epicatechin, isoquercetin, rutin, phloridzin, procyanidin B2, phloretin, quercetin | WNT inhibitors and reduced WNT activity elicited by WNT5A | NM | [157] |
| Coix lacryma-jobi var. ma-yuen | Leaf | HCT116 | 0.5, 1 mg/mL | Aqueous | Coixspirolactam A, coixspirolactam B, coixspirolactam C, coixlactam, methyl dioxindole-3-acetate | NM | Inhibited migration, invasion, and adhesion via repression of the ERK1/2 and Akt pathways under hypoxic conditions | [158] |
| Mesua ferrea | Stem, bark | HCT116, HT-29 | 3.3, 6.6, and 11.8 μg/mL | NM | Fractions (α-amyrin, SF-3, n-Hex) | Downregulation of multiple tumor promoter | Upregulation of p53, Myc/Max, and TGF-β signaling pathways | [159] |
| Taraxacum | Root | SGC7901, BGC823 | 3 mg/mL | Aqueous | NM | NM | Proliferation and migration through targeting lncRNA-CCAT1 | [160] |
| Portulaca oleracea | Leaf | HT-29 CSCs | 2.25 μg/mL | Alcoholic | Oxalic, malic acid | NM | Inhibited expression of the Notch1 and β-catenin genes, regulatory and target genes that mediate the Notch signal transduction pathway | [161] |
| Hordeum vulgare L. | NM | HT-29 | NM | Aqueous & juice | Protein, dietary fiber, the B vitamins, niacin, vitamin B6, manganese, phosphorus, carbohydrates |
|
Free radical scavenging activity | [162] |
| Paraconiothyrium sp. | NM | COLO 205 and KM12 | 12.5 μM | Methyl ethyl ketone extract | n-Hexane, CH2Cl2, EtOAc, and MeOH fractions (A−D) |
|
NM | [163] |
| Mentha×piperita | Leaf | HCT116 | 5, 10, 20, 30, 40, 50 μg/mL | Aqueous | Polyphenols | NM | Inhibited replication of DNA and transcription of RNA which induce the ROS | [164] |
| Mammea longifolia Planch. and Triana | Fruit | SW480 | 25, 50, 100 μg/mL | Methanolic | NM | NM | Mitochondria-related apoptosis and activation of p53 | [165] |
| Rollinia mucosa (Jacq.) Baill. | NM | HCT116, SW-480 | <4, <20 μg/mL | EtOH | Rollitacin, jimenezin, membranacin, desacetyluvaricin, laherradurin | Cytotoxic activity | NM | [54] |
| Annona diversifolia Saff. | NM | SW-480 | 0.5 μg/mL | NM | Cherimolin-2 | Cytotoxic activity | NM | [54] |
| A. purpurea Moc. & Sessé ex Dunal | NM | HT-29 | 1.47 μg/mL | CHCl3-MeOH | Purpurediolin, purpurenin, annoglaucin, annonacin A | Cytotoxic activity | NM | [54] |
| Viguiera decurrens (A.Gray) A. Gray | NM | NM | 3.6 μg/mL | Hex; EtOAc; MeOH | β-Sitosterol-3-O-β-D-glucopyranoside; β-D-glucopyranosyl oleanolate; β-sitosterol-3-O-β-D-glucopyranoside, and oleanolic acid-3-O-methyl-β-D-glucuronopyranoside ronoate | Cytotoxic activity | NM | [54] |
| Helianthella quinquenervis (Hook.) A. Gray | NM | HT-29 | 2-10 μg/mL | NM | Demethylencecalin | Cytotoxic activity | NM | [54] |
| Smallanthus maculatus (Cav.) H. Rob. | NM | HCT15 | <20 μg/mL | Acetone | Fraction F-4, fraction F-5, ursolic acid | Cytotoxic activity | NM | [54] |
| Bursera fagaroides (Kunth) Engl. | NM | HF6 | 1.8×10-4 to 2.80 μg/mL | Hydroalcoholic | Podophyllotoxin, β-peltatin-A methyl ether, 5 ′-desmethoxy-β-peltatin-A methyl ether, desmethoxy-yatein, deoxypodophyllotoxin, burseranin, acetyl podophyllotoxin | NM |
|
[54] |
| Viburnum jucundum C.V. Morton | NM | HCT15 | <20 μg/mL | Acetone | Ursolic acid | Cytotoxic activity | NM | [54] |
| Hemiangium excelsum (Kunth) A.C.Sm. | NM | HCT15 | <10 (μg/mL) | MeOH | PE, EtOAc, MeOH | Cytotoxic activity | NM | [54] |
| Hyptis pectinata (L.) Poit. | NM | Col2 | <4, <20 μg/mL | NM | Pectinolide A, pectinolide B, pectinolide C, α-pyrone, boronolide, deacetylepiol-guine | Cytotoxic activity | NM | [54] |
| H. verticillata Jacq. | NM | Col2 | <4,<20 μg/mL | NM | Dehydro-β-peltatin, methyl ether dibenzylbutyrolactone, (-)-yatein, 4 ′-demethyl-deoxypodophyllotoxin | Nonspecific cytotoxic activity | NM | [54] |
| H. suaveolens (L.) | NM | HF6 | 2.8-12 μg/mL | Chloroform and butanol | β-Apopicropodophyllin | Nonspecific cytotoxic activity | NM | [54] |
| Salvia leucantha Cav. | Leaf, root, stem | HF6, HT-29, HCT15 | 14.9, 12.7, 9.9 μg/mL | CHCl3 | NM | Cytotoxic activity | NM | [54] |
| Vitex trifolia L. | NM | HCT15 | 3.5 to <1 (μg/mL) | Hexane and dichloromethane | Salvileucalin B, Hex: leaf, Hex: stem, DCM: leaf, DCM: stem | Cytotoxic activity | NM | [54] |
