Effect of Dopant Compensation on the Behavior of Dissolved Iron and Iron-Boron Related Complexes in Silicon
Abstract
The behavior of iron, iron-boron (FeB) pairs, and iron-boron-phosphorus (FeB-P) complexes has been studied in B-doped Czochralski silicon with phosphorus (P) compensation and compared with that in uncompensated material. The interstitial iron concentration has been measured at temperatures from 50 to 270°C. The apparent binding energy (Eb) of FeB in compensated silicon is (0.25 ± 0.03) eV, significantly lower than the (0.53 ± 0.02) eV in uncompensated silicon. Possible reasons for this reduction in binding energy are discussed by experimental and calculation methods. The results are important for understanding and controlling the behavior of Fe in compensated silicon.
Low-cost silicon feedstock for photovoltaic (PV) industry contains dopant species and metal impurities, in most cases boron (B), phosphorous (P), and iron (Fe) [1–3]. In some cases, a significant dopant compensation also exists, which affects the properties of the silicon substrate and reduces the efficiency of the solar cells [4, 5]. One of the unresolved issues of compensated B-doped silicon is the influence of compensation on the behavior and properties of dissolved iron and of iron-boron pairs (FeB). The stable configuration of FeB is that an interstitial Fe atom (Fei) occupies a tetrahedral (T) interstitial site close to a substitutional B atom (Bs) whereby the positively charged Fe atom is attracted by the negatively charged B atom [6, 7]. In any B-doped p-type silicon wafer, even when it is strongly compensated, Fei is always positively charged and will therefore form pairs with the negatively charged acceptor atoms [8]. As the B atoms are quasi immobile in the relevant temperature range and the pairing reaction itself is very fast, the formation kinetics of FeB are determined only by diffusion [9, 10] which is little or not affected by the presence of compensating dopants [8]. The migration energy of , which is close to the formation energy of FeB, has been reported to be in the range of 0.58 to 0.81 eV [7]. For compensated silicon, Istratov et al. [7] reported that the effect of P on migration energy can be ignored. The equilibrium concentration of (N()) depends on the binding energy (Eb) of FeB, the temperature, and the B concentration [11], whereby N() can be monitored by the variation of carrier lifetime before and after the association of FeB [11]. Eb is in the range of 0.45 to 0.65 eV as reported in a previous study [7, 12]. For compensated silicon, first-principles calculations show that there is a binding energy of 0.82 eV when Bs pairs with substitutional P (Ps) located at its second-neighbor sites [13]. This pairing of B and P, which can occur during crystallization from a melt, will also lead to a different binding energy of Fe with the B-P pair due to the fact that also the apparent thermodynamic properties of FeB (in fact of the carrier recombination centers associated with the FeB-P and FeB complexes), as determined from lifetime measurements, will be different in compensated material. Comparing the Fe behavior in compensated silicon with that in uncompensated silicon will therefore yield information on Eb of iron-boron-phosphorus (FeB-P) related complexes in compensated material.
At temperatures below 100°C, the formation of FeB is the dominant process in uncompensated material, while a recovery of Fei species and a reduction in the concentration of FeB are observed at temperatures between 100 and 200°C [7]. At temperatures above 200°C, acceptors can no longer provide stable traps for iron and iron will diffuse towards more stable sinks such as dislocations, precipitates, or even the sample surface [7, 14]. Zeng et al. [14] reported that iron forms small precipitates from 300 to 700°C in as-received Czochralski (CZ) silicon and that Fe precipitation is a diffusion-limited process that follows closely Ham’s law [15]. Krain et al. [16] reported that the precipitation activation energy is close to the Fei migration energy. To limit the influence of Fe precipitation, Eb is determined at temperatures below 200°C in our experiment. To completely dissociate FeB for B concentrations above 1016 cm−3, however, the temperature must be higher than 300°C in uncompensated Si [17]. The total iron concentration can be determined before and after the light induced dissociation of FeB (or Fe-B related complexes) both in compensated and in uncompensated silicon [8].
In this letter, the effect of dopant compensation in silicon on the behavior and properties of Fe is investigated at temperatures between 50 and 270°C. The concentration of single positively charged interstitial iron atoms N() in B and P codoped silicon has been compared with that in uncompensated silicon in this temperature range.
