Substituted 4-Acyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazones with Antioxidant Properties: X-Ray Crystal and Spectroscopic Studies
Abstract
Phenylhydrazine was reacted with synthesized acylpyrazolone derivatives 4-ethyl-5-methyl-2-phenyl-pyrazol-3-one and 4-propyl-5-methyl-2-phenyl-pyrazol-3-one, to obtain two new azomethine phenylhydrazones, a study in continuation of our probe into the effects of acyl group substitutions on the physicochemical and free radical scavenging properties of acylpyrazolone Schiff bases. The keto imine tautomers of 4-ethyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone (Empp-Ph) and 4-propyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone (Prmpp-Ph) according to single X-ray crystallography data which precipitated in good yield are reported. Furthermore they have been characterized by elemental analysis, FTIR, 13C and 1H NMR, and mass-spectroscopy techniques. Both phenylhydrazone Schiff bases crystallize in a triclinic crystal system, each with a space group of P-1 (number 2) having short intramolecular N3—H3…O1 hydrogen interaction between the first hydrazine hydrogen H3 and the pyrazolone oxygen O1. The antioxidant free radical scavenging activities of titled compounds against 2,2-diphenyl-1-picrylhydrazyl (DPPH) showed a positive response almost as good as that of vitamin c under the same conditions, with the propyl substituted 4-propyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone (Prmpp-Ph) having a stronger activity (calculated IC50 value of 175.66 μg/ml).
1. Introduction
The synthesis of new compounds with unique properties has continued to be one of the scientific approaches employed by coordination chemists towards the search for alternative therapeutic agents to address the problems of medicinal drugs toxicity effects and disease resistance [1–3]. Similarly, these compounds mostly organic/inorganic based, as ligands or/and their metal complexes, are obtained through careful functional groups modifications by way of formulation, which invariably affects their properties and influence specific applications [4–6]. Acylpyrazolone derivatives, mostly known for their use as analytical reagents in the extraction and separation of various metal ions, made possible because of their chelating properties, are a group of bidentate diketones that have been well researched [7–10]. They are important precursors in the synthesis of a more superior group of acylpyrazolone Schiff bases, obtained by their reaction with amines in a condensation process [11]. Since its discovery, Schiff bases in the midst of its numerous applications have been receiving profound research interest because of its pharmacological properties, a characteristic that is attributed to the azomethine C=N functional group that it possesses [2, 12, 13]. The available lone pair electrons in sp2 hybridized orbital of the C=N nitrogen atom interfere with the normal cell membrane by forming hydrogen bonds with these active centers of cell constituents to inhibit its growth.
Schiff bases either in its form or as coordination metal complexes have gained research interest as free radical scavenging agents (antioxidant properties) [14]. Free radicals are highly unstable atoms, molecules, or ions having unpaired electrons which are derived from oxygen, nitrogen, or sulfur, forming reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS), respectively. The oxidative properties impact of these free radicals present within the human metabolic system has been of great concern as it is revealed to be the leading cause of most life endangering ailments [15]; as such the need for a closer look at understanding their origin and modes of action amongst other characteristics, which will in turn give room for strategic approach in scavenging them, is most important. Tridentate 3-formyl chromone Schiff bases showed good antioxidant activities against DPPH at different concentrations with as low as 1.27 g/ml IC50 value compared to standard drug BHT (IC50 value of 0.67 g/ml) [16]. More recently, Karthik and fellow researchers synthesized a series of Schiff bases with 4-(methylthio)benzaldehydes that have shown to highly scavenge free radicals [17]. Acylpyrazolone Schiff bases synthesized from the condensation reaction of some acylpyrazolone derivatives with sulfanilamide that showed antioxidant properties have been reported by our group [18]. Latest on our findings was the synthesis of new stable Schiff bases with dinitrophenylhydrazine having interesting structural hydrogen bonding and spectroscopic characteristics [2]. In this paper however, it is revealed that ethyl and propyl substitution on acylpyrazolone Schiff bases greatly influence their free radical scavenging behavior, and these results were carefully attained from a study of the antioxidant activities of these new phenylhydrazones, starting with synthesis and followed by their characterization as well as structural elucidation.
