Diffusion-Controlled Growth of Oxygen Bubble Evolved from Nanorod-Array TiO2 Photoelectrode
Abstract
Nanorod-array structure gains its popularity in photoelectrode design for water splitting. However, the structure’s effects on solid-liquid interface interaction and reaction product transportation still remain unsolved. Gas bubble generally evolved from photoelectrodes, which provides a starting point for the problem-solving. Based on this, investigations on the gas-evolving photoelectrode are carried out in this paper. By experimental studies of wettability on the photoelectrode nanorod-array surface and oxygen bubble growth from anode, we analyzed the interaction affecting the gas-solid-liquid contact behaviors and product transportation mechanism, which is controlled by diffusion due to the concentration gradient of dissolved gases in the aqueous electrolyte and the microconvection caused by the bubble interface movement. In the end, based on the bubble growth characteristics of RB(t) ~ t0.5 in the experiment, a model describing the product transport mechanism was presented.
1. Introduction
Hydrogen production by photoelectrochemical water splitting is a promising source for carbon-free energy [1–3]. In order to increase the energy conversion efficiency, a lot of research on transfer process enhancement of photo-electron has been carried out with great progress by photoelectrode surface modification with special micro-nano structure [4–7]. But for this kind of heterogeneous photocatalytic or photochemical conversion system, many issues concerning interface of such micro-nano structure semiconductor and liquid still remain unsolved, such as the nature of the active sites, mechanism for heterogeneous photocatalytic reaction of water splitting, and effects of interfacial interaction during reaction product transfer.
Although much progress has been made on the bubble growth, effects of the solid surface structure are still to be understood. Wei et al. investigated the bubble behavior on micro-pin-fined surface and considered a large capillary force leading to easy bubble detachment [14]. Yang et al. studied the bubble-solid interaction indicating the importance of electrolyte concentration and DLVO surface forces [15]. The interaction between the micro-nano structure surface with electrolyte and gas-liquid interface should be investigated more deeply.
In this paper, to investigate nanostructure effects on bubble behavior evolved from nanoscale structure photoelectrode, a study on nanorod-array structure surface characteristics and single oxygen bubble growth dynamics is carried out.
2. Experimental
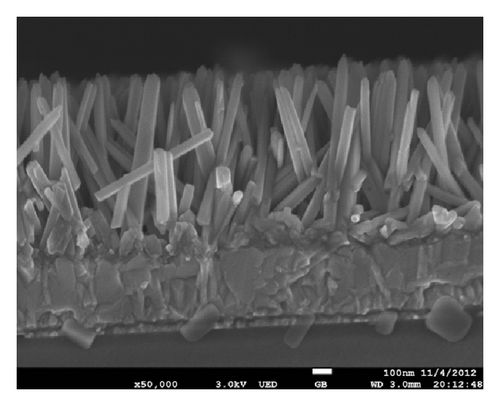
TiO2 film modified by nanorod-array was used as the anode in the investigation, and its SEM shows in Figure 1 with rod height of about 870 nm and rod diameter of nearly 120 nm. The optical contact angle meter model SL200 was applied to study the wettability of its surface.

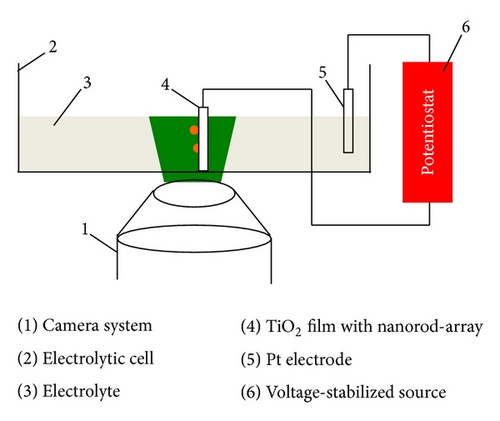
The experiments on bubble evolution were conducted in an electrolytic cell, as shown in Figure 2. Na2SO4 in deionized water with different concentrations of 0.495, 0.354, 0.261, 0.206, and 0.170 mol/L was used as the electrolyte. Pt was used as a cathode. The bubble evolution process was observed by the microscope camera system. The image intense CCD whose resolution is 1376*1040 and objective lens is 5x /0.15 was applied. All the experiments were conducted under the conditions of ambient temperature 25°C and ambient pressure 1 atm.
3. Results and Discussion
3.1. Wettability of Nanorod-Array TiO2 Film
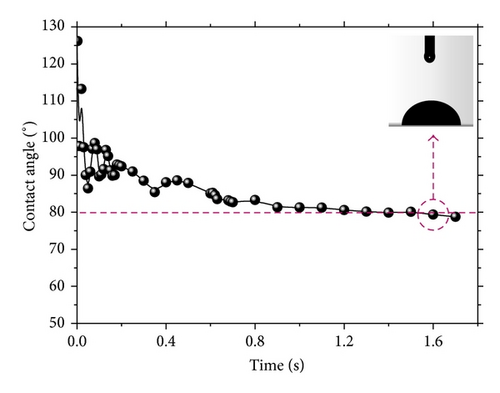
In order to investigate the interaction, especially wettability between nanorod-array film and water, we carry out the experiment on contact angle firstly. Figure 3 shows measurement results of about 80° ± 1°, indicating a general hydrophilic ability.
