On the Stability and Equilibrium Points of Multistaged SI(n)R Epidemic Models
Abstract
This paper relies on the concept of next generation matrix defined ad hoc for a new proposed extended SEIR model referred to as SI(n)R-model to study its stability. The model includes n successive stages of infectious subpopulations, each one acting at the exposed subpopulation of the next infectious stage in a cascade global disposal where each infectious population acts as the exposed subpopulation of the next infectious stage. The model also has internal delays which characterize the time intervals of the coupling of the susceptible dynamics with the infectious populations of the various cascade infectious stages. Since the susceptible subpopulation is common, and then unique, to all the infectious stages, its coupled dynamic action on each of those stages is modeled with an increasing delay as the infectious stage index increases from 1 to n. The physical interpretation of the model is that the dynamics of the disease exhibits different stages in which the infectivity and the mortality rates vary as the individual numbers go through the process of recovery, each stage with a characteristic average time.
1. Introduction
- (a)
If it ranges from zero to one, the disease-free equilibrium is asymptotically stable and the infection asymptotically vanishes without requiring any external action on the system. In this case, the Jacobian of the matrix of the dynamics of the epidemic model defining the linearized state-trajectory has all its eigenvalues in the stable region. If it exceeds one, the disease-free equilibrium is unstable so that the state trajectories can converge asymptotically to the endemic equilibrium or exhibit an oscillatory behavior.
- (b)
The infection cannot progress if the reproduction number is less than one since the minimum available initial infected population to propagate the disease is below its critical value for spreading.
However, the explicit expression of the reproduction number is neither direct nor easy to obtain in sophisticated epidemic models. Therefore, more advanced techniques, based on the so-called next generation matrix, have been proposed to define such a reproduction number; see, for instance, [8, 33–35]. More recent studies on epidemic models are described in [36, 37]. The importance of demographics is taken into account in [36], while the stability of the equilibrium points of a delayed disease is described in [37]. This paper addresses the concept of the next generation matrix for a new proposed extended continuous time SEIR model referred to as SI(n)R model with delays where there are n-successive stages of infectious subpopulations, each one acting at the exposed subpopulation of the next infectious stage in a cascade global disposal. Since the susceptible subpopulation is common to all infectious stages, its impact on each of those stages is modeled with an increasing delay as the infectious stage index increases from 1 to n. A second related model is used without the above delay. Both models are also reformulated with discretized versions. The physical interpretation of the SI(n)R models is that the incubating period of any disease is not identical for all the susceptible individuals so that they can become infected at different times. So, each of the infectious stages is an infectious class which includes a certain number of individuals which become infected at times centered about a reference average time instant which is considered common for the whole group. The stability analysis is performed by defining a basic reproduction number from an ad hoc next generation matrix for this model. The basic properties which are proved are as follows. (1) All the trajectories remain bounded since the differential system is nonnegative, as it is the alternative discrete model, in the sense that all the subpopulations are nonnegative for all time, and the total population is uniformly bounded for all time. (2) If the defined basic reproduction number is less than one, then the disease-free equilibrium point is globally asymptotically stable as it is proved through the definition and use of a Lyapunov function. In this case, the endemic equilibrium point is locally unstable and, furthermore, it is unfeasible (i.e., nonreachable by any trajectory) since it is not compatible with the system positivity. (3) If such a basic reproduction number is equal to one, then the endemic equilibrium does not exist as such since it is identical to the disease-free equilibrium and then globally stable since the system is positive and the total population is uniformly bounded for all the time so that none of the subpopulation can be unbounded. (4) If the basic reproduction number exceeds one then the disease-free equilibrium point is locally unstable.
2. The Model
This section contains the description and main properties of the introduced models. Previous models of multistaged infectious diseases have been described in [38] including (a) the so-called epidemic models with multistaged infectious period where the susceptible population influences the first infectious stage, that one, the second infectious stage, and so on; (b) the epidemic models with several types of infective subpopulations which are influenced in a parallel disposal and all of them have transitions to a unique removed population; (c) the epidemic among a number of homogeneous groups (each susceptible group generates its own SIR model). It can be considered, in a general context, that the structure proposed in this paper lies in the first of the above classes of the multistaged models.
2.1. SI(n)R Model with No Delays
Now the positivity, existence of equilibrium points, and stability are studied for this model.
2.1.1. Positivity and Boundedness
Proposition 1. All the subpopulations remain nonnegative for all time for any given nonnegative initial conditions, xi ≥ 0 ∀ i ∈ [1, …, n + 2] and all t ≥ 0.