2. Experimental
2.1. Materials, Physical Measurements, and Methods
3-Methyl-1-phenyl-2-pyrazolin-5-one, propionyl chloride, and butyryl chloride were purchased from Sigma Aldrich and used as received for the synthesis of the 4-acylpyrazolone precursors. Phenylhydrazine and other reagents/solvents of standard analytical grade were obtained from the same supplier. 1H and 13C NMR spectra were recorded in deuterated DMSO on a Varian Unity Inova 600 NMR spectrometer with a 1H frequency of 600 MHz and a 13C frequency of 150 MHz using trimethylsilane TMS as internal standard. Spectra data were collected in a 5 mm dual channel IDpfg probe and chemical shifts were expressed in ppm (δ scale). The Mestrenova 9.0 software package was used for processing of NMR spectra. Mass spectra were analyzed with atmospheric-pressure chemical ionization (APCI) method using a direct insertion probe (DIP) on the Bruker micrOTOF-Q II 10390 mass spectrometer. An external calibration with sodium formate was performed to attain the correct accurate mass. FTIR spectroscopy was measured within the range of 4000–370 cm−1 on the new PerkinElmer Spectrum Two FTIR UATR Spectroscopy with the spectrum 10.5.3 software used for spectra preparation. The uncorrected melting point was determined using the GallenKamp melting point apparatus and the LECO.TRUSpec Micro CHNS analyzer was employed for the elemental analyses of synthesized ligands. Single crystal X-ray diffraction studies were performed at 200 K using a Bruker Kappa Apex II diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 Å).
2.2. Synthesis of Phenylhydrazones
2.2.1. 4-Ethyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone Empp-Ph
A mixture of 4-ethyl-5-methyl-2-phenyl-pyrazol-3-one Empp (2 mmol, 0.46 g) in methanol (40 mL) and a solution of phenylhydrazine (2.0 mmol, 0.22 g) in methanol (20 mL) was stirred under reflux for 6 hours. The obtained solution with a yellow solid was filtered and the product precipitate was washed with methanol, followed by a small amount of diethyl ether. The yellow crystalline solid was recrystallized in methanol, dried at room temperature, and stored over fused calcium chloride in a desiccator. Yield: 79%. Mol. Wt.: 320.38 g/mol. 1H NMR (DMSO-d6, 600 MHz), δ (ppm) 12.24 (s, 1H), 8.52 (s, 1H), 8.02–7.97 (m, 2H), 7.38 (dd, J = 8.6, 7.3 Hz, 2H), 7.28 (dd, J = 8.6, 7.3 Hz 2H), 7.15–7.09 (m, 1H), 6.90 (t, J = 7.3 Hz, 1H), 6.86–6.81 (m, 2H), 2.82 (q, J = 7.6 Hz, 2H), 2.37 (s, 3H), 1.24 (t, J = 7.6 Hz, 3H). 13C NMR (600 MHz, DMSO): δ (ppm) = 172.7 (s, C=O), 165.5 (s, C=N), 147.4–112.7 (m, aromatic carbons), 96.2 (s, pyrazolone CH3), 16.3 (s, acyl CH2–CH3), 12.8 (s, acyl CH2–CH3). M.p.: 161−163°C. Elemental analysis Calc. for C19H20N4O: C 71.23%; H 6.29%; N 17.49%. Found: C 71.50%; H 5.98%; N 17.46%. IR vmax (cm−1): v (N–H): 3295, v (C–H): 2975, v (C=N): 1616, v (C=O): 1534, v (C–O): 1382, v (C–N): 1063. APCI-MS: m/z 321.17 ([M + H]+, 100%).
