Effects of Aluminum Powder on Ignition Performance of RDX, HMX, and CL-20 Explosives
Abstract
As a kind of high explosives, aluminized explosive cannot release the energy maximumly, which is a key problem. Using DTA-TG equipment, the ignition performance of three kinds of aluminized explosives (RDX, HMX, and CL-20) with different mass percentages of aluminum powder (0%, 10 wt.%, 20 wt.%, and 30 wt.%) was investigated. The results showed that the energy release of the HMX/Al composite explosive with 10 wt.%, 20 wt.%, and 30 wt.% aluminum powder was only equivalent to 80%, 65%, and 36% of pure HMX, respectively. It was similar to RDX/Al and CL-20/Al composite explosives, except the CL-20/Al mixture with 10% aluminum powder. Rather than participating in the ignition and combustion, the aluminum powder does effect the complete reaction of RDX, HMX, and CL-20 in the initial stage of ignition or in the lower temperature area of the boundary.
1. Introduction
Aluminum powder can be incorporated into explosives to raise the reaction temperature, enhance the heat of detonation, increase bubble energies in underwater weapons, improve air blast, and create an incendiary effect [1]. Therefore, the aluminized explosive is usually called high explosive and has attracted much attention. Vadhe et al. [2] summarized the development trend of aluminized explosives and considered adequately their low sensitivity and good mechanical properties. Tao et al. [3] studied the early dynamic characteristics of Al powder which was heated after the detonation of aluminized explosives. Stromsoe et al. [4] proposed that both the bubble energy and the shock wave energy of aluminized explosives were higher than those of pure explosives in a certain range of Al content. Peng et al. [5] found that the size, the activity, and the shape of Al powder could significantly affect the energy level of aluminized explosives. Hwang et al. [6] reported the application characteristics of nickel-coated Al powder in the explosives, whose results showed that nickel was able to reduce the ignition temperature of Al powder and improved the impulse, the pressure, and the temperature field. However, the above researches highlight a problem that aluminized explosives show nonideal behavior. The anticipated energy of aluminized explosives is very hard to be utilized fully [7–9]. The combustion of Al powder is incomplete in the actual explosion process, resulting in the incomplete release of energy. Thus, Gogulya et al. [10] tried to promote the reaction of Al powder using Teflon and Viton.
The purpose of the present work is to investigate the effect of Al powder on the ignition property based on thermogravimetric analysis and differential thermal analysis (TG-DTA). The samples are cyclotrimethylene trinitramine (RDX), cyclotetramethylenetetranitramine (HMX), and hexanitrohexaazaisowurtzitane (CL-20), with different mass percentages of Al powder. On this basis, the underlying reason is explored that aluminized explosives showed nonideal behavior in the process of practical application.
2. Experimental
2.1. Reagents and Instruments
2.1.1. Reagents
Al powder (350 mesh, particle size < 40 μm) was obtained from Tangshan Weihao Magnesium Powder Co., Ltd. RDX (80 mesh), HMX (80 mesh), and CL-20 (80 mesh) were obtained from Qingyang Chemical Co., Ltd.
2.1.2. Instrument
DTA-50-type differential thermal analyzer (NETZSCH-Gerätebau GmbH) and Hot Disk TPS 2500S thermal conductivity meter (Swedish Kaigenasi Company) were used.
2.2. Sample Preparation
Mix different mass percentages of Al powder (0%, 10 wt.%, 20 wt.%, and 30 wt.%), respectively, with RDX, HMX, and CL-20 uniformly.
2.3. TG-DTA Parameters
Differential thermal analysis was performed on STA449C high-temperature thermal analyzer. Experimental conditions are as follows: sample weight was 0.5 mg, carrier gas was air, gas velocity was 100 mL/min, Al2O3 crucibles, and gradient heating at a constant rate of 10°C/min ranging from normal temperature to 600°C. The α-Al2O3 was used as the reference sample to correct the reference instrument, and the crucibles were exposed during the whole process. The equipment was corrected before each test.
3. Results and Discussion
3.1. Ignition Temperature
Figures 1–3 show the TG-DTA curves of the three groups of samples, corresponding to the formulations of RDX/Al, HMX/Al, and CL-20/Al, respectively.