| Persea americana Mill. | NM | HT-29 | <4 μg/mL and <20 μg/mL | Ethanolic | 1,2,4-trihydroxynonadecan, 1,2,4-trihydroxyheptadec-16-ene, 1,2,4-trihydroxyheptadec-16-yne | Cytotoxic activity | NM | [54] |
| Linum scabrellum | Roots, aerial parts | HF6 | 0.2, 0.5, 2.3 μg/mL | Chloroform and butanol | DCM: MeOH, 6MPTOXPTOX | NM |
|
[54] |
| Phoradendron reichenbachianum (Seem.) Oliv. | NM | HCT15 | 3.6, 3.9, and 4.3 μg/mL | NM | Moronic acid | Cytotoxic activity | NM | [54] |
| Cuphea aequipetala Cav. | NM | HCT15 | 18.70 μg/mL | Acetone | NM | Cytotoxic inactivity | NM | [54] |
| Galphimia glauca Cav. | NM | HCT15 | 0.63, 0.50, 1.99 μg/mL | EtOH, MeOH, aqueous | NM | Cytotoxic activity | NM | [54] |
| Mimulus glabratus Kunth | NM | HF6 | 12.64 μg/mL | MeOH | NM | Cytotoxic activity | NM | [54] |
| Picramnia antidesma Sw. | NM | HCT15 | 0.6 to 4.5 μM | NM | 10-Epi-uveoside, uveoside, picramnioside E, picramnioside D | Cytotoxic activity | NM | [54] |
| Penstemon barbatus (Cav.) Roth | NM | HF6 | 15.19 μg/mL | MeOH | NM | Cytotoxic activity | NM | [54] |
| P. campanulatus (Cav.) Willd. | NM | HF6 | 6.74 μg/mL | MeOH | NM | Cytotoxic activity | NM | [54] |
| Veronica americana Schwein. ex Benth. | NM | HF6 | 0.169 and 1.46 μg/mL | MeOH | NM | Cytotoxic activity | NM | [54] |
| Zea mays L. | NM | HCT116, SW-480, SW-620 | NM | NM | 13-Hydroxy-10-oxo-trans-11-octadecenoic acid | Cytotoxic activity | NM | [54] |
| Colubrina macrocarpa (Cav.) G. Don | NM | HCT15 | 10, 2.1, 9.1 μg/mL | PE, EtOAc, MeOH | NM | Cytotoxic activity | NM | [54] |
| Coix lacryma-jobi | Seed, endosperm, and hull | HT-29 | 0.1–1,000 μg/mL | Methanolic, hexane | Phytosterols (campesterol, stigmasterol, and β-sitosterol), gamma-linolenic acid (GLA), arachidonic acid (AA), eicosapentaenoicacid (EPA) and docosahexaenoic acid (DHA), linoleic acid | NM |
|
[166] |
| Abutilon indicum | Leaf | HT-29 | 210 μg/mL | Aqueous | Flavonoids (4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl, 2-ethoxy-4-vinylphenol, N,N ′-dimethylglycine, lup-20(29)-en-3-one, linolenin, 1-mono-, 9-hexadecanoic acid methyl ester, linolenic acid methyl ester), phenolic (amino acids, terpenoids, fatty acids, methyl palmitoleate) | NM | (i) Increase in the levels of reactive oxygen species and simultaneous reduction in cellular antioxidant, mitochondrial membrane loss, DNA damage, and G1/S phase cell cycle arrest | [167] |
| Galla rhois | NM | HCT116, HT-29 | 12.5, 25, 50, 100, 200 μg/mL | Aqueous with steaming process | Gallotannins | Increased contents of gallic acid and ellagic acid |
|
[168] |
| Artemisia annua Linné | Powder | HCT116 | 20, 30, 40, 60, 80, 100 μg/mL | Ethanolic | Phenolic compounds | Inhibited cell viability and increased LDH release |
|
[169] |
| Nelumbo nucifera stamen | Powder | HCT116 | 100, 200, 400 μg/mL | Ethanolic crude | NM | NM |
|
[170] |
| Corn silk | NM | LoVo, HCT116 | 1.25, 2.5, 5, 10, 20 μg/mL | Aqueous | Proteins, polysaccharides, flavonoid, vitamins, tannins, alkaloids, mineral salts, steroids | NM | (i) Increase in the Bax, cytochrome c, caspases 3 and 9 levels | [171] |
| Lycium barbarum L. | Powder | HT-29 | 1, 2, 3, 4, 5 μg/mL | NM | Neoxanthin, all-trans-β-cryptoxanthin, polysaccharides, carotenoids, flavonoids | NM |
|
[172] |
| Chrysobalanus icaco L. | Freeze-dried fruit | HT-29 | 1, 2.5, 5, 10, 20 μg/mL | Crude ethyl acetate | Delphinidin, cyanidin, petunidin, and peonidin | NM |
|
[173] |
| Zanthoxylum piperitum De Candolle | Fruit | Caco-2, DLD-1 | 200 μg/mL | Aqueous | NM | NM | (i) Increased the phosphorylation of c-Jun N-terminal kinase (JNK) | [174] |
| Celtis aetnensis (Tornab.) Strobl (Ulmaceae) | Twigs | Caco-2 | 5, 50, 100, 250, or 500 μg/mL | Methanolic | Flavonoid and triterpenic compounds | NM |
|
[175] |
| Rosa canina | Peel and pulp | Caco-2 | 62.5, 125, 250, 500 μg/mL | Total extract (fraction 1), vitamin C (fraction 2), neutral polyphenols (fraction 3), and acidic polyphenols (fraction 4) | Polyphenols | Decreased production of reactive oxygen species (ROS) | NM | [176] |
| Rhazya stricta | Leaf | HCT116 | 47, 63, 79, and 95 μg/cm2 | Crude alkaloid | Alkaloids | NM |
|
[177] |