2.2.2. 4-Propyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone Prmpp-Ph
A mixture of propyl-5-methyl-2-phenyl-pyrazol-3-one Prmpp (2 mmol, 0.49 g) in methanol (40 mL) and a solution of phenylhydrazine (2.0 mmol, 0.22 g) in methanol (20 mL) was stirred under reflux for 6 hours. The obtained solution with a yellow solid was filtered and the product precipitate was washed with methanol, followed by a small amount of diethyl ether. The yellow crystalline solid was recrystallized in methanol, dried at room temperature, and stored over fused calcium chloride in a desiccator. Yield: 72%. Mol. Wt.: 334.41 g/mol. 1H NMR (600 MHz, DMSO-d6) δ 12.30 (s, 1H), 8.50 (s, 1H), 8.03–7.98 (m, 2H), 7.38 (dd, J = 8.6, 7.3 Hz, 2H), 7.27 (t, J = 7.7 Hz, 2H), 7.12 (t, J = 7.4 Hz 1H), 6.90 (t, J = 7.3 Hz, 1H), 6.83 (d, J = 8.0 Hz, 2H), 2.81–2.75 (m, 2H), 2.37 (s, 3H), 1.65 (q, J = 7.8 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (600 MHz, DMSO): δ (ppm) = 171.3 (s, C=O), 165.5 (s, C=N), 147.4–112.9 (m, aromatic carbons), 96.6 (s, pyrazolone CH3), 21.9 (s, acyl CH2–CH2–CH3), 16.4 (s, acyl CH2–CH3), 14.0 (s, acyl CH2–CH2–CH3). M.p.: 172−174°C. Elemental analysis Calc. for C20H22N4O: C 71.83%; H 6.63%; N 16.75%. Found: C 71.75%; H 6.20%; N 16.69%. IR vmax(cm−1): v (N–H): 3299, v (C–H): 2965, v (C=N): 1613, v (C=O): 1582, v (C–O): 1378, v (C–N): 1075. APCI-MS: m/z 335.19 ([M + H]+, 100%).
2.2.3. X-Ray Diffraction Studies of Empp-Ph and Prmpp-Ph
Single crystal X-ray diffraction analyses were performed at 200 K using a Bruker Kappa Apex II diffractometer with monochromated Mo Kα radiation (λ= 0.71073 Å). APEX2 [19] was used for data collection and SAINT [20] for cell refinement and data reduction. The structures were solved by direct methods using SHELXS–2013 [21] and refined by least-squares procedures using SHELXL-2013 [21] with SHELXLE [22] as a graphical interface. Data were corrected for absorption effects using the numerical method implemented in SADABS [20]. All nonhydrogen atoms were refined anisotropically. Carbon-bound H atoms were placed in calculated positions (C–H bond lengths of 0.95 Å for aromatic carbon atoms; 0.99 Å for methylene) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C). The H atoms of the methyl groups were allowed to rotate with a fixed angle around the C–C bond to best fit the experimental electron density (HFIX 137 in the SHELX program suite [21, 23]) with Uiso(H) set to 1.5Ueq(C) and C–H bond lengths of 0.98 Å. The nitrogen-bound H atoms were located on a different Fourier map and refined freely.
2.2.4. Antioxidant (Free Radical Scavenging) Activity
3. Results and Discussion
3.1. Synthesis, Physical Properties, and Elemental Analysis
The general synthesis scheme for the reported phenylhydrazones is shown in Figure 1. Air stable, nonhygroscopic ligands were precipitated in high yield from the condensation reaction of previously synthesized ethyl and propyl acylpyrazolone derivatives with phenylhydrazine in a simple one-pot synthesis set-up. They were recrystallized from methanol and with slow evaporation of their dry samples in ethanol solution; single crystals suitable for X-ray crystallography were grown.
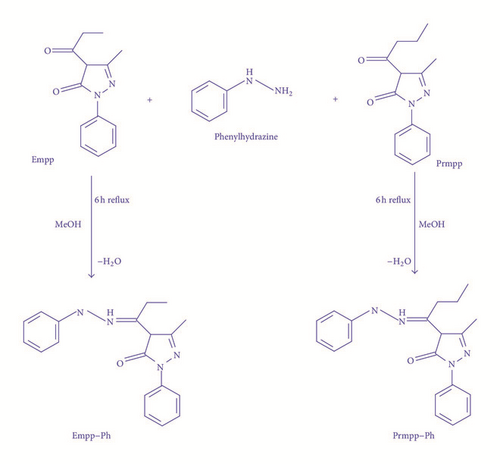
Obtained elemental analysis data were in agreement with calculated percentage composition of CHN values. The titled compounds were generally soluble in basic organic solvents with short range melting point presented in the experimental section.
3.2. 1H and 13C NMR Spectroscopy
1H and 13C NMR spectroscopy data have been used to verify both the hydrogen and carbon environments of the structures of reported phenylhydrazones as obtained from single crystal X-ray crystallography. The 1H NMR signals associated with aromatic protons (ArH) resonated at a downfield region between 8.52 and 6.83 ppm in the NMR spectra of ligands [18], with an integration values of 11 protons each. A singlet each resonating at 12.24 ppm in Empp-Ph and at 12.30 ppm in Prmpp-Ph further downfield is due to the protonation of the azomethine nitrogen HN=C, Figure 2. Signals due to alkyl group protons resonate at an upfield region of 2.82, 2.37, and 1.24 ppm in Empp-Ph and at 2.75, 2.37, 1.65, and 1.00 ppm in Prmpp-Ph.

