Topological Model on the Inductive Effect in Alkyl Halides Using Local Quantum Similarity and Reactivity Descriptors in the Density Functional Theory
Abstract
We present a topological analysis to the inductive effect through steric and electrostatic scales of quantitative convergence. Using the molecular similarity field based in the local guantum similarity (LQS) with the Topo-Geometrical Superposition Algorithm (TGSA) alignment method and the chemical reactivity in the density function theory (DFT) context, all calculations were carried out with Amsterdam Density Functional (ADF) code, using the gradient generalized approximation (GGA) and local exchange correlations PW91, in order to characterize the electronic effect by atomic size in the halogens group using a standard Slater-type-orbital basis set. In addition, in this study we introduced news molecular bonding relationships in the inductive effect and the nature of the polar character in the C–H bond taking into account the global and local reactivity descriptors such as chemical potential, hardness, electrophilicity, and Fukui functions, respectively. These descriptors are used to find new alternative considerations on the inductive effect, unlike to the binding energy and dipole moment performed in the traditional organic chemical.
1. Introduction
The inductive effect is one of the most important electronic effects in chemistry [1]. According to the International Union of Pure and Applied Chemistry (IUPAC) the inductive effect is defined as transmission effect of charge on a chain of atoms via electrostatic induction [2]. The inductive effect is experimentally observable via molecular polarization of polar covalent bonds produced by electrostatic induction, due to differences in electronegativity between the atoms involved [3]. In this contribution, our attention will be focused in the quantification of the molecular polarization from the point of view of the molecular bond in alkyl halides molecules such as 3HC–X, (X = F, Cl, Br) taken as example. Using an atomic approach of molecular quantum similarity performed by Cioslowski and Nanayakkara [4] and the Molecular Quantum Similarity Indexes (MQSI) based on the electron density proposed by Carbó-Dorca and coworkers [5–9].
Several concepts and theories have been proposed in the literature in order to explain the molecular chemical bond [10–19]. Nowadays, the most used theories are the molecular orbital theory [20, 21] and the valence bond method [22, 23]. In this paper was used the concept of chemical bond as minimization of equilibrium interactions determined by the bond distances at the experimental level. We propose to relate these experimental distances with the dis(similarity) electrostatic and steric, using quantum similarity descriptors such as overlap and Coulomb indexes, with their Euclidean distances.
On the other hand, we present in this contribution a theoretical investigation in order to relate the inductive effect in these alkyl halides and analyze its dependence on the bond distance with respect to the variation of electronegativity of substituent atoms (–F, –Cl, –Br). This approach could shed some light onto some molecular aspects that can contribute to the knowledge behind interactions of these molecules.
Another reason for carrying out this study is to relate the dis(similarity) between the inductive effect and the nature of chemical bond with differences of electronegativity between atoms. In addition, this approach suggests a new perspective to the treating of the chemical bond with the inductive effect, taking into account that the search for new interpretations is a field of great interest in organic physical chemistry; in addition to the inductive effect also can be used to determine the stability of molecule with respect to their charge distribution [24–26].
2. Theory and Computational Details
2.1. Similarity Indexes
Reviews about concepts and applications in quantum similarity can be found elsewhere (see, for example, [5–9, 27, 28]); only general definitions will be given at this point. Quantum similarity provides a quantitative measure of the resemblance between two quantum objects A and B.
2.2. Local Similarity Indexes
2.2.1. The Hirshfeld Approach
The Hirshfeld approach [32–38]. This approach is based on partitioning the electron density ρ(r1) of a molecule in contributions ρA(r1). In this study, the Hirshfeld approach is used to express the global similarity index (4) to local level.
2.2.2. Description of Molecular Bond Proposed
To study the effects by varying electronegativity by substituents, we propose a particular characterization of the chemical bond which can be considered complementary to the Hirshfeld partition, considering that the inductive effect is dependent on the interatomic bond length [2].
3. Global and Local Reactivity Descriptors
4. Molecular Alignment and Computational Details
The principal problem with the calculation of the quantum similarity measures (QSM) is the dependence the relative orientation of the molecules. The problem has been addressed through the use of several methodologies and approaches. Many critera have been used in order to deal with this problem, such as aligning the molecules according to common chemical features (e.g., three-dimensional molecular fields such as steric, electrostatic, or hydrophobic fields, among others) [65].