3.2. Oxygen Bubble Behavior Evolved from Nanorod-Array TiO2 Film
Figure 4 shows the general characteristics of bubble growth. Compared to bubble growth during boiling and common electrolysis, bubble evolved from nanorod-array surface stays longer and smaller, which is determined by the forces acting on them. In our experiment, the proper effects include gravity, gas-liquid interfacial surface tension, marangoni force, electric force (with the possibility of gas-liquid interface being charged), and interfacial interaction with elastic materials. Among those, the most possible and important effects to prevent the bubble departure and keep bubble staying are marangoni force due to nonhomogeneous gas adsorption at the interface and the capillary force caused by contact behavior with nanorod-array surface structure.
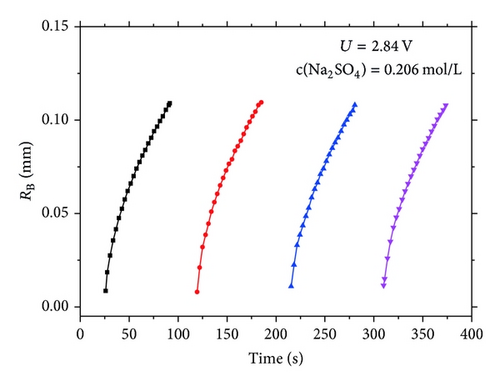
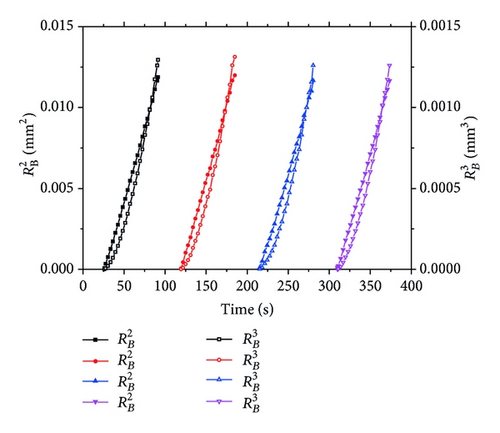
Besides the dynamics aspect, we are much concerned about the mechanism driving the bubble growth. We investigate the relationship of RB(t) ~ tx, as show in Figure 5. It indicates the RB(t)2 is linear to the time, which is to say x = 0.5 for equation RB(t) ~ tx.
3.3. Oxygen Bubble Growth Model from Nanorod-Array TiO2 Film
The bubble growth is driven by the concentration difference between the electrolyte bulk and the bubble interface, which is an analogy to the bubble growth during boiling. But the acquisition of the concentration data, key to electrolysis, is much difficult than that of the temperature data for boiling. So the modelling of bubble growth driven by the concentration difference is less developed than that driven by the temperature difference.
From the above analysis, we obtained RB(t) ~ t0.5 relationship, which is first growth mechanism showed in Figure 6. According to Vogt’s theory [12, 13], effects on bubble growth in one circle, meaning the process of bubble departure and movement excluded, include two parts, which are the diffusion due to the concentration gradient of dissolved gases in the aqueous electrolyte and the microconvection caused by the bubble interface movement.
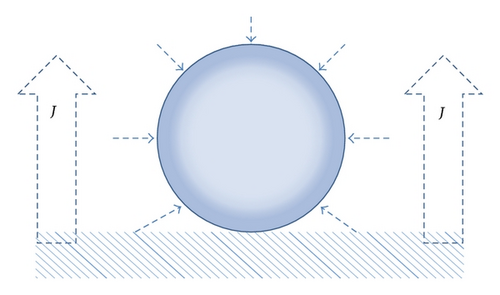
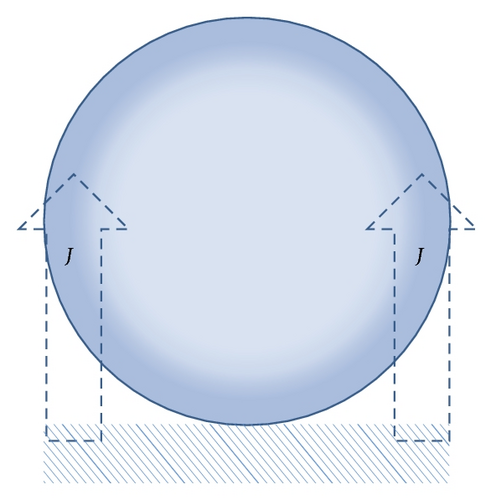
4. Conclusions
In this paper, we carried out research on nanorod-array structure surface characteristics and single oxygen bubble growth dynamics. By experimental studies of wettability on the photoelcectrode nanorod-array surface and oxygen bubble growth from anode, we analyzed the interaction affecting the gas-solid-liquid contact behaviors and product transportation mechanism, which is controlled by diffusion due to the concentration gradient of dissolved gases in the aqueous electrolyte and the microconvection caused by the bubble interface movement and we emphasize the importance of marangoni force due to nonhomogeneous gas adsorption at the interface and the capillary force caused by contact behavior with nanorod-array surface structure on bubble growth period. In the end, based on the bubble growth characteristics of RB(t) ~ t0.5 in the experiment, a model describing the product transport mechanism was presented.
Abbreviations
-
- RB:
-
- Bubble radius, m
-
- ci:
-
- Concentration at interface, mol/L
-
- c0:
-
- Concentration in bulk liquid, mol/L
-
- :
-
- Diffusion coefficient of oxygen gas, m/s
-
- J:
-
- Mass-transfer rate, mol/s
-
- Vmol:
-
- Molar volume, m3/mol
-
- VB:
-
- Bubble volume, m3.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (no. 51306147, no. 51236007, no. 51323011, and no. 51102194) and China Postdoctoral Science Foundation (no. 2013M532043). One of the authors (Xiaowei Hu) was supported by the “Fundamental Research Funds for the Central Universities.” And the authors sincerely thank Dr. J. Su for helpful discussions.