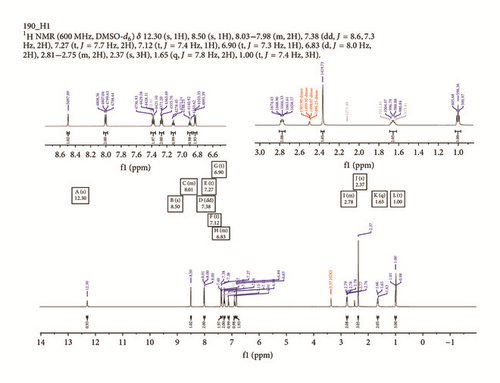
In the 13C NMR spectra of synthesized compounds, Figure 3, carbon atoms due to keto C=O and azomethine C=N groups display resonance signals at 165.5 and 147.4 ppm in Empp-Ph and Prmpp-Ph, respectively [24]. Also the signals due to aromatic carbon are observed as multiplets and resonate in the chemical shift range of 147.4 and 112.9 ppm. The substituted aliphatic carbon environment resonates as singlets in the chemical shift region between 21.9 and 12.8 ppm.
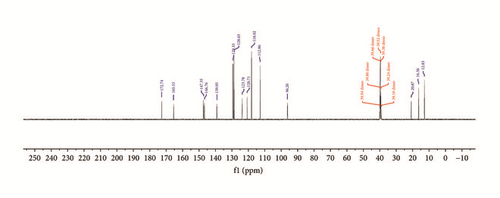
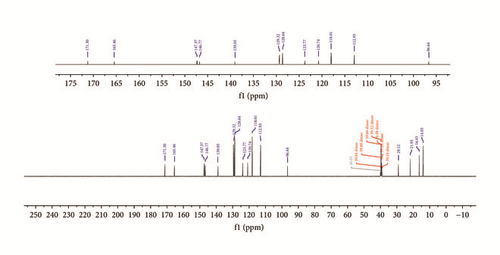
3.3. Mass-Spectroscopy
The APCI-mass spectra with molecular ion peak intensities of 100% are observed for Empp-Ph and Prmpp-Ph having molecular ion values of 321.17 and 335.19 m/z, respectively, equivalent to a protonation of the ligands [M + H]+ (Figure 4). Also, in the mass-spectrum of Empp-Ph, the peak with m/z at 641.33 corresponds to the molecular weight of 2 molecules of Empp-Ph, which may be due to the formation of a dimer. However the molecular ions observed are in close agreement with calculated theoretical molecular weight plus a proton [2].


3.4. Infrared Spectroscopy
IR spectra of ligands have been carefully obtained using their pure powder samples on the new PerkinElmer Spectrum Two FTIR UATR Spectroscopy and the spectra were processed using the spectrum 10.5.3 software. Strong bands due to azomethine v (C=N) and keto v (C=O) at 1616 and 1534 cm−1, respectively, peculiar with acylpyrazolone Schiff bases are observed in the IR spectrum of Empp-Ph which were in agreement with obtained crystal structure. These frequency vibrations are observed in Prmpp-Ph at 1613 cm−1 for v (C=N) and 1582 cm−1 for v (C=O) which is consistent with previous findings [23]. Further, vibration peaks observed as medium bands at 3295 and 2975 cm−1 in Empp-Ph due to v (N–H) and v (C–H), respectively, vibrate at 3299 and 2965 cm−1 in the IR spectrum of Prmpp-Ph. In addition to the confirmation of the successful synthesis of reported acylpyrazolone phenylhydrazones, the IR spectra have displayed relevant functional group vibrations at appropriate wave numbers cm−1 as presented in the experimental section.
