Characteristics of Boron Decorated TiO2 Nanoparticles for Dye-Sensitized Solar Cell Photoanode
Abstract
Different boron weight percents on mixed-phase (anatase and rutile) TiO2 nanoparticles were synthesized to investigate structure morphology, defect states, luminescence properties, and energy conversion. The measured results indicate that boron doping of TiO2 both increases the crystallite size and rutile-phase percent in an anatase matrix. Decreasing the band gap by boron doping can extend the absorption to the visible region, while undoped TiO2 exhibits high UV absorption. Oxygen vacancy defects generated by boron ions reduce Ti+4 and affect electron transport in dye-sensitized solar cells. Excess electrons originating from the oxygen vacancies of doped TiO2 downward shift in the conduction band edge and prompt the transfer of photoelectrons from the conduction band of the rutile phase to the lower energy anatase trapping sites; they then separate charges to enhance the photocurrent and Jsc. Although the resistance of the electron recombination (Rk) between doped TiO2 photoanode and the electrolyte for the doped TiO2 sample is lower, a longer electron lifetime (τ) of 19.7 ms with a higher electron density (ns) of 2.1 × 1018 cm−3 contributes to high solar conversion efficiency.
1. Introduction
Because of the unusual weather induced by enormous carbon dioxide emissions and undisciplined energy utilization, environmental issues have gradually emerged as an important topic. Recently, highly efficient energy conversion products, which are an attempt to eliminate air pollution and to improve environmental qualities, have attracted much attention as replacements for traditional energy utilization devices. Titanium dioxide (TiO2) is the most promising semiconductor because of its long-term stability, nontoxicity, and low cost; thus, TiO2 is widely used in various fields, such as dye-sensitized solar cells (DSSCs), removal of organic and inorganic pollutants, and photocatalytic splitting of water for green-energy hydrogen production [1–3]. Among these applications, dye-sensitized solar cells (DSSCs) are advantageous in terms of both technology and the economy because they are low in cost, as compared to inorganic photovoltaic (PV) devices, have higher energy conversion efficiency, and are simple to fabricate. Since 1991, when O’Regan and Grätzel announced the operating principle of a DSSC [4], many studies have extensively focused on the enhanced light collection ability and conductivity of electrons in photoanodes and charge transport behaviors. Unwanted factors reduce DSSC efficiency, including electrons scavenged from photoanodes and the recombination of back electrons with oxidized redox species I3− [5, 6]. The photoexcited capability of oxidized dye molecules has also been studied in order to realize the carrier transport mechanism [7–9]. Recently, many efforts have focused on creating novel structures to enhance PV performance, and the working mechanism of the DSSC system has been gradually realized. Particularly, TiO2 photoanodes, including core-shell nanowire structures [10, 11], surface treatments and synergistic, mixed-phase TiO2, and hybrid structures [12–14], which provide a feasible synthesis and a simple method, have been widely utilized to enhance DSSC efficiency. On the other hand, decorated TiO2 photoanode technologies have emerged to improve DSSC performance by means of metal/nonmetal doping [15–17].