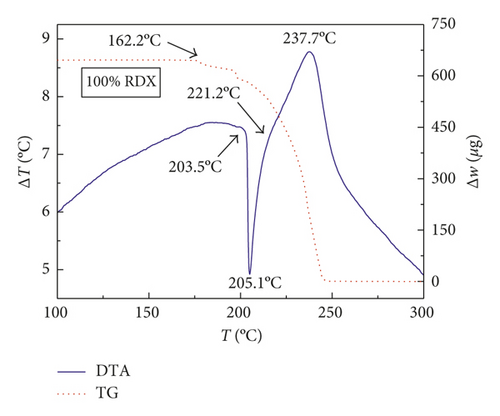
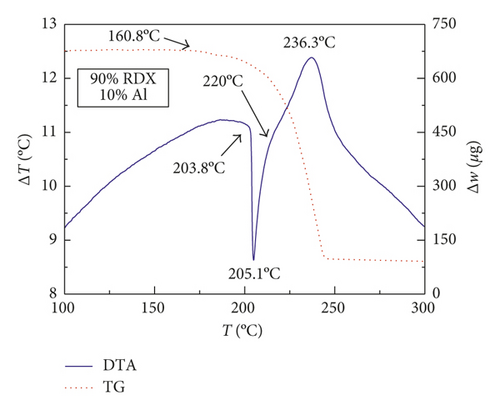
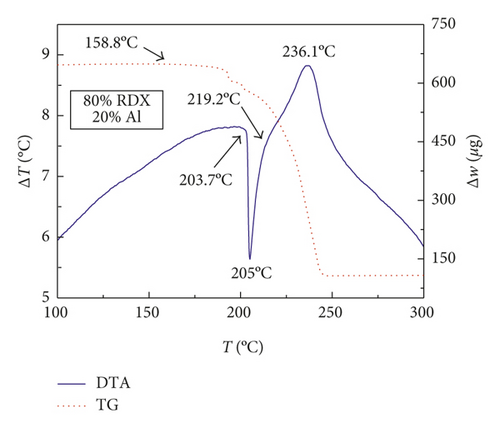
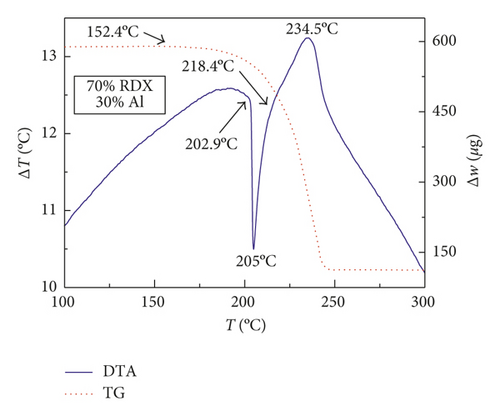
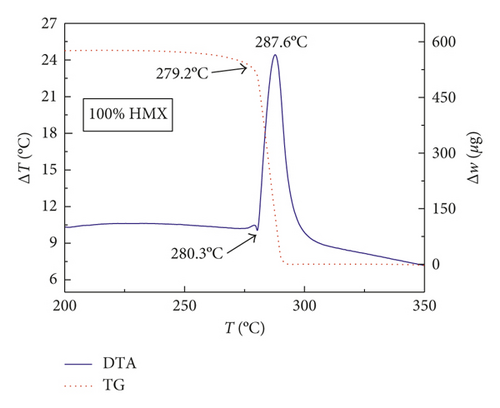
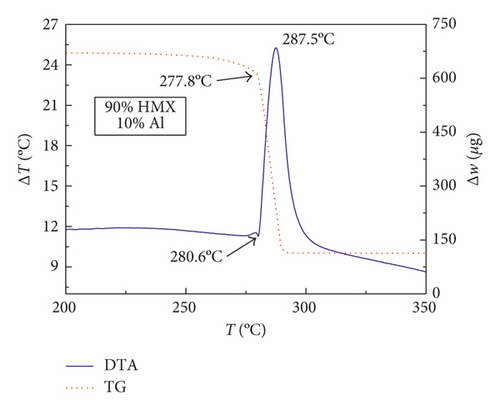
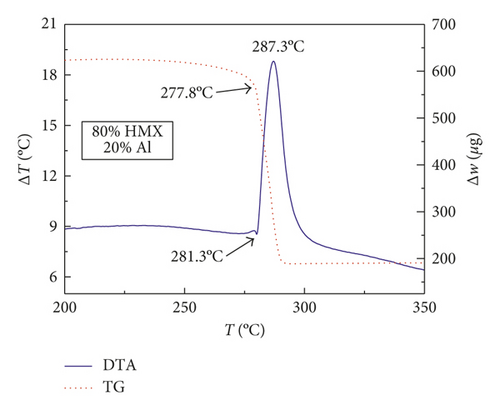
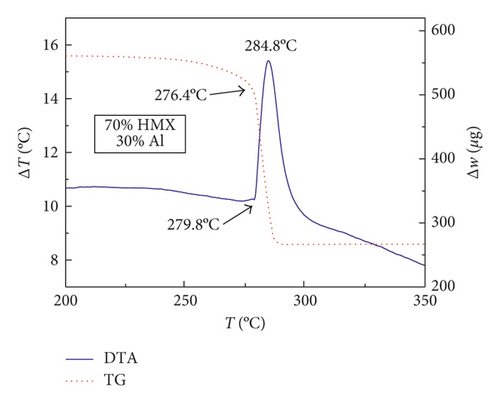
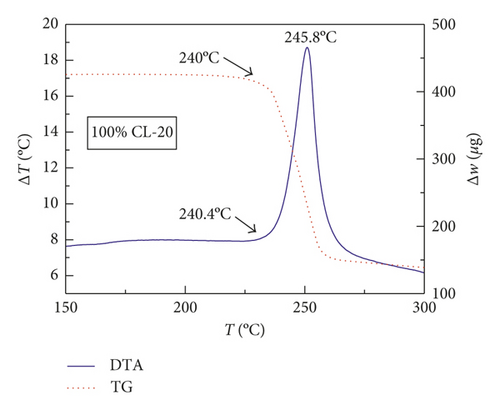
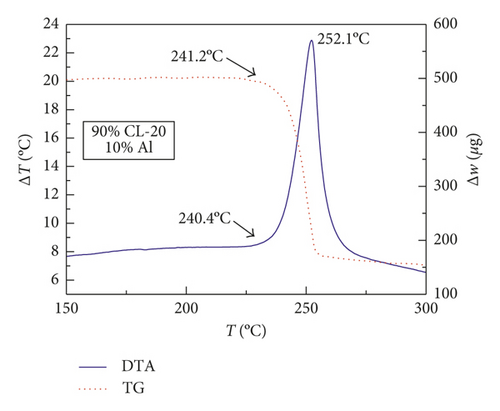
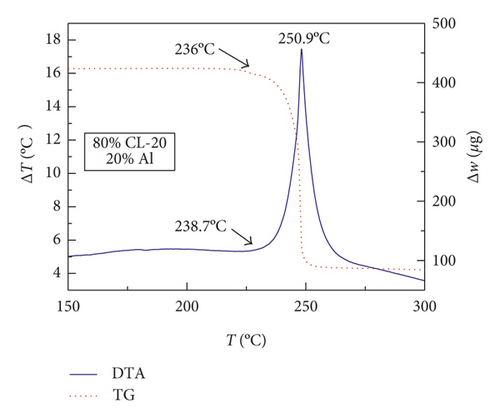
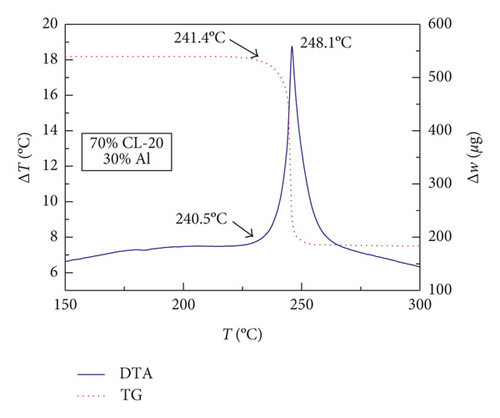
According to the TG-DTA curves, the first strong exothermic peak of the DTA curves generally corresponds to a significant loss of weight in TG curve, which can be extrapolated by ignition temperature (Te). The Te of the three groups of samples is shown in Table 1 based on