| Green coffee | NM | Caco-2 | 10-1,000 μg/mL | NM | 5-Caffeoylquinic acid (5-CQA), 3,5-dicaffeoylquinic acid (3,5-DCQA), ferulic acid (FA), caffeic acid (CA), dihydrocaffeic acid (DHCA), dihydroferulic acid (DHFA) | Reduced viability of cancer cells | NM | [178] |
| Flourensia microphylla | Leaf | HT-29 | NM | Ethanolic and acetone | Phenolic compounds | NM |
|
[179] |
- ∗NM: not mentioned.
3.1.2. Studies in Animal Models
The most used animal model is the murine one (Tables 2(a) and 2(b)). In particular, studies were carried out above all on HT-29 and HCT116 cells. The effects of the different medicinal plants and their extracts are essentially the same detected in in vitro studies. In particular, plant extracts were able to induce apoptosis and inhibit proliferation and tumor angiogenesis by regulating p53 levels and checkpoint proteins with consequent cell cycle arrest and antiproliferative and antiapoptotic effects on cancerous cells.
| Scientific name | Parts used | Model | Dose | Type of extract | Important compounds | Cellular effect | Mechanisms | References | |
|---|---|---|---|---|---|---|---|---|---|
| Vitis vinifera | Seed | In vivo (murine) | Caco-2 |
|
Aqueous | Procyanidins |
|
Reduced MPO (myeloperoxidase) activity | [180] |
| Seed | In vivo | HT-29, SW480 | 5 mg/kg | Aqueous | NM | NM | Decreased VEGF, TNF, MMP-1, MMP-3, MMP-7, MMP-8, MMP-9, and MMP-13 protein expression | [181] | |
| Skin | In vivo | NM | 7.5, 30, 60 μg/mL | Methanolic | 4 ′-Geranyloxyferulic acid | NM | NM | [30] | |
| Seed | In vivo (murine) | NM | 0.12% w/w | NM | Catechin, epicatechin | NM |
|
[31] | |
| Camellia sinensis | Leaf | In vivo (murine) | HT-29 |
|
Aqueous | Catechin, epigallocatechin gallate | 1.9-fold increase in tumor endothelial cell apoptosis | Inhibited the ERK-1 and ERK-2 activation, VEGF expression, and VEGF promoter | [182] |
| In vivo (murine) | HCT116 | 0.5% | NM | NM | Reduced basement membrane | Inhibition of MMP-9 and VEGF secretion | [183] | ||
| In vivo (murine) | Caco-2, HT-29 | 300 μM | Aqueous | Theaflavins (TF-2, TF-3, TF-1) | Induced apoptosis of human colon cancer cells | Inhibition of edema formation correlated to attenuation of COX-2 expression and promoter analysis revealed modulation of NFκB, AP-1, CREB, and/or NF-Il-6 (C/EBP) | [36] | ||
| In vivo (murine) | HT115 | 25 μg/mL | Hydroethanolic | Phenolic compounds (p-hydroxyphenyl ethanol, pinoresinol & dihydroxyphenyl ethanol) | NM | Inhibition via reduced expression of a range of α5 & β1 | [184] | ||
| Sasa quelpaertensis | Leaf | In vivo | HT-29, HCT116 | 0, 100, 200, 300 mg/L | Ethanolic | p-Coumaric acid, tricin | Inhibition of colony formation |
|
[41] |
| Anoectochilus | NM | In vivo | CT26 | Oral dose of 50 & 10 mg/mouse per day | Aqueous | Kinsenoside | Stimulated proliferation of lymphoid tissues | Activation of phagocytosis of peritoneal macrophages | [185] |
| Purple-fleshed potatoes | Fruit | In vivo | Colon cancer stem cells | 5.0 μg/mL | Ethanol, methanol, ethyl acetate | Anthocyanin, β-catenin, cytochrome c | Reduction in colon CSCs number and tumor incidence |
|
[58] |
| Phaseolus vulgaris | Leaf | In vivo | HT-29 | Nm | Ethanolic | Polysaccharides, oligosaccharides | Induction of apoptosis and inhibit proliferation |
|
[59] |
| Rosmarinus officinalis L. | Leaf | In vivo | HT-29 | SC-RE 30 μg/mL and CA 12.5 μg/mL | Ethanolic | Polyphenols (carnosic acid (CA) and carnosol) |
|
||
| Leaf | In vivo (rat) | NM | NM | Ethanolic | Rosmanol and its isomers, carnosol, rosmadial, carnosic acid, and 12-methoxycarnosic acid, carnosic acid, carnosol | Interactions with the gut microbiota and by a direct effect on colonocytes with respect to the onset of cancer or its progression | NM | ||
| Wasabia japonica | Rhizomes | In vivo | COLO 205 | 5 mg/mL | Methanolic | 6-(Methylsulfinyl)hexyl isothiocyanate | Anticolon cancer properties through the induction of apoptosis and autophagy |
|
[186] |
| Zingiberaceae | Rhizome | HT-29 | HT-29 | 5 g/kg | Dichloromethanic | Turmerone | Suppressed the proliferation of HT-29 colon cancer cells |
|
[187] |
| Panax quinquefolius | Root | In vivo (murine) | NM | 30 mg/kg | Ethanolic | Ginsenosides (protopanaxadiol or protopanaxatriol) | Attenuated azoxymethane/DSS-induced colon carcinogenesis by reducing the colon tumor number and tumor load |