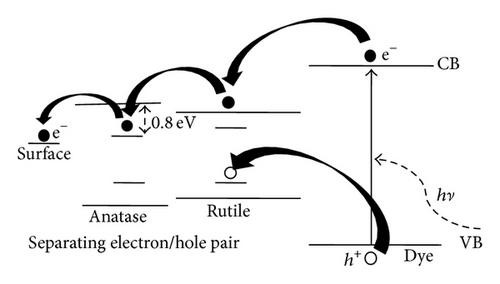
Metal doping commonly tailors semiconductor properties by, for example, redistributing defects and trapping levels in the band gap and by changing the conduction band position [18–20]. However, metal-doped TiO2 is impaired due to its thermal instability and increased electron recombination facilities. Instead of metal doping, nonmetal elements such as nitrogen and carbon doping on TiO2 can change oxygen vacancies and surface-deficiency-related defects and modify the electronic properties. Oxygen plasma and annealing treatments reduce defect trapping and crystallize TiO2, which results in lower resistivity and contact resistance on the carrier transport pathway. If the surfaces trapping sites and/or energies of lattice are taken into consideration, the anatase trapping site has been shown to be 0.8 eV lower in energy than that of the conduction band, locating it below the rutile conduction band [21, 22]. The synergistic effect in an adequate mixed-phase TiO2 between heterogeneous anatase and rutile phases facilitates electron migration from the rutile conduction band to the anatase trapping site due to lower energy of anatase trapping sites below the rutile conduction band [23]. The aforementioned methods to modify photoanode TiO2 result in increased resistance at the TiO2 anode-electrolyte interfaces and reduced electron recombination, which improves the open-circuit voltage. Among the nonmetallic elements, boron incorporated into rutile TiO2 leads to the reduction of Ti+4 to Ti+3, which improves photocatalytic activity, because Ti+3 acts as a photogenerated electron trap [24]. On the other hand, Gopal et al. reported that boron doping was likely to improve the conductivity of TiO2 because of the reduced valence of the titanium ion and the concomitant increase in the oxygen vacancy with the formation of excess electrons [25, 26]. Recently, researchers have devoted much effort to investigate various photoanode materials and structures, which were expected to suppress interfacial recombination and to improve the parameters of electron transport for increasing power conversion efficiency. Numerous studies have focused on the enhanced light collection ability and conductivity of electrons in photoanode and charge transport behaviors. Although methods of boron doping TiO2 nanotubes/nanoparticles structures to tailor the position of the conduction band (CB), which redistribute the defect density and trapping level, have been reported [27], the relationship between carriers transport within conduction bands, energy conversion efficiency, and factors negatively affecting DSSC efficiency has not been verified in detail.
In this paper, the content is divided into three sections: characteristics of boron doping of mixed-phase TiO2; photovoltaic performance; and pathway of photoelectrons. In the first section, crystallite characteristics and changes in the mixed phase associated with various boron doping samples are analyzed by light absorption, luminescence, and defect states. The photovoltaic performances of assembled DSSC devices using various doping TiO2 photoanodes are verified in the second section. In the third section, we analyze the pathway of photoexcited electrons and correlate the available parameters of electron transport with various boron doping of TiO2 (B-TiO2) photoanode on DSSC devices. Under various boron doping conditions on TiO2, the electrochemical impedance spectrum (EIS) is measured by stressing the open-circuit voltage (Voc) to determine the related parameters, such as electron lifetime (τ), electron density (ns) in CB, and interfacial resistance (Rk) between TiO2 and the electrolytes. The obtained PV characteristics relating to the photoanode properties are analyzed in terms of EIS results, deficiencies in photoanodes, and incident photon-to-current conversion efficiency (IPCE).
2. Experimental Details
2.1. Preparation of TiO2 Electrodes
Prior to the fabrication of the B-TiO2 nanoparticles, the fluorine-doped tin oxide (FTO) glass substrates were cleaned by the same volume ratio of acetone and isopropyl alcohol mixture in an ultrasonic water bath for 30 min. To prepare B-TiO2 photoanodes, first, boric acid (H3BO3) was added into 50 mL of distilled water under stirring at room temperature to obtain various concentrations of boric acid solution. Subsequently, 1 g of TiO2 nanoparticles was added dropwise to 20 mL of boric acid mixed solution and the resulting solution was then put into oven in air at 200°C for 36 hours to vaporize water and obtained B-TiO2 powder. TiO2 colloidal solution was prepared by adding 0.5 mL of ethanol and distilled water (v/v = 1/1) mixture solution to 0.5 g of B-TiO2 powder and 0.15 g of polyethylene glycol (PEG). The mixture was ultrasonicated for 3 h to form the TiO2 colloidal solution. The TiO2 photoanodes were prepared layer-by-layer coating of the as-synthesized TiO2 colloidal solution on the fluorine-doped SnO2 (FTO) glasses using a glass rod. The purpose of PEG is used to generate mesoporous TiO2 photoelectrode for future dye adhesion. All as-prepared TiO2 nanoparticles were calcined at 450°C for 30 min in air atmosphere to form microstructure and the thickness was about 14 μm.