the results of Figures 1–3. As shown in Table 1, the changes of Te are not obvious after adding different mass percentages of Al powder, which means that Al powder does not change the Te of these three kinds of explosives.
| Sample | Te (°C) | |||
|---|---|---|---|---|
| Al-0% | Al-10 wt.% | Al-20 wt.% | Al-30 wt.% | |
| RDX/Al | 205.1 | 205.1 | 205.0 | 205.0 |
| HMX/Al | 280.3 | 280.6 | 281.3 | 279.8 |
| CL-20/Al | 240.4 | 240.4 | 238.7 | 241.5 |
3.2. Thermal Conductivity
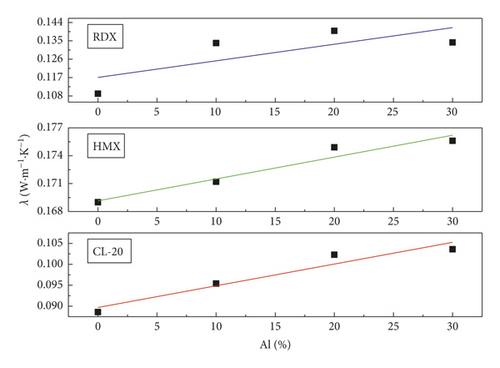
The thermal conductivity tests of samples were carried out after the samples were pressed into flakes. The thermal conductivity of pure Al and shell steel was measured simultaneously, and the testing values were 226 W·m−1·K−1 and 8.25 W·m−1·K−1.