Proof. Let ts > 0 be so that S(ts) = 0 and R(ts), Ii(ts) ≥ 0 ∀ i ∈ [1, …, n]. Then, from 1
Now, let t1 > 0 be so that I1(t1) = 0 and S(t1), Ii(t1) ≥ 0 ∀ i ∈ [2, …, n]. Then, from 1
For the next (n − 1) infectious subpopulations the same operation is made: let tj > 0 be so that Ij(tj) = 0 and S(tj), Ii(tj) ≥ 0 ∀ i ≠ j. Then, from 1
Finally, let tr > 0 so that R(tr) = 0 and S(tr), Ii(tr) ≥ 0, ∀i ∈ [1, …, n]. Then, from 1
Not only does the epidemic system possess nonnegative solutions under nonnegative initial conditions, but also the total population and all the subpopulations are bounded for all time with a uniform finite upper-bound as it is discussed in the subsequent two results.
Proposition 2. The total population is finitely upper-bounded for any given nonnegative initial conditions.
Proof. If we sum up 1, we obtain
Corollary 3. All the subpopulations are finitely upper-bounded for all time for any given nonnegative initial conditions, obeying the constraints , where is the total population at the disease-free equilibrium.
2.1.2. Equilibrium Points
2.1.3. Stability Analysis: Next Generation Matrix
2.2. Discrete SI(n)R Model with No Delays
Proposition 4. The discrete system described in 17 is nonnegative for any nonnegative initial conditions if the step size τ is small enough to satisfy
Proof. Given a set of nonnegative subpopulations at the kth-sample, from the second equation of 17, it can be deduced that
In order to guarantee the nonnegativity of the susceptible subpopulation, first a maximum value is set for Nk. From 17
So from the first equation at 17 it is known that
Thus, the disease-free equilibrium state will be locally stable when the reproduction number is less than 1 and unstable otherwise. From known previous calculations of the stability of similar models [35, 40], this decomposition technique for stability analysis can be compared to previous ones such as the study of the stability made in [35] for the SEIR model, which is a specific version of a continuous SI(n)R model in which n = 2 β1 = 0, ν1 = ν2 = b, and γ1 = κ. From the definition in 33 it is obtained that R0 = β2Sdfeκ/(b + κ)(b + γ2), which agrees with the study of the eigenvalues of the Jacobian in a continuous-time model [26].
2.3. SI(n)R Model with Delays
2.3.1. Construction of the Model
2.3.2. Positivity and Boundedness
Proposition 5. The model equation 40 is nonnegative for any initial nonnegative conditions. Thus,
Proof. Since βi ≥ 0, it is then deduced from 41 that f(ω) ≥ 0 ∀ ω ∈ (−∞, t0). Assume that
From 38 assume that ∃t1 ≥ t0 such that Im(t1) = 0 for the first time and the value ζ ≥ 0 as small as desired such that I(t1 − ζ) > 0; I(t1 + ζ) < 0. Then it can be said that
Since f(t1 − ζ) ≥ 0 and knowing that f(t) is continuous and derivable for all t, from the mean value theorem, it can be deduced that
Since the lower bound of the subpopulations is established as 0, an upper-bound for all the subpopulations is set in the following proposition.
Proposition 6. S(t) ≤ Ndfe, Im(t) ≤ Ndfe, R(t) ≤ Ndfe , for any given initial conditions .
2.3.3. Equilibrium Points
Take into account that if S* = α/b, xend = xdfe, the endemic, and the disease-free equilibrium are the same, so there is only one equilibrium point in the system.
2.3.4. Stability of the Disease-Free Equilibrium (DFE) Point
The local stability of the DFE point is proved in this section after introducing the reproductive number R0, the average number of new cases that produce an infected individual during the average duration of the disease. In order to find this number easily, a next generation is constructed as follows.
Proposition 7. The DFE is globally asymptotically stable if R0 < 1.
Proof. A candidate for a Lyapunov function Φ is defined as Φ = ϕ1 + ϕ2 + ϕ3, having the auxiliary functions ϕ1, ϕ2, ϕ3 been defined as
This integral function can be reconfigured as
Given this definition of Ii(t), the parameter σi(t) is defined as
2.3.5. Stability of the Endemic Equilibrium Point (END) Model with n = 1
2.3.6. Stability of the Endemic Equilibrium Point (END) Model with n = 2
- (1)
gi > 0 ∀ i = 0,1, 2,3
- (2)
g1g2 > g3g0.