3.5. X-Ray Crystallography
Single crystal structures of synthesized phenylhydrazone derivatives were obtained from a slow evaporation of their ethanol solution. Crystal data summary are presented in Table 1.
| Compound | Empp-Ph | Prmpp-Ph | Pmpp-Ph [25] |
|---|---|---|---|
| Formula | C19H20N4O | C20H22N4O | C23H20N4O |
| Crystal colour and form | Yellow/block | Golden yellow/block | Yellow/block |
| Formula weight | 320.39 | 334.42 | 368.43 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | P-1 | P-1 | P21/c |
| a | 8.8820(2) (Å) | 9.0474(3) (Å) | 8.6806(2) (Å) |
| b | 9.5593(3) (Å) | 9.4350(3) (Å) | 20.4319(4) (Å) |
| c | 10.4678(3) (Å) | 10.5546(3) (Å) | 10.6100(2) (Å) |
| α | 80.690(1)° | 81.162(1)° | 90° |
| β | 81.658(1)° | 78.850(1)° | 99.713(1)° |
| γ | 81.484(1)° | 82.568(2)° | 90° |
| V | 860.69(4) (Å3) | 868.84(5) (Å3) | 1854.83(7) (Å3) |
| Z | 2 | 2 | 4 |
| D(calc) | 1.236 (Mg cm−1) | 1.278 (Mg cm−1) | 1.319 (Mg cm−1) |
| F (000) | 340 | 356 | 776 |
| θ range | 2.2, 28.3 (°) | 2.0, 28.4 (°) | 2.4, 28.3 (°) |
| Crystal size | 0.25 × 0.33 × 0.40 (mm) | 0.31 × 0.32 × 0.47 (mm) | 0.33 × 0.40 × 0.45 (mm) |
| Dataset | −11 : 11; −12 : 12; −13 : 13 | −12 : 11; −12 : 12; −14 : 14 | −11 : 11; −27 : 26; −14 : 14 |
| Tot., uniq. data, R(int) | 19169, 4275, 0.014 | 15195, 4294, 0.019 | 17965, 4607, 0.014 |
| Observed data [I > 2 σ(I)] | 3705 | 3632 | 3891 |
| Temperature | 200 (K) | 200 (K) | 200 (K) |
| Parameters | 227 | 237 | 262 |
| wR2, S | 0.1035, 1.05 | 0.1027, 1.04 | 0.1079, 1.04 |
The structure of reported compounds, 4-ethyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone Empp-Ph and 4-propyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone Prmpp-Ph, is similar to previously described 4-phenyl-5-methyl-2-phenyl-pyrazol-3-one-phenylhydrazone Pmpp-Ph [25]. Structures Empp-Ph and Prmpp-Ph (Figure 5) are isostructural in the P-1 space group, while structure Pmpp-Ph has the P21/c space group.

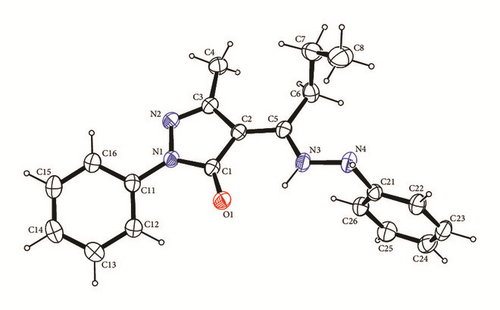
The methyl-pyrazolone-hydrazine cores are essentially planar with RMS deviation of fitted atoms of 0.0281 for Empp-Ph, 0.0285 for Prmpp-Ph, and 0.0493 for Pmpp-Ph indicating that the phenyl group in Pmpp-Ph exhibits greater steric hindrance [25]. Core planarity is also aided by a short intramolecular N3—H3…O1 hydrogen interaction between the first hydrazine hydrogen H3 and the pyrazalone oxygen O1 with interaction lengths of 1.935(14) for Empp-Ph, 1.928(16) for Prmpp-Ph, and 1.994(18) Å for Pmpp-Ph. In terms of graph-set analysis [19, 26] the descriptor is (6) on the unary level.
The phenyl groups bonded to the pyrazalone groups are turned slightly out of the methyl-pyrazolone-hydrazine core planes by 21.43(5)° for Empp-Ph, 21.21(6)° for Prmpp-Ph, and 12.15(6)° for Pmpp-Ph. Each structure has an intramolecular C12—H12…O1 interaction which can be described by a (6) descriptor on the unary level and limits the rotation of the phenyl group. The bond lengths are 2.35, 2.45, and 2.26 Å for Empp-Ph, Prmpp-Ph, and Pmpp-Ph, respectively.