The fitting results of testing values are shown in Figure 4, indicating the variation trend of the thermal conductivity with the mass percentage change of Al powder. We find that the thermal conductivities of different explosives are all improved gradually as the mass percentage of Al powder increases, originating from the higher thermal conductivities of Al.
3.3. Calculation Formula
The weight of the samples was normalized to directly evaluate the effects of different mass percentages of Al powder on the energy release of explosives.
3.3.1. Mass Normalization Formula
3.3.2. Theoretical Exothermic Enthalpy Percentage of Composite Explosives
| Pure substance | ΔH (kJ·kg−1) |
|---|---|
| RDX | 5400 |
| HMX | 5673 |
| CL-20 | 7100 |
| Al | 30,222.22 |
3.4. Ignition Release Energy
After correcting the baseline of curves in Figures 1–3 and normalizing the mass of samples, the results are shown in Figures 5–7. Notably, Figure 5 presents the DTA curves of RDX mixed with different mass percentages of Al powder, in which the exothermic peaks reduce gradually with the increasing mass percentages of Al powder. The declining trend of exothermic peaks is more obvious in the DTA curve of HMX/Al shown in Figure 6. However, CL-20 exhibits different characteristics compared with those of RDX and HMX shown in Figure 7, of which exothermic peaks change irregularly.
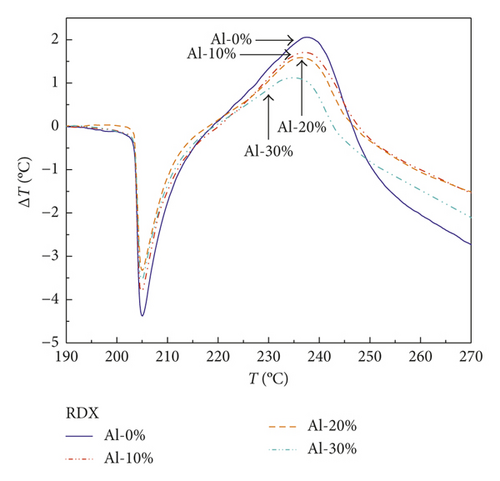
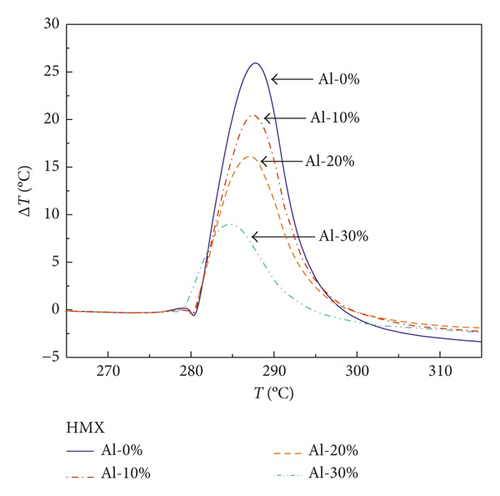
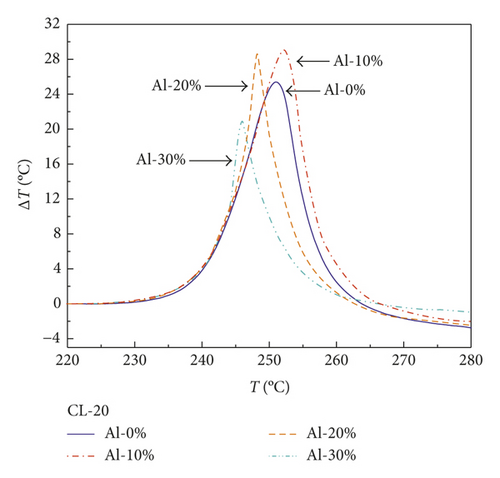
Table 3 lists the exothermic enthalpy data (ΔH) [11, 12] of the three groups of samples which can be calculated by integral processing of exothermic peaks in DTA curves.