|
[188] |
| Myrtaceae | Leaf | In vivo (murine) | HCT116 | 100 μg/mL (in vitro) 200 and 100 μg/disc (in vivo) | Methanolic | Phenolics, flavonoids, betulinic acid | Inhibition of tumor angiogenesis |
|
[82] |
| Spica prunellae | Leaf | In vivo | HT-29 | 200 mg/mL (in vitro), 600 mg/mL (in vivo) | Ethanolic | Rosmarinic acid | Induction of apoptosis and inhibition of cell proliferation and tumor angiogenesis |
|
[83] |
| Gymnaster koraiensis | Aerial part | In vivo (murine) | NM | 500 μmol/kg | Ethanolic | Gymnasterkoreaynes B, C, E, 2,9,16-heptadecatrien-4,6-dyne-8-ol | Anti-inflammatory and cancer preventive activities |
|
[189] |
| Allium fistulosum | Edible portions | In vivo (murine) | CT26 | 50 mg/kg b.w. | Hot water | p-Coumaric acid, ferulic acid, sinapic acid, quercitrin, isoquercitrin, quercetol, kaempferol | Suppression of tumor growth and enhanced survival rate of test mice |
|
[190] |
| Annona squamosa Linn | Leaf | In vivo (animal) | HCT116 | 8.98 μg/mL | Crude ethyl acetate | Acetogenins (annoreticuin & isoannoreticuin) and alkaloids dopamine, salsolinol, and coclaurine | (i) Inhibited growth and proliferation of tumor cells | Reactive oxygen species (ROS) formation, lactate dehydrogenase (LDH) release, and caspases 3/7, 8, 9 activation | [191] |
| Eupatorium cannabinum | Aerial parts | In vivo (murine) | HT-29 | 25 μg/mL | Ethanolic | Pyrrolizidine alkaloids (senecionine, senkirkine, monocrotaline, echimidine) | Cytotoxicity against colon cancer cells |
|
[96] |
| Flacourtia indica | Aerial parts | In vivo (murine) | HCT116 | 500 μg/mL | Methanolic | Phenolic glucoside (flacourticin, 4 ′-benzoylpoliothrysoside) | Antiproliferative and proapoptotic effects in HCT116 cells | Apoptosis via generation of ROS and activation of caspases (PARP) | [192] |
| Sorghum bicolor | The dermal layer of stalk | In vivo (murine) | HCT116 & colon cancer stem cells | >16 and 103 μg/mL | Phenolic, acetone | Apigeninidin & luteolinidin | Antiproliferative effect | (i) Target p53-dependent and p53-independent pathways | [97] |
| Gleditsia sinensis | Thorn | In vivo (murine) | HCT116 | 800 μg/mL | Aqueous | Flavonoid, lupine acid, ellagic acid glycosides | Inhibited proliferation of colon cancer |
|
[90] |
| Thorn | In vivo (murine) | HCT116 | 600 μg/mL | Ethanolic | NM | Inhibitory effect on the proliferation of human colon cancer HCT116 cells | (i) Caused G2/M phase cell cycle arrest | [91] | |
| Zingiber officinale | Rhizome | In vitro/in vivo (murine) | HCT116 | 5 μM | Ethanolic | 6-Paradol, 6- and 10-dehydrogingerdione, 6- and 10-gingerdione, 4-, 6-, 8-, and 10-gingerdiol, 6-methylgingerdiol, zingerone, 6-hydroxyshogaol, 6-, 8-, 10-dehydroshogaol, diarylheptanoids | Inhibitory effects on the proliferation of human colon cancer cells |
|
[103] |
| Cucumaria frondosa | The enzymatically hydrolyzed epithelium of the edible | In vivo (murine) | HCT116 | <150 μg/mL | Hydroalcoholic | Monosulphated triterpenoid glycoside frondoside A, the disulphated glycoside frondoside B, the trisulphated glycoside frondoside C |
|
|
[105] |
| Rolandra fruticosa | Leaf & twigs | In vivo (murine) | HT-29 | 10 and 5 mg/kg/day | Methanolic | Sesquiterpene lactone (13-acetoxyrolandrolide) | Antiproliferative effect against human colon cancer cells | (i) Inhibition of the NFκB pathway, subunit p65 (RelA) and upstream mediators IKKβ and oncogenic K-ras | [106] |
| Cydonia oblonga Miller | Leaf & fruit | In vivo (murine) | Caco-2 | 250–500 μg/mL | Methanolic | Phenolic compound (flavonol and flavone heterosides, 5-O-caffeoylquinic acid) | Antiproliferative effect against human kidney and colon cancer cells |
|
[107] |
| Sedum kamtschaticum | Aerial part | In vivo (murine) | HT-29 | 0–0.5 mg/mL | Methanolic | Buddlejasaponin IV | Induced apoptosis in HT-29 human colon cancer cells | (i) Induced apoptosis via mitochondrial-dependent pathway triggered by downregulation of Bcl-2 protein levels, caspase 3 activation, and subsequent PARP cleavage | [109] |
