A Fractional Order Model for Viral Infection with Cure of Infected Cells and Humoral Immunity
Abstract
In this paper, we study the dynamics of a viral infection model formulated by five fractional differential equations (FDEs) to describe the interactions between host cells, virus, and humoral immunity presented by antibodies. The infection transmission process is modeled by Hattaf-Yousfi functional response which covers several forms of incidence rate existing in the literature. We first show that the model is mathematically and biologically well-posed. By constructing suitable Lyapunov functionals, the global stability of equilibria is established and characterized by two threshold parameters. Finally, some numerical simulations are presented to illustrate our theoretical analysis.
1. Introduction
The immune response plays an important role to control the dynamics of viral infections such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and human T-cell leukemia virus (HTLV). Therefore, many mathematical models have been developed to incorporate the role of immune response in viral infections. Some of these models considered the cellular immune response mediated by cytotoxic T lymphocytes (CTL) cells that attack and kill the infected cells [1–5] and the others considered the humoral immune response based on the antibodies which are produced by the B-cells and are programmed to neutralize the viruses [6–11]. However, all these models have been formulated by using ordinary differential equations (ODEs) in which the memory effect is neglected while the immune response involves memory [12, 13].
Fractional derivative is a generalization of integer derivative and it is a suitable tool to model real phenomena with memory which exists in most biological systems [14–16]. The fractional derivative is a nonlocal operator in contrast to integer derivative. This means that if we want to compute the fractional derivative at some point t = t1, it is necessary to take into account the entire history from the starting point t = t0 up to the point t = t1. For these reasons, modeling some real process by using fractional derivative has drawn attention of several authors in various fields [17–22]. In biology, it has been shown that the fractional derivative is useful to analyse the rheological proprieties of cells [23]. Furthermore, it has been deduced that the membranes of cells of biological organism have fractional order electrical conductance [24]. Recently, much works have been done on modeling the dynamics of viral infections with FDEs [25–31]. These works ignored the impact of the immune response and the majority of them deal only with the local stability.
On the other hand, system (1) becomes a model with ODEs when α = 1, which improves and generalizes the ODE model with bilinear incidence rate [42], the ODE model with saturated incidence rate [43], and the ODE model with specific functional response [44].
The rest of the paper is organized as follows. The next section deals with some basic proprieties of the solutions and the existence of equilibria. The global stability of equilibria is established in Section 3. To verify our theoretical results, we provide some numerical simulations in Section 4, and we conclude in Section 5.
2. Basic Properties and Equilibria
In this section, we will show that our model is well-posed and we discuss the existence of equilibria.
Theorem 1. Assume that the initial conditions satisfy (3). Then there exists a unique solution of system (1) defined on [0, +∞. Moreover, this solution remains nonnegative and bounded for all t ≥ 0.
Proof. First, system (1) can be written as follows:
It is important to note that when α = 1, (4) becomes a system with ODEs. In this case, we refer the reader to [45] for the existence of solutions and to the works [46–50] for the stability of equilibria. In the case of FDEs, we will use Lemma 2.4 in [31] to prove the existence and uniqueness of solutions. Hence, we put
- (i)
If α0 ≠ 0, F(X) can be formulated as follows:
()-
where
() -
Hence,
()
-
- (ii)
If α1 ≠ 0, we can write F(X) in the form
()-
where
() -
Moreover, we get
()
-
- (iii)
If α2 ≠ 0, we have
()-
where
() -
Further, we obtain
()
-
- (iv)
If α3 ≠ 0, we have
()-
where
() -
Then
()
-
Finally, we prove the boundedness of solutions. We define the function
Hence if R0 > 1, (30) has a unique root x1 ∈ (0, λ/d). As a result, when R0 > 1 there exists an equilibrium E1(x1, l1, y1, v1, 0) satisfying x1 ∈ (0, λ/d), l1 = (λ − dx1)/(m + γ), y1 = γ(λ − dx1)/a(m + γ), and v1 = kγ(λ − dx1)/aμ(m + γ).
We summarize the above discussions in the following theorem.
Theorem 2.
- (i)
If R0 ≤ 1, then system (1) has one infection-free equilibrium of the form E0(x0, 0,0, 0,0), where x0 = λ/d.
- (ii)
If R0 > 1, then system (1) has an infection equilibrium without humoral immunity of the form E1(x1, l1, y1, v1, 0), where x1 ∈ (0, λ/d), l1 = (λ − dx1)/(m + γ), y1 = γ(λ − dx1)/a(m + γ), and v1 = kγ(λ − dx1)/aμ(m + γ).
- (iii)
If R1 > 1, then system (1) has an infection equilibrium with humoral immunity of the form E2(x2, l2, y2, v2, w2), where x2 ∈ (0, λ/d − ahμ(m + γ)/dkgγ), l2 = (λ − dx2)/(m + γ), y2 = γ(λ − dx2)/a(m + γ), v2 = h/g, and w2 = kγg(λ − dx1)/aqh(m + γ) − μ/q.
3. Global Stability of Equilibria
In this section, we focus on the global stability of equilibria.
Theorem 3. If R0 ≤ 1, then the infection-free equilibrium E0 is globally asymptotically stable and it becomes unstable if R0 > 1.
Proof. The proof of the first part of this theorem is based on the construction of a suitable Lyapunov functional that satisfies the conditions given in [51, Lemma 4.6]. Hence, we define a Lyapunov functional as follows:
Calculating the fractional derivative of L0(t) along solutions of system (1) and using the results in [52], we get
The proof of the instability of E0 is based on the computation of the Jacobean matrix of system (1) and the results presented in [53–55]. The Jacobean matrix of (1) at any equilibrium E(x, l, y, v, w) is given by
Theorem 4.