1 × 1 cm2 p-type 〈100〉 crystalline silicon samples are prepared. One set of samples (labeled CZ-1) is only B-doped, with a B concentration (NB) of 1.1 × 1016 cm−3. The second set of samples (labeled CZ-2) is B and P codoped, with NB of 4.10 ± 0.04 × 1016 cm−3 and P concentration (NP) of 1.20 ± 0.01 × 1016 cm−3, as determined by secondary ion mass spectroscopy (SIMS). The interstitial oxygen concentration in all the samples is about 1018 cm−3, as determined by Fourier transform infrared spectroscopy (FTIR) using the 3.14 × 1017 cm−2 calibration factor. After dipping in 0.1 mol/L ferric nitric acid (Fe(NO3)3) solution, Fe was diffused into the samples by annealing in an argon ambient at 800°C for 2 h, followed by quenching in air. Fe concentration that can be introduced into the samples at that temperature is of the order of 1012 cm−3 according to the solubility of iron in silicon [7]. After that, the samples were subjected to chemical polishing and surface-passivation with SiNx:H films by plasma-enhanced chemical vapor deposition (PECVD) technique. Then, the samples were kept in the dark for more than 24 h to allow the dissolved iron atoms to diffuse and form complexes with dopant atoms until an equilibrium is established at room temperature. Subsequently, the samples were annealed at different temperatures in the range between 50 and 270°C until equilibrium was reached at each temperature, followed by cooling in water. The applied anneal times were at 50°C/2 h, at 100°C/1 h, at 120°C/40 min, at 140°C/30 min, at 160°C/25 min, at 180°C/17 min, and above 200°C/10 min. After 200°C/10 min anneal, the annealing times (<200°C) were determined when the carrier lifetimes reached the equilibrium state at each temperature. Note that the effects of the B-O complexes were eliminated after 200°C/10 min anneal, since the B-O complexes were dissociated. The evolution of carrier lifetime with the anneal temperature is measured by the microwave-detected photoconductance decay (MW-PCD) technique using Semilab WT2000 instrument. In all experiments the change of the carrier density after FeB dissociation is less than 6%.
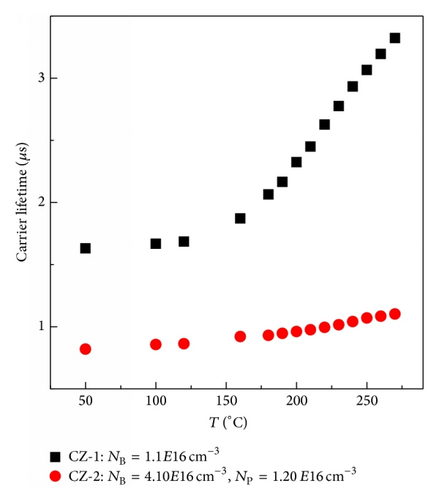
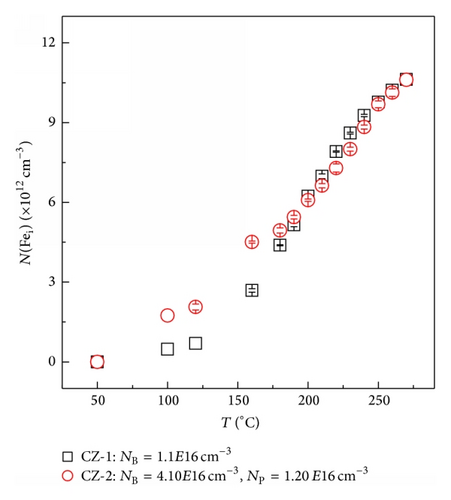
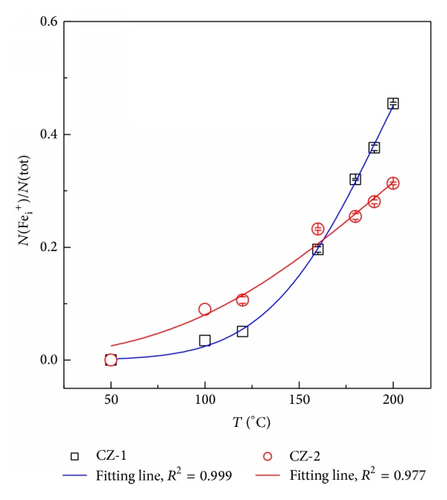
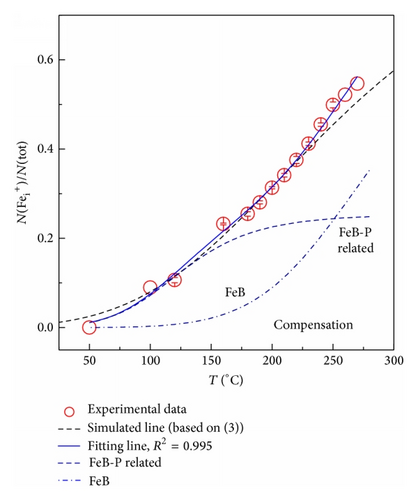