| Ganoderma lucidum | Caps & stalks | In vivo (murine) | HT-29 | 0-0.1 mg/mL | Triterpene extract (hot water) extract | Polysaccharides (mainly glucans & glycoproteins), triterpenes (ganoderic acids, ganoderic alcohols, and their derivatives) | Cytokine expression inhibited during early inflammation in colorectal carcinoma | Induced autophagy through inhibition of p38 mitogen-activated kinase and activation of farnesyl protein transferase (FPT) | [193] |
| Ginkgo biloba | Fruit & leaf | In vivo (murine) | HT-29 | 20–320 mg/L | Aqueous | Terpene lactones and flavonoid glycosides | Inhibited progression of human colon cancer cells induced HT-29 cell apoptosis | (i) Activation in caspase 3, reduction in Bcl-2 expression, and elevation in p53 expression | [112] |
| Rubus occidentalis | Fruit | In vivo (murine) | JB6 Cl 41 | 25 μg/mL | Methanolic | β-Carotene, α-carotene, ellagic acid, ferulic acid, coumaric acid | Inhibited tumor development | (i) Impaired signal transduction pathways leading to activation of AP-1 and NFB RU-ME fraction | [194] |
| Oryza sativa | Seed | In vivo (murine) | HT-29, SW 480, HCEC | 100 μg/mL | Ethyl acetate extract | Phenolic compound (tricin, ferulic acid, caffeic acid, and methoxycinnamic acid) | Inhibited growth of human colon cancer cells |
|
[113] |
| Cistanche deserticola | Dried stem | In vivo (murine) | SW480 |
|
Aqueous | Polysaccharides, phenylethanoid glycosides | Decreased mucosal hyperplasia and helicobacter infection |
|
[133] |
| Rehmannia glutinosa | NM | In vivo (male C57BL6 mice and Sprague-Dawley rats) | CT26 | 28 mg/kg | NM | Catalpol | (i) Inhibited proliferation, growth, and expression of angiogenic markers | (i) VEGF, VEGFR2, HIF-1α, bFGF inhibited the expressions of inflammatory factors such as IL-1β, IL-6, and IL-8 | [143] |
| Olea europaea | Olive mill wastewater | In vivo (murine) | NM | NM | Methanolic | Hydroxytyrosol | Interferes with tumor cell growth | NM | [195] |
| Leaf | In vivo (xenograft model) (murine) | HCT116, HCT8 | 0, 5, 10, 20, 30, 50, and 70 μg/mL | Phenolic | Oleuropein and hydroxytyrosol | NM |
|
[196] | |
| Ginkgo biloba L. | Leaf | In vivo (rat) | NM | 0.675 and 1.35 g/kg | Methanolic | Flavonoid glycosides, terpene lactones, and ginkgolic acids | (i) Suppressed tumor cell proliferation, promoted apoptosis, and mitigated inflammation | NM | [197] |
| Rhus trilobata Nutt. | NM | In vivo (hamster) | NM | 400 mg/kg, 100 mg/kg | Aqueous | Tannic acid, gallic acid | Cytotoxic activity | NM | [54] |
| Annona diversifolia Saff. | NM | In vivo (mice) | SW-480 | 1.5, 7.5 mg/kg/day | NM | Laherradurin | Cytotoxic activity | NM | [54] |
| A. muricata L. | NM | In vivo (rat) | NM | 250/500 mg/kg | EtOAc | A, B, and C, and cis- and trans-annomuricin-D-ones | Cytotoxic activity | NM | [54] |
| Plumeria acutifolia Poir. | NM | In vivo (hamster) | NM | 400 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Lasianthaea podocephala (A. Gray) K. M. Becker | NM | In vivo (hamster) | NM | 200 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Flourensia cernua DC. | NM | In vivo (hamster) | NM | 350 mg/kg/day | Aqueous | Flavonoids, sesquiterpenoids, monoterpenoids, acetylenes, p-acetophenones, benzopyrans, benzofurans | Cytotoxic activity | NM | [54] |
| Ambrosia ambrosioides (Cav.) W. W. Payne | NM | In vivo (hamster) | NM | 400 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Alnus jorullensis Kunth | NM | In vivo (hamster) | NM | 175 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Dimorphocarpa wislizeni (Engelm.) Rollins | NM | In vivo (hamster) | NM | 100 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Euphorbia pulcherrima Willd. ex Klotzsch | NM | In vivo (hamster) | NM | 200 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Acalypha monostachya Cav. | NM | In vivo (hamster) | NM | 400 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Crotalaria longirostrata Hook. & Arn. | NM | In vivo (hamster) | NM | 400 mg/kg/day, 350 mg/kg/day | EtOH-CHCl3 | NM | Cytotoxic activity | NM | [54] |
| Asterohyptis stellulata (Benth.) Epling | NM | In vivo (hamster) | NM | 50 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Acacia constricta A. Gray | NM | In vivo (hamster) | NM | 400 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Holodiscus dumosus A. Heller | NM | In vivo (hamster) | NM | 350 mg/kg/day | Aqueous | NM | Cytotoxic activity | NM | [54] |