2.2. Fabrication of the DSSC Devices
Synthesis of undoped and B-TiO2 nanoparticles were pasted on conducting FTO glass substrate with an active area of 0.5 cm × 0.5 cm and then immersed in a 3.0 × 10−3 M solution of the ruthenium based dye [RuL2(NCS)2]TBA2 for overnight, where Ru is ruthenium, L stands for 2,2′-bipyridyl-4,4′-dicarboxylic acid, NCS represents isothiocyanate, and TBA is tetra-n-butylammonium (N719 dye, Everlight Chemical, Taiwan). The specimens were washed with ethanol after immersion in N719 dye solution. A thin Pt sputtered on an FTO glass was used as the counter electrode. The iodide/tri-iodide (I−/) electrolyte (lodolyte R-150) was cast onto the dye absorbed TiO2 electrodes. The TiO2 electrodes and the Pt coated electrodes were clamped together to assemble the DSSC devices.
2.3. Characteristics Measurement
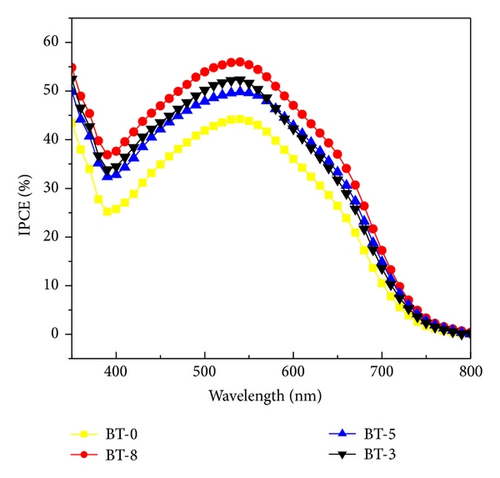
In order to study the physical and electrical properties, the decorated boron of TiO2 nanoparticles was characterized. The field emission scanning electron microscope (FESEM, JEOL 7600F) with energy dispersive X-ray spectroscopy (EDS) was used to determine the surface morphology, thickness of the films, and boron weight percentage in TiO2. The crystalline phases of the obtained titania electrodes were analyzed by Raman spectra and X-ray diffractometer with a Cu-Kα line target (λ = 1.54 Å) and Ni filter at a scanning rate of 2°/min from 2θ = 20° to 80°. Photoluminescence spectroscopy (PL) was used to observe the change of defect states and band characteristics with increasing boron doping. The photovoltaic characteristics of DSSC devices were measured from an illuminated area of 0.5 cm × 0.5 cm under standard AM 1.5 sunlight illumination (XES-151S, San-Ei, Japan) with 100 mW/cm2 light source from the solar simulator and Keithley 2420 SourceMeter. The EIS results was obtained using electrochemical analyzer (CHI 6173b, CH Instruments Co., USA) in the range from 0.01 Hz to 100 kHz within the alternating current amplitude of 10 mV open-circuit voltage (Voc) under the condition of zero electric current. The IPCE was measured within the range of 350–800 nm wavelengths using an IPCE system (Enlitech, Taiwan) that was specifically designed for DSSCs.
3. Results and Discussion
The properties of boron-doped TiO2 nanoparticles are discussed and then analyze the performance of the assembled DSSC devices using various boron-doped TiO2 nanoparticles as photoanode.