Figure 2 shows the ratio of N() to N(tot) at temperatures between 50 and 200°C for the compensated and uncompensated samples. Note that Fei concentration is equal to N() as the Fermi level is below the defect level Ev + 0.39 eV of Fe0/+, for all temperatures studied [7]. It can be seen from Figure 2 that the value of N()/N(tot) increases with increasing temperature for both samples. But the increase rate of N()/N(tot) for the uncompensated material is larger than that for the compensated one if temperature is above 100°C.
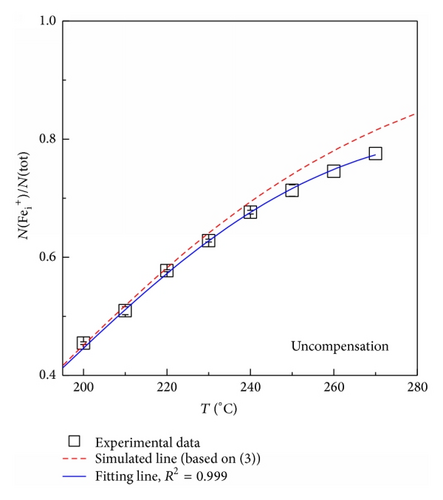
For further support of the above mentioned model, the binding energy of FeB and FeB-P complexes has been calculated using the density-functional theory (DFT). All calculations were performed with the Vienna ab initio simulation package (VASP) [23]. A 216-atom supercell, Brillouin-zone sampling with 2 × 2 × 2 Monkhorst-Pack k-point sampling [24], and the plane wave basis set with kinetic energy cutoff of 480 eV were used. The generalized gradient approximation (GGA) is applied with the projector augmented wave (PAW) [25, 26] method and the Perdew-Burke-Ernzerhof exchange-correlation functional [27]. The structures were optimized until the forces on each atom were smaller than 0.01 eV/Å. The convergence criterion for energy was chosen as 10−5 eV. Finally, the GGA + U method [28, 29] was used to account for on-site correlation at Fe sites. The effective Coulomb exchange interaction Ueff ( = U − J) is about 3.6 ( = 4 − 0.4) eV for the Fe atom [30]. Eb of () pairs is 0.71 eV, while it is 0.61 eV for Fe and B in () complexes. The difference of 0.10 eV agrees well with 0.08 eV deduced from the experimental results. This illustrates again that P located close to B weakens the Eb of () pairs. Furthermore, the Fei atom does not pair with Ps since Eb is smaller than zero.
In summary, Fei behavior and properties in B-P codoped silicon have been studied. The apparent binding energy of iron-dopant complexes in compensated silicon of (0.25 ± 0.03) eV is 0.28 eV lower than that of FeB in uncompensated silicon. The binding energy for Fe with the B-P pair is (0.45 ± 17) eV compared with 0.53 eV for FeB in uncompensated silicon. Calculation results show that P atom reduces Eb of FeB of 0.10 eV. The results are of significance for understanding the behavior of FeB in compensated silicon.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This project is supported by the National Natural Science Foundation of China (nos. 51532007, 61574124, and 61274057), and the authors are also grateful to Professor Duanlin Que for his supervision.