2.4. Discrete SI(n)R Model with Delay
3. Simulation
A set of Matlab simulations are made based on the models described in the previous section, in order to contrast the predictions on their respective endemic and disease-free equilibrium states. A large enough number of infectious subpopulations are chosen, n = 3, so the stability of the models will be tested regarding their reproductive numbers. A common initial condition is set to all the susceptible subpopulation in the disease-free equilibrium plus a 0.1 of that value of infected subpopulation at the first stage of the disease. For exposition and calculations convenience, the mortality rate b and the birth rate α will be both taken as equal to the inverse of the average lifespan of a human b = α = (1/70) year−1. The mortality rate of the three stages of the infection will be ν1 = 2b, ν2 = 3b, ν3 = 3b/2, and the average time which an individual spends in each stage will be 19, 29, and 61 days, respectively. The infectious ratios βi will be altered in order to obtain reproductive numbers below and above 1, but the ratios between them will be constant, as 4β1 = 7β2 = 4β3. When simulating the discrete models, an appropriate time step size is chosen in order to guarantee the nonnegativity of model with no delays. As for the discrete model with delays, given that nonnegativity of the subpopulations in the continuous model has been proved in Proposition 5, an algorithm is established during the simulation so that the discrete dynamics approximates to the continuous one in any critical positivity points. A loop in which the following point is evaluated is introduced, acting over the time step size. While any of the subpopulations of the next point present a negativity xk+1,i < 0 for any i ∈ [1, …, n + 2], then the step size is reduced , with j being the jth loop iteration, , and λ ∈ (0,1). Another loop is set after this resetting the time step size while the next point is nonnegative or the time step size is equal to or below the original value.
3.1. SI(3)R Model with No Delays
Given the parameters previously commented, the transition ratios between the different subpopulations are obtained as the inverse of their average times during each stage. Thus, γ1 = 365/19 year−1, γ2 = 365/29 year−1, γ3 = 365/61 year−1, and the final transition from recovered to susceptible again will be set as γ4 = 365/670 year−1. Given a value of the transition rates the reproduction number will be set to 0.5 and 1.5, respectively. For the continuous model, two graphics representing the evolution of each subpopulation for both situations are presented in Figures 1 and 2 while, for the discrete model, a step time τ is set to 0.1 years and the simulations are run with the same parameters. The results of these simulations are presented in Figures 3 and 4.
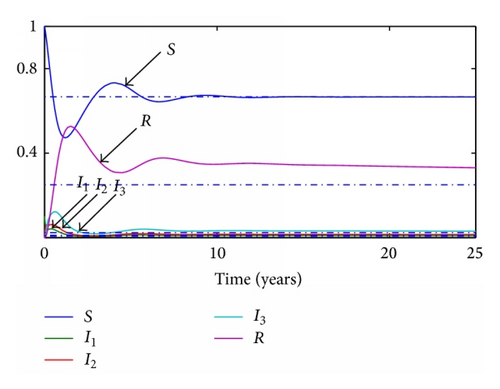
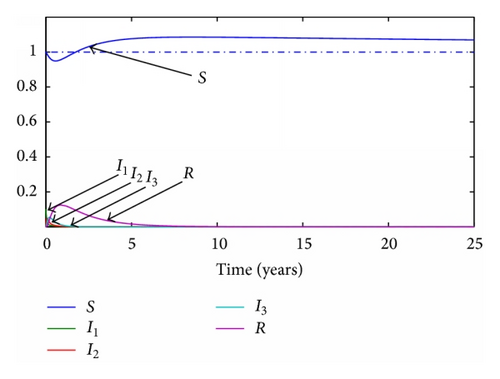
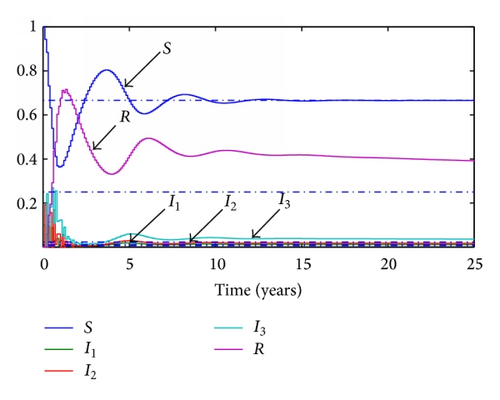
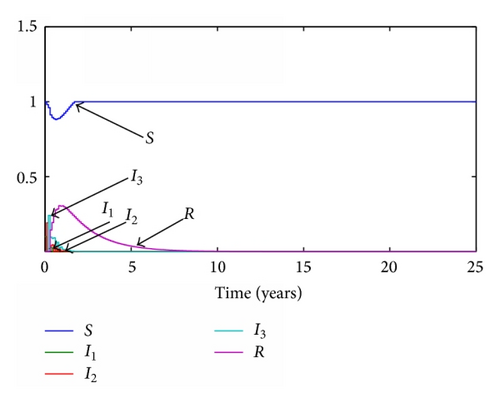
3.2. SI(3)R Model with Delays
The common parameters are set as in the previous cases, but this time the delayed times will be equal to their respective average times τ1 = 19/365 years, τ2 = 29/365 years, and τ3 = 61/365 years. The delay of the transition between the recovered and the susceptible subpopulations will be changed from 610/365 to 80/365 years in order to let the simulation achieve the simulation at a reasonable time. The values of the transmission rates will maintain their proportionality, but the range of the possible reproductive number is not so wide as in the previous case. Their values are changed to now be R0 = 1.01 and R0 = 0.92, respectively. The evolution of the subpopulations is presented in Figures 5 and 6.