| Butea monosperma | Flower | In vivo (rat) | HT-29 | 150 mg/kg | n-Butanol extract | Isocoreopsin, butrin, and isobutrin | Free radical scavenging and anticancer activities | NM | [198] |
| Taraxacum spp. | Root | In vivo (xenograft murine model) | HT-29, HCT116 | 40 mg/kg/day | Aqueous | α-Amyrin, β-amyrin, lupeol, and taraxasterol | Induced programmed cell death | NM | [199] |
| Scientific name | Parts used | Model | Dose | Type of extract | Important compounds | Cellular effect | Mechanisms | References | |
|---|---|---|---|---|---|---|---|---|---|
| Allium sativum | Root | In vivo (murine) | NM | 2.4 mL of daily | Ethanolic | Allicin, S-allylmercaptocysteine | Significantly suppressed both the size and number of colon adenomas | Enhancement of detoxifying enzymes: SAC and GST activity | [200] |
| Olea europaea | Fruit | In vivo | Caco-2 | 50 μM | Aqueous | Phenolic compounds, authentic hydroxyl tyrosol (HT) |
|
Increase in Cnr1 gene expression, CB1 protein levels | [201] |
| In vivo (murine) | HT115 | 25 μg/mL | Hydroethanolic | Phenolic compounds (p-hydroxyphenyl ethanol, pinoresinol & dihydroxyphenyl ethanol) | NM | Inhibition via reduced expression of a range of α5 & β1 | [184] | ||
| Origanum vulgare L. | Leaf | In vivo (murine) | NM | 20, 40, 60 mg·kg−1 | Aqueous | Rosmarinic acid, caffeic acid, flavonoids | Antioxidant status |
|
[202] |
| Hazelnut | Skin | In vivo | NM | The flow rate 0.21 mL/min and injection volume 9.4 μL | Aqueous | Flavan-3-ols, in monomeric and polymeric forms, and phenolic acids |
|
Increase of the total antioxidant capacity of plasma | [203] |
| Apples and apple juice | Fruit | In vivo | NM | 90 mg/L | Aqueous | Phenolic acids, flavonoids, tannins, stilbenes, curcuminoids | NM | NM | [204] |
| Grifola frondosa | Fruit | In vivo (murine) | HT-29 | 10 ng/mL | Aqueous | Phenolic compounds (pyrogallol, caffeic acid, myricetin, protocatechuic acid, etc.) | Inhibition of TNBS-induced rat colitis | (i) Induced cell cycle progression in G0/G1 phase and apoptotic death | [104] |
| Ruta chalepensis | Leaf | In vivo (human) | NM | 250 μg/mL | Ethanolic | Rutin, gallic acid, catechin hydrate, naringin | Oxidative profile in patients with colon cancer | NM | [205] |
| Cannabis sativa | Dry flower & leaf | In vivo (murine) | DLD-1 and HCT116 | 0.3–5 μM | Methanolic | Cannabidiol, phytocannabinoids | NM |
|
[121] |
| Melia toosendan | Fruit | In vivo (murine) | SW480, CT26 | 0, 10, 20, 30, 40, 50 μg/mL | Ethanolic | Triterpenoids, flavonoids, polysaccharide, limonoids | NM |
|
[123] |
| Smallanthus sonchifolius | Root | In vivo (murine) | NM | 73.90, 150.74, 147.65, and 123.26 mg/kg | Aqueous | Fructans | NM | Reduction incidence of colon tumors expressing altered β-catenin | [206] |
| Punica granatum | Peel | In vivo (adult male Wistar rats) | NM | 4.5 g/kg | Methanolic | Gallic acid, protocatechuic acid, cateachin, rutin, ellagic acid, punicalagin | NM |
|
[207] |
| Linum usitatissimum | Seed | In vivo (male Sprague-Dawley rats) | NM | 500 mg/kg | Alkaline | Secoisolariciresinol diglucoside, carbohydrates, proteins, and tannins | Reduced the serum fasting glucose levels | Significantly reduced the HbA1c, insulin levels, and proinflammatory cytokines | [208] |
| Diospyros kaki | Fruit | In vivo (male CD-1 mice) | NM | 15 mg/kg | Hydroacetone | Polyphenol |
|
Decreased expression of COX-2 and iNOS in the colonic tissue | [147] |
| Muntingia calabura | Leaf | In vivo (rat) | NM | 50, 250, 500 mg/kg | Methanolic | Rutin, gallic acid, ferulic acid, and pinocembrin | Reduction of the colonic oxidative stress, increasing the antioxidants levels possibly via the synergistic action of several flavonoids | NM | [209] |
| Portulaca oleracea | NM | In vivo (murine) | HT-29 CSCs | 2.25 μg/mL | Alcoholic | NM | Regulatory and target genes that mediate the Notch signal transduction pathway | Inhibition of expression of the Notch1 and β-catenin genes | [161] |
| Aloe vera | Gel | In vivo (murine) | NM | 400 mg/kg/day | Gel | Polysaccharides | NM |
|
[210] |