3.1. TiO2 Nanoparticle Properties
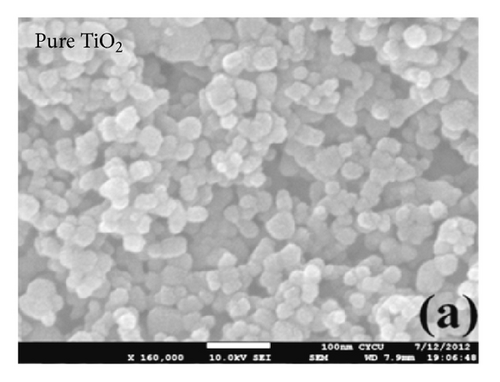

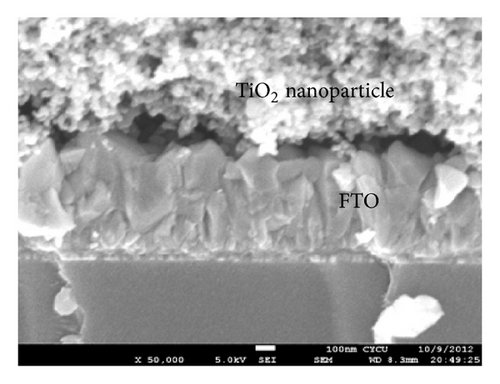
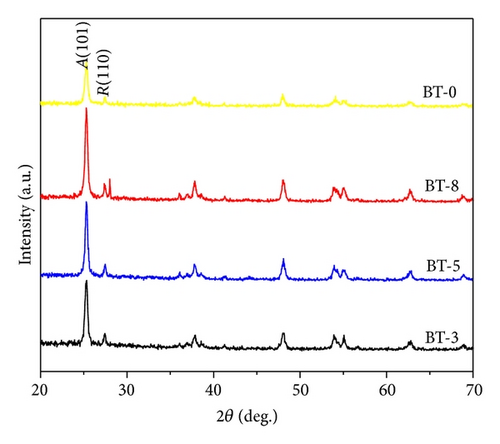
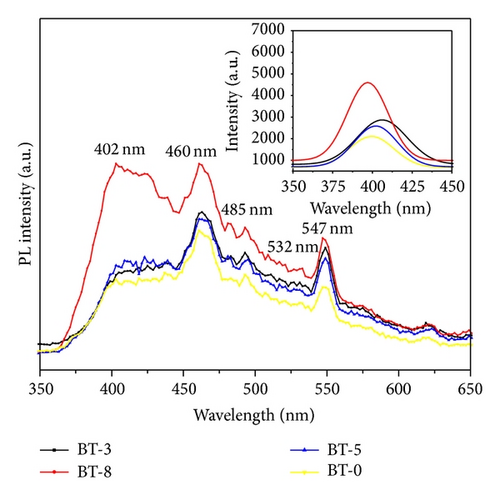
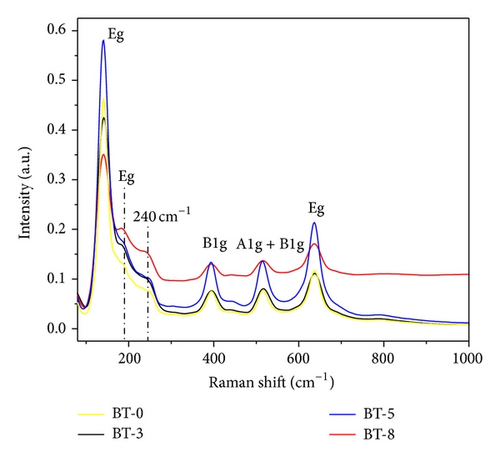

3.2. Photovoltaic Performance of DSSC
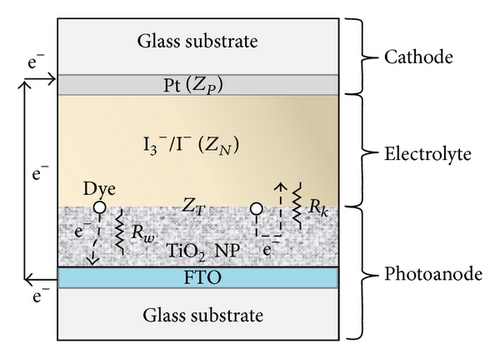
As shown in Figure 1(d), decorated TiO2 that acted as photoanode on DSSC device was investigated. Figure 6 shows the photocurrent density-voltage characteristics of the DSSC with various boron doping of TiO2 photoanodes under a simulated solar light of 100 mW/cm2. Among them, BT-8 has an obvious increase in Jsc and the highest energy conversion efficiency (η) of 4.6% is 21% higher than that of BT-0 sample. Because the Fermi energy position of the anatase trapping site is below the rutile conduction band, in an adequate doping concentration in mixed-phase TiO2, the excess electrons accumulate and there is a downward shift in the conduction band edge at the anatase-rutile grain boundary. The downward shift in the conduction band self-generates a built-in potential and facilitates electrons injected from the rutile phase into the lower energy level of the anatase trapping site, leading to a charge separation. The open-circuit voltage (Voc = I × Rk) values are not obviously different between experimental samples which will be explained by following EIS section. The energy diagram shown in Figure 7 depicts the pathway of excited photoelectrons injected from the dye to the rutile conduction band, anatase trapped level in the band gap and arrive photoanode surface.