- (i)
The infection equilibrium without humoral immunity E1 is globally asymptotically stable if R0 > 1, R1 ≤ 1, and
() - (ii)
When R1 > 1, E1 is unstable.
Proof. Define a Lyapunov functional as follows:
At E1, the characteristic equation of (40) is given as follows:
Theorem 5. The infection equilibrium with humoral immunity E2 is globally asymptotically stable if R1 > 1 and
Proof. Consider the following Lyapunov functional:
It is important to note that when ρ is sufficiently small or γ is sufficiently large, the two conditions (44) and (52) are satisfied. Then, we have the following corollary.
Corollary 6. Assume that R0 > 1. When ρ is sufficiently small or γ is sufficiently large, then we have the following:
- (i)
The infection equilibrium without humoral immunity E1 is globally asymptotically stable if R1 ≤ 1.
- (ii)
The infection equilibrium with humoral immunity E2 is globally asymptotically stable if R1 > 1.
4. Numerical Simulations
In this section, we validate our theoretical results to HIV infection. Firstly, we take the parameter values as shown in Table 1.
| parameters | values | parameters | values | parameters | values |
|---|---|---|---|---|---|
| λ | 10 | a | 0.27 | h | 0.2 |
| d | 0.0139 | γ | 0.01 | g | 0.0001 |
| β | 0.00024 | k | 800 | α0 | 1 |
| ρ | 0.01 | μ | 3 | α1 | 0.1 |
| m | 0.0347 | q | 0.01 | α2 | 0.01 |
| α3 | 0.00001 |
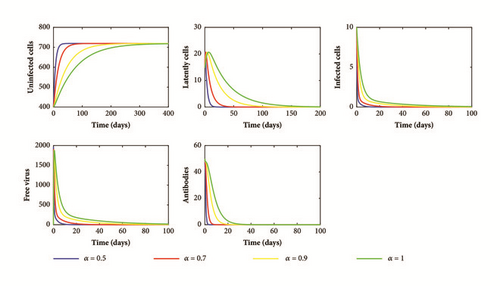
By calculation, we have R0 = 0.4274 ≤ 1. Then system (1) has an infection-free equilibrium E0(719.4245,0, 0,0, 0). By Theorem 3, the solution of (1) converges to E0 (see Figure 1). Consequently, the virus is cleared and the infection dies out.
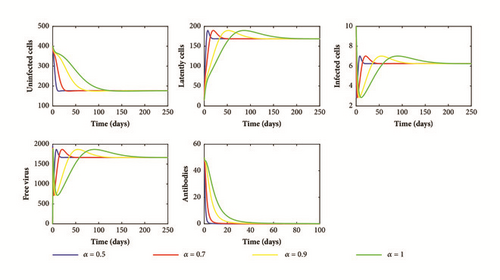
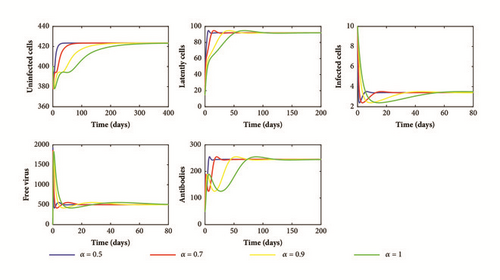
Next, we take g = 0.0004 and do not change the other parameter values. In this case, we have R1 = 3.3338, ρβh = 0.0000024, and d(m + ρ + γ)(α0g + α2h) + ρλ(α1g + α3h) = 0.000006. Hence, condition (52) is satisfied. Consequently, system (1) has an infection equilibrium with humoral immunity E2(423.4261,92.0442,3.4090,500,245.4473) which is globally asymptotically stable. Figure 3 illustrates this result. We can observe that the activation of the humoral immune response increases the healthy cells and decreases the productive infected cells and viral load to a lower levels but it is not able to eradicate the infection.
5. Conclusion
In the present paper, we have studied the dynamics of a viral infection model by taking into account the memory effect represented by the Caputo fractional derivative and the humoral immunity. We have proved that the solutions of the model are nonnegative and bounded which assure the well-posedness. We have shown that the proposed model has three infection equilibriums, namely, the infection-free equilibrium E0, the infection equilibrium without humoral immunity E1, and the infection equilibrium with humoral immunity E2. By constructing suitable Lyapunov functionals, the global stability of these equilibria is fully determined by two threshold parameters R0 and R1. More precisely, when R0 ≤ 1, E0 is globally asymptotically stable, whereas if R0 > 1, it becomes unstable and another equilibrium point appears, that is, E1, which is globally asymptotically stable whenever R1 ≤ 1 and condition (44) is satisfied. In the case that R1 > 1, E1 becomes unstable and there exists another equilibrium point E2 which is globally asymptotically stable when condition (52) is satisfied. In addition, we remarked that when ρ is sufficiently small or γ is sufficiently large, conditions (44) and (52) are verified, and then the global stability of E1 and E2 is characterized only by R0 and R1.
From our theoretical and numerical results, we deduce that the order of the fractional derivative α has no effect on the dynamics of the model. However, when the value of α decreases (long memory), the solutions of our model converge rapidly to the steady states (see Figures 1–3). This behavior can be explained by the memory term 1/Γ(1 − α)(t − u) α included in the fractional derivative which represents the time needed for the interaction between cells and viral particles and the time needed for the activation of humoral immune response. In fact, the knowledge about the infection and the activation of the humoral immune response in an early stage can help us to control the infection.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.