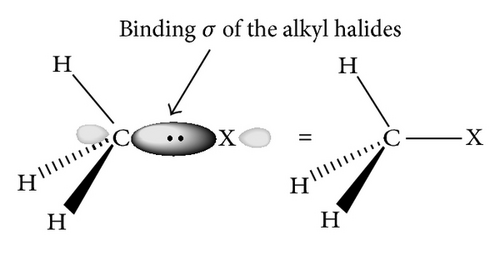
Also these molecules can be aligned based on topological and geometrical features as has been implemented in the Topo-Geometrical Superposition Algorithm (TGSA) [66]. In this method the alignment is based on the recognition of a common skeleton, taking only the types of atoms and bond length [67–70], in this paper the recognition of the common carbon atom was used as is shown in Figure 1.
4.1. Density Functional Calculations
In order to characterize the effects by atomic size, all calculations were carried out by using the Amsterdam Density Functional (ADF) program package [71, 72]. ADF is a program for first-principle electronic structure calculations that make use of (DFT) [50]. All the molecular structures were fully optimized using the gradient generalized approximation (GGA) [73–76] local exchange correlations PW91 [77–79]. The geometry optimizations were calculated using a standard Slater-type-orbital basis set with triple-quality plus double-polarization functions for all the atoms.
5. Results and Discussion
The results reported in this work are those associated with the molecular quantum similarity indexes, followed by results of the relationship of bond proposed, and finally the results of the reactivity descriptors.
5.1. Molecular Quantum Similarity Analysis
In Tables 1–4 the (MQS) matrices for the compounds analyzed are shown, taken as an example.
| Compound | CH3–F | CH3–Cl | CH3–Br |
|---|---|---|---|
| CH3–F | 1.00 | ||
| CH3–Cl | 0.82 | 1.00 | |
| CH3–Br | 0.77 | 0.98 | 1.00 |
| Compound | CH3–F | CH3–Cl | CH3–Br |
|---|---|---|---|
| CH3–F | 1.00 | ||
| CH3–Cl | 0.98 | 1.00 | |
| CH3–Br | 0.97 | 0.99 | 1.00 |
| Compound | CH3–F | CH3–Cl | CH3–Br |
|---|---|---|---|
| CH3–F | 0.00 | ||
| CH3–Cl | 1.31 | 0.00 | |
| CH3–Br | 1.46 | 0.27 | 0.00 |
| Compound | CH3–F | CH3–Cl | CH3–Br |
|---|---|---|---|
| CH3–F | 0.00 | ||
| CH3–Cl | 2.79 | 0.00 | |
| CH3–Br | 3.34 | 0.72 | 0.00 |
In Tables 1 and 2, is possible to see that the CH3–Cl and CH3–Br have the higher quantum similarity with an overlap value of 0.98 and a Coulomb value of 0.99. With a Euclidean distance of Overlap of (0.27, see Table 3) and a Euclidean distance of Coulomb of 0.72 (see Table 4). The high values associated with the (MQS) of Coulomb are justified, taking into account that the alkyl halides are taken as examples of hydrocarbon derivatives in which one or more C–H bonds are replaced by C–X bonds (X = F, Cl, Br), and the similarity of C–X bond is the result of dis(similarity) in the carbon sp3 hybrid orbital; in this sense, studies based on the molecular orbital theory suggest that the hybrid orbital of the halogen is due to one small proportion p and to the s character [80]; see Figure 2.
To obtain the relations proposed in this paper the bond lengths obtained from reference [31] were used (Table 5).
| Compound | Bond length (pm) | Δ electronegativity |
|---|---|---|
 |
134 | 1.5 |
 |
176 | 0.5 |
 |
193 | 0.3 |
We can calculate the relations of bonding described in this study, for (MQSI) of the CH3–Cl and CH3–Br with respect to CH3–F, taking into account that the strong differences in electronegativity determine the molecular polarization effects quantified by the MQSI Coulomb (Table 2).