In contrast the phenyl groups bonded to the hydrazine are all almost perpendicular to the core plane by 88.46° for Empp-Ph, 89.24° for Prmpp-Ph, and 85.34° for Pmpp-Ph. The phenyl hydrazine group is held in place by two C—H…π ring interactions: one with a phenyl (C11–C16) ring and the other with a pyrazole (N1 N2 C1 C2 C3) ring with hydrogen to centroid distances of 2.84 and 2.65 Å, respectively, Figure 6. The hydrogen bonding and parking diagrams of Prmpp-Ph are not presented as they have the same structural behavior and crystal orientation as Empp-Ph.
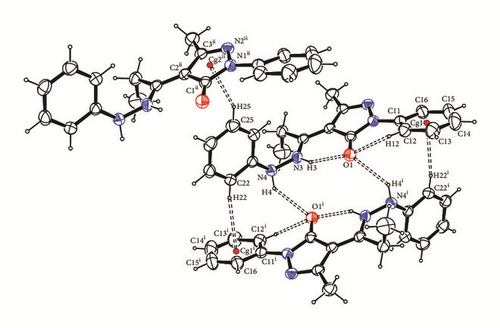
Between two adjacent molecules in structures Empp-Ph and Prmpp-Ph, there are two N4—H4…O1 intermolecular hydrogen bonds which can be described with a (14) descriptor on the unary level. The bond lengths are 2.321(15) for Empp-Ph and 2.368(16) for Prmpp-Ph. For structure Pmpp-Ph the second hydrazine hydrogen H4 is not involved with a hydrogen bond, but instead between two adjacent molecules where there are two intermolecular N4—H4…π ring interactions of 2.728(17) Å to phenyl groups bonded to the pyrazalones.
3.6. Antioxidant Studies against DPPH
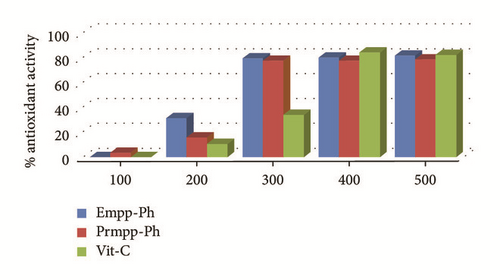
The extent to which synthesized Schiff bases can scavenge free radicals of DPPH was carefully carried out in vitro in triplicate and the average of absorbance values of test samples at different concentrations was measured at a constant wavelength of 517 nm. Interesting antioxidant activities have been exhibited by the reported phenylhydrazones compared to the standard drug, vitamin C. A progressive increase in the percentage antioxidant activity with increasing concentration of test compounds as depicted in Table 2 and Figure 7 was recorded [17]. Based on the calculated IC50 values, Prmpp-Ph has shown a stronger antioxidant property overall, with an IC50 value of 175.66 μg/ml followed by Empp-Ph with an IC50 of 193.90 μg/ml. It is understood that Prmpp-Ph showed a stronger scavenging potential because of the presence of more electron donating hydrogen environment giving room for the ease of releasing protons responsible for free radical reduction.
| Test compounds | Percentage antioxidant activity % | IC50 μg/ml | R2 | ||||
|---|---|---|---|---|---|---|---|
| 100 μg/ml | 200 μg/ml | 300 μg/ml | 400 μg/ml | 500 μg/ml | |||
| Empp-Ph | 0.29 | 31.65 | 79.52 | 80.36 | 82.00 | 193.90 | 0.82 |
| Prmpp-Ph | 3.61 | 16.15 | 77.78 | 77.87 | 78.8 | 175.66 | 0.79 |
| Vit. C | 0.26 | 10.71 | 34.22 | 84.51 | 82.67 | 87.54 | 0.91 |
4. Conclusions
New substituted acylpyrazolone phenylhydrazones with excellent antioxidant properties relative to standard drug vitamin C have been identified based on their abilities to scavenge free radicals of DPPH. The propyl phenylhydrazone derivative Prmmp-Ph showed a stronger antioxidant activity which has been attributed to the presence of more hydrogen atoms that can be released in a process of deprotonation to scavenge free radical. Spectroscopic techniques data has corroborated their single crystal X-ray structures. In addition, their good hydrogen bonding properties according to X-ray crystallography studies could be a factor of their stability and as such they may become a significant contribution to the pull of synthesized compounds that may qualify as clinical trials for potential anticancer candidates.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The financial support of the South Africa Medical Research Council and the University of Fort Hare is appreciated.