| Artemisia annua Linné | Powder | In vivo (xenograft murine model) | HCT116 | 20, 40 mg/kg/day | Ethanolic | Phenolic compounds | NM |
|
[169] |
| Hordeum vulgare | Powder | In vivo (xenograft murine model) | HT-29 | 2 g/kg and 1 g/kg | Aqueous (fermented) | β-Glucan, protein, amino acids, phenolic compounds | NM |
|
[211] |
| Dendrophthoe pentandra | Leaf | In vivo (murine) | NM | 125, 250, 500 mg/kg | Ethanolic | Quercetin-3-rhamnose | NM |
|
[212] |
| Aquilaria crassna | Stem, bark | In vivo (murine) | HCT116 |
|
NM | Resin and essential oils | NM | NM | [213] |
| Berberis integerrima | NM | In vivo (murine) | NM | 50 and 100 mg/kg | Hydroalcoholic | NM | NM | NM | [214] |
| Salix aegyptiaca | Bark | In vivo (murine) | NM | 100 and 400 mg/kg | Ethanolic | Catechin, catechol, and salicin | NM | Decreased level of EGFR, nuclear β-catenin, and COX-2 | [215] |
- ∗NM: not mentioned.
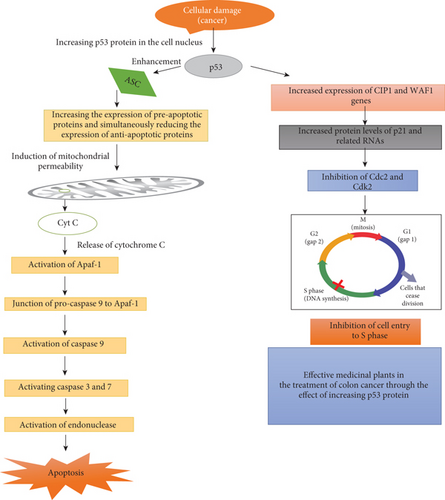
The main mechanisms of action of medicinal plants are summarized in Figure 1.
In in vitro studies, it has been found that grapes, which contain substantial amounts of flavonoids and procyanidins, play a role in reducing the proliferation of cancer cells by increasing dihydroceramides and p53 and p21 (cell cycle gate keeper) protein levels. Additionally, grape extracts triggered antioxidant response by activating the transcriptional factor nuclear factor erythroid 2-related factor 2 (Nrf2) [27].
Grape seeds contain polyphenolic and procyanidin compounds, and their reducing effects on the activity of myeloperoxidase have been shown in in vitro and in vivo studies. It has been suggested that grape seeds could inhibit the growth of colon cancer cells by altering the cell cycle, which would lead eventually to exert the caspase-dependent apoptosis [180].
Another plant that attracted researchers’ attention was soybean, which contain saponins. After 72 h of exposure of colon cancer cells to the soy extract, it was found that this extract inhibited the activity and expression of protein kinase C and cyclooxygenase-2 (COX-2) [34]. The density of the cancer cells being exposed to the soy extract significantly decreased. Soybeans can also reduce the number of cancer cells and increase their mortality, which may be due to increased levels of Rab6 protein [216].
Green tea leaves have also attracted the researchers’ attention in these studies. Green tea leaves, with high levels of catechins, increased apoptosis in colon cancer cells and reduced the expression of the vascular endothelial growth factor (VEGF) and its promoter activity in in vitro and in vivo studies. The extract increased apoptosis (programmed cell death) by 1.9 times in tumor cells and 3 times in endothelial cells compared to the control group [182]. In another in vitro study, the results showed that green tea leaves can be effective in the inhibition of matrix metalloproteinase 9 (MMP-9) and in inhibiting the secretion of VEGF [183].
Garlic was another effective plant in this study. Its roots have allicin and organosulfur compounds. In an in vitro study, they inhibited cancer cell growth and induced apoptosis through the inhibition of the phosphoinositide 3-kinase/Akt pathway. They can also increase the expression of phosphatase and tensin homolog (PTEN) and reduce the expression of Akt and p-Akt [32]. Garlic roots contain S-allylcysteine and S-allylmercaptocysteine, which are known to exhibit anticancer properties. The results of a clinical trial on 51 patients, whose illness was diagnosed as colon cancer through colonoscopy, and who ranged in age from 40 to 79 years, suggest that the garlic extract has an inhibitory effect on the size and number of cancer cells. Possible mechanisms suggested for the anticancer effects of the garlic extract are both the increase of detoxifying enzyme soluble adenylyl cyclase (SAC) and an increased activity of glutathione S-transferase (GST). The results suggest that the garlic extract stimulates mouse spleen cells, causes the secretion of cytokines, such as interleukin-2 (IL2), tumor necrosis factor-α (TNF-α), and interferon-γ, and increases the activity of natural killer (NK) cells and phagocytic peritoneal macrophages [200].