| Sample | ΔH (uV·s·mg−1) | |||
|---|---|---|---|---|
| Al-0% | Al-10 wt.% | Al-20 wt.% | Al-30 wt.% | |
| RDX/Al | −332.2 | −247.3 | −204.3 | −135.4 |
| HMX/Al | −1483 | −1191 | −960.6 | −535.0 |
| CL-20/Al | −2120 | −2230 | −1684 | −1169 |
For comparison, the relative exothermic enthalpy percentage of samples is calculated based on the exothermic enthalpy of pure explosives listed in Table 4. The data in parentheses are theoretical exothermic enthalpy percentages of composite explosives.
| Sample | ΔH% | |||
|---|---|---|---|---|
| Al-0% | Al-10 wt.% | Al-20 wt.% | Al-30 wt.% | |
| RDX/Al | 100 (100) | 74.4 (145.97) | 61.5 (191.93) | 40.8 (237.9) |
| HMX/Al | 100 (100) | 80.3 (143.27) | 64.8 (186.55) | 36.1 (229.82) |
| CL-20/Al | 100 (100) | 105.2 (132.57) | 79.4 (165.13) | 55.1 (197.7) |
It can be seen from Table 4 that the released energy of the RDX/Al composite explosive with 10 wt.% Al powder (i.e., containing 90 wt.% RDX) is merely 74.4% of pure RDX, which means the Al powder does not react and even block the complete reaction of RDX. The gap between the actual value and the theoretical value widens as the mass percentage of Al powder increases. The released energy of RDX/Al with 20 wt.% Al is merely 61.5% of pure RDX, and the value of RDX/Al with 30 wt.% Al is only 40.8%. The HMX/Al composite explosive exhibits the same rule as RDX/Al. The released energy of HMX/Al with 10 wt.% Al is merely 80.3% of pure HMX, not even reaching 90%, and the values of HMX/Al with 20 wt.% and 30 wt.% Al are 64.8% and 36.1%, respectively. These results illustrate that the Al powder in RDX/Al and HMX/Al composite explosives cannot burn at the initial stage of ignition but impact the burning property of explosives around the Al particles.
Unlike RDX and HMX, the released energy of CL-20/Al composite explosive with 10 wt.% Al powder is 105.2% of pure CL-20, exceeding 90% but lower than the theoretical value (132.57%), indicating that part of the Al powder joined the reaction and released energy. However, as the mass percentage of Al powder increases, the released energy is reduced obviously. The released energy of CL-20/Al with 20 wt.% and 30 wt.% Al is merely equal to 79.4% and 55.1% of pure CL-20, respectively, which suggests that part of CL-20 does not react. This may be attributed to the higher thermal conductivity of Al, which makes part of Al particles dissipate the heat by themselves and CL-20 around them simultaneously, resulting in unachievable reaction temperature condition and the end of the reaction.
The result of this experiment indicates that Al particles in RDX and HMX cannot react under low-temperature condition or the ignition temperature condition. Due to the high melting point (660.4°C), boiling point (2467°C), and low saturated vapor pressure, Al is difficult to volatilize [13], which makes Al nonreactive in gaseous form under the low-temperature condition. Although explosives release energy in the form of high temperature, high pressure, and high-speed detonation, the process could still be regarded as a continuous ignition process. Due to low temperature, the Al particles far from the explosion core do not react, and at the same time, it dissipates the heat around, which makes incomplete combustion of reactants.
Therefore, to make Al particles and explosives around them combust completely and fast in the microscale, they should be under the high-temperature condition or in the existence of an urge medium such as CL-20 and ammonium perchlorate [10, 12]. A further study can be carried out to investigate the mechanism of CL-20 promoting the combustion of Al particles in ignition process.
4. Conclusions
- (1)
There are no obvious effects of Al powder (≤30 wt.%) on the ignition temperature of RDX, HMX, and CL-20.
- (2)
Al powder blocks the energy release processes of RDX and HMX evidently and may not react at the initial stage of ignition.
- (3)
The CL-20/Al composite explosive with 10 wt.% Al powder can release more energy than that of pure CL-20, which may be caused by the energy release of partial Al powder promoted by CL-20.
The thermal conductivity of aluminized explosives increases accordingly with the increasing mass percentage of Al powder, which accelerates the heat dissipation. Therefore, part of the explosives in touch with the Al particle is not easy to approach spontaneous reaction temperature compared with the others, resulting in termination of reaction or time difference of energy release on the chemical reaction.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Science Foundation of China (approval number 51676100).