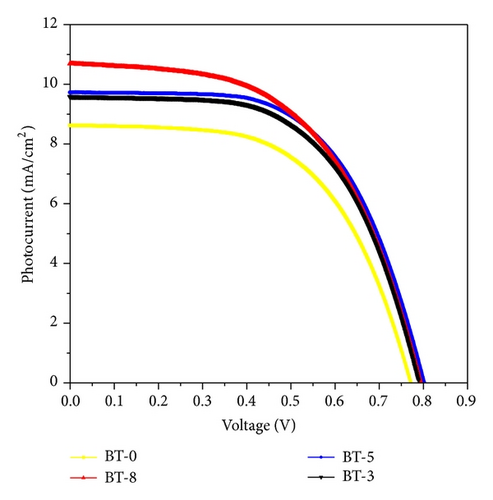
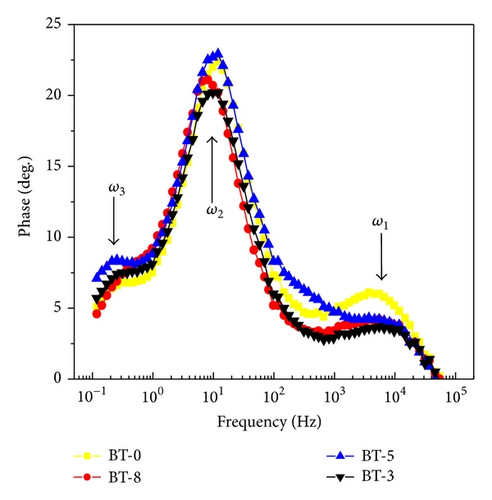
3.3. Parameters of Electron Transport Determined by EIS
Regarding the assembling DSSC device, the pathway of the carrier transport, the conductivity of TiO2 nanoparticles, the behavior of the photoelectron injection, and back-electron recombination correlated with PV parameters are now discussed in terms of the EIS analysis.
| Photovoltaic and EIS parameter of DSSC with various anodes | |||||||
|---|---|---|---|---|---|---|---|
| Anode | Jsc (mA/cm2) |
Voc (V) |
FF | η (%) |
Rk (Ω) |
τeff (ms) |
ns (cm−3) |
| BT-0 | 8.62 | 0.77 | 0.6 | 3.83 | 29.6 | 13.3 | 1.3 × 1018 |
| BT-8 | 10.69 | 0.79 | 0.5 | 4.61 | 27.1 | 19.7 | 2.1 × 1018 |
| BT-5 | 9.72 | 0.80 | 0.6 | 4.62 | 27.5 | 15.2 | 1.6 × 1018 |
| BT-3 | 9.58 | 0.79 | 0.6 | 4.42 | 26.4 | 16.4 | 1.8 × 1018 |

4. Conclusions
Boron doping with mixed-phase TiO2 photoanodes relating to photovoltaic performance and electrochemical impedance spectrum on DSSC was investigated in this study. Boron doping in TiO2 nanoparticles changes the composition of anatase-rutile. Rich oxygen vacancies and excess electrons within TiO2 nanoparticles also facilitate the fast electrons injected from the rutile conduction band into the lower energy at the anatase trapping site, finally prompting the charge separation and enhancing the photocurrent and Jsc. However, oxygen vacancies also reduce the resistance of the electron recombination between photoanodes/electrolytes. On the other hand, the electron lifetime and electron density in oxygen-vacancy-rich B-TiO2 specimens conquer the drawback of electron recombination and improve energy conversion efficiency.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.