The results of in vitro studies on olive fruit showed that it can increase peroxide anions in the mitochondria of HT-29 cancer cells due to the presence of 73.25% of maslinic acid and 25.75% of oleanolic acid. It also increases caspase 3-like activity up to 6 times and induces programmed cell death through the internal pathway [217]. Furthermore, the olive extract induces the production of reactive oxygen species (ROS) and causes a quick release of cytochrome c from mitochondria to cytosol.
The pomegranate fruit contains numerous phytochemicals, such as punicalagins, ellagitannins, ellagic acid, and other flavonoids, including quercetin, kaempferol, and luteolin glycosides. The results of an in vitro study indicate the anticancer activity of this extract through reduction of phosphorylation of the p65 subunit and subsequent inhibition of nuclear factor-κB (NFκB). It also inhibits the activity of TNF receptor induced by Akt, which is needed for the activity of NFκB. The fruit juice can considerably inhibit the expression of TNF-α-inducing proteins (Tipα) in the COX-2 pathway in cancer cells [43]. The effective and important compounds in pomegranate identified in these 104 studies are flavonoids, polyphenol compounds, such as caffeic acid, catechins, saponins, polysaccharides, triterpenoids, alkaloids, glycosides, and phenols, such as quercetin and luteolin, and kaempferol and luteolin glycosides.
In a systematic review of the plants being studied, some mechanisms were mainly common, including the induction of apoptosis by means of an increase of expression and levels of caspase 2, caspase 3, caspase 7, caspase 8, and caspase 9 in cancer cells, increasing the expression of the proapoptotic protein Bax and decreasing the expression of the antiapoptotic proteins.
Many herbal extracts block specific phase of the cell cycle. For instance, the extract prepared from the leaves of Annona muricata inhibits the proliferation of colon cancer cells and induces apoptosis by arresting cells in the G1 phase [53]. They can also prevent the progress of the G1/S phase in cancer cells [74]. In general, the herbal extracts reported here have been able to stop cancer cells at various stages, such as G2/M, G1/S, S phase, G0/G1, and G1 phase, and could prevent their proliferation and growth.
Other important anticancer mechanisms are the increase of both p53 protein levels and transcription of its gene. Even the increase of p21 expression is not without effect [137]. In an in vitro study on the Garcinia mangostana roots, the results were indicative of the inhibitory effect of the extract of this plant on p50 and P65 activation [93]. Moreover, reduction of cyclin D1 levels and increase of p21 levels are among these mechanisms [137], as well as inhibition of NFκB and reduction of the transcription of its genes, which contribute to reduce the number of cancerous cells [127]. Other important anticancer mechanisms are the inhibition of COX-2, as well as the reduction of the protein levels in this pathway [34]. In addition to this, in some cases, the inhibition of MMP-9 can be mentioned as the significant mechanism of some herbal extracts to kill cancer cells [183].
4. Conclusion and Perspectives
The findings of this review indicate that medicinal plants containing various phytochemicals, such as flavonoids, polyphenol compounds, such as caffeic acid, catechins, saponins, polysaccharides, triterpenoids, alkaloids, glycosides, and phenols, such as quercetin and luteolin, and kaempferol and luteolin glycosides, can inhibit tumor cell proliferation and also intduce apoptosis.
Plants and their main compounds affect transcription and cell cycle via different mechanisms. Among these pathways, we can point to induction of superoxide dismutase to eliminate free radicals, reduction of DNA oxidation, induction of apoptosis by inducing a cell cycle arrest in S phase, reduction of PI3K, P-Akt protein, and MMP expression, reduction of antiapoptotic Bcl-2, Bcl-xL proteins, and decrease of proliferating cell nuclear antigen (PCNA), cyclin A, cyclin D1, cyclin B1, and cyclin E. Plant compounds also increase the expression of both cell cycle inhibitors, such as p53, p21, and p27, and BAD, Bax, caspase 3, caspase 7, caspase 8, and caspase 9 proteins levels. In general, this study showed that medicinal plants are potentially able to inhibit growth and proliferation of colon cancer cells. But the clinical usage of these results requires more studies on these compounds in in vivo models. Despite many studies’ in vivo models, rarely clinical trials were observed among the studies. In fact, purification of herbal compounds and demonstration of their efficacy in appropriate in vivo models, as well as clinical studies, may lead to alternative and effective ways of controlling and treating colon cancer.
Conflicts of Interest
There is no conflict of interest regarding the publication of this paper.
Authors’ Contributions
Dr. Paola Aiello and Maedeh Sharghi contributed equally to this work. Shabnam Malekpour Mansourkhani and Azam Pourabbasi Ardekan contributed equally to this work.
Acknowledgments
The authors appreciate and thank Dr. Moahammad Firouzbakht for his cooperation in draft editing.