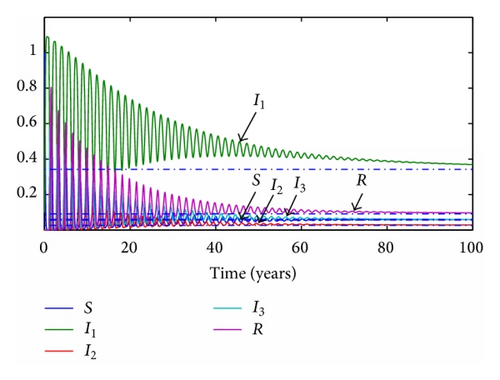
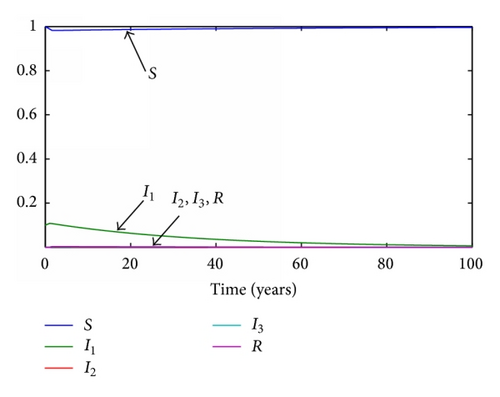
In the discrete model, the same time step as before is set to τ = 0.1 years. The evolution of the subpopulations for each reproductive number can be seen in Figures 7 and 8, respectively.
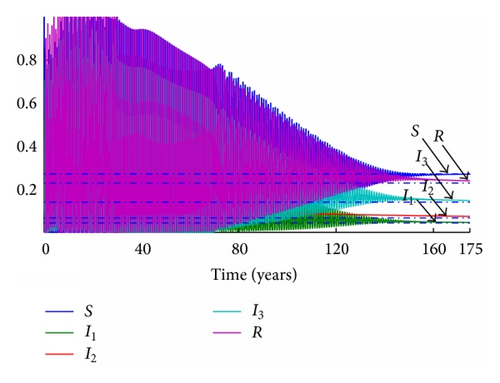
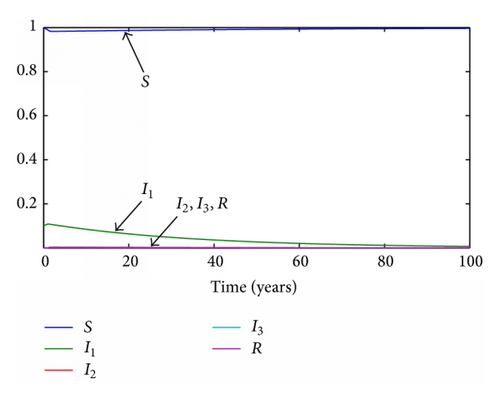
Observe that the subpopulations rapidly tend to the disease-free equilibrium in Figure 8. While it is seen that the discrete-time and continuous-time models reach the same final states when the conditions are equal, the irregularity of the dynamics in the discrete models over the continuous has also been noticed. This fact reveals that the discretization procedure along with the time step must be selected carefully prior to simulating the discrete-time model.
4. Conclusions
A model of a disease spreading with multiple infectious stages has been studied in depth. The next generation techniques have been proven to be quite useful when operating with models with complex interactions as in the variations of the model presented in this work. The reproduction numbers obtained in each case have also proven to predict correctly the final stable state of the subpopulations when they are properly simulated. The simulations have also shown that the different discretizations of a continuous model can cause significant discrepancies in the resulting dynamics. An appropriate integration step should be considered taking this into account as well as other factors like the computation time available, or, if the model is being used in a controlled system, the available time for data acquisition.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was partially supported by the Spanish Ministry of Economy and Competitiveness through Grant no. DPI2012-30651, the Scholarship support BES-2010-035160 for Raul Nistal, the Basque Government, through Grant no. IE378-10, and by the University of the Basque Country through Grant nos. UFI 11/07, PES13/21, and GIU14/07. Raul Nistal is grateful to the doctorate program “Molecular biology and Biomedicine” of UPV/EHU for its support for him as a Ph.D. student. Finally, the authors are also grateful to the reviewers for their useful suggestions.