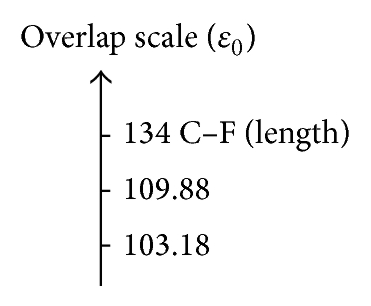
In Table 6 the values associated with the bond relationships proposed in this study are depicted to quantify the inductive effect; with the overlap index, the values associated with the bond relationships are 109.88 pm when comparing the CH3–F with the CH3–Cl and 103.18 pm when compared with the CH3–Br (see Figure 3(a)); these results are consistent with the quantum similarity indexes of overlap (see Table 1). Comparing the values associated with the relationship of bond proposed we can see that the Coulomb descriptor has the same trend (CH3–Cl > CH3–Br).
| CH3–F versus | Overlapa (εO) | Coulombb (εC) | ED-overlapc (εEDO) | ED-coulombd (εEDC) |
|---|---|---|---|---|
| CH3–Cl | 109.88 | 131.32 | 175.54 | 373.86 |
| CH3–Br | 103.18 | 129.98 | 195.64 | 447.56 |
- aBonding relationship of overlap.
- bBonding relationship of coulomb.
- cEuclidean distances of overlap.
- dEuclidean distances of coulomb.
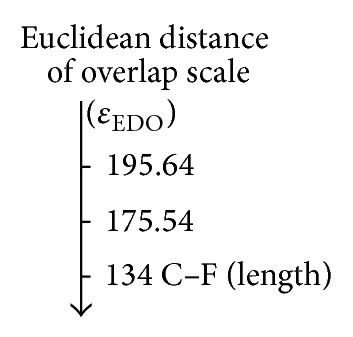
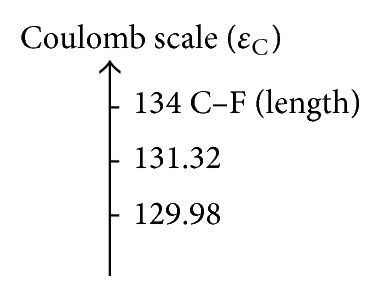
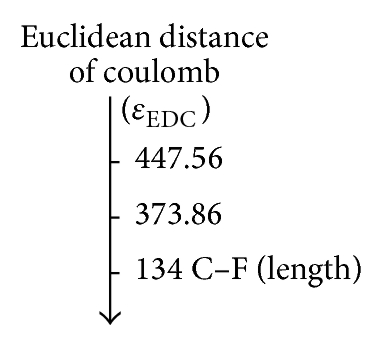
On the other hand, the predicted values for the Euclidean distance ((b) and (d)) are closest to the reference value (134 pm) of CH3–F with respect to CH3–Cl and CH3–Br, respectively, reflecting the strong correlation between the molecular set from the electrostatic point of view, such that trends were sketched by the proposed scales and ae in agreement with the theoretical and experimental studies about the effect of the halogen substituent [81–83].
5.2. Analysis of Global and Local Reactivity Descriptors
Although the C–H bonds of alkyl halides are covalent and have some polar character, because the halogens are more electronegative than the carbon; in order to characterize from the chemical reactivity point of view, the polar character (C–H) is calculated in the global and local reactivity descriptions based in DFT.
In Table 7 we can see that the CH3–Cl is the most stable compound with chemical potential of μ = −4.09 eV and the most reactive compound is the CH3–F with μ = −3.14 eV. While the CH3–Br have a chemical potential intermediate with a value μ = −3.81 eV, the analysis of the chemical potential leads to some general comments. The difference in chemical potential and electronegativity among the alkyl halides is also shown in the greatest opposition to the electronic flow from the electron cloud, and this conclusion is obtained with the value of chemical hardness to CH3–F with η = 5.97 eV and η = 3.60 eV to CH3–Br. On the other hand, such trend is also observed with the values of ionization potential of the CH3–Br with a value of IP = 7.41 eV with the most low energy to remove electrons from the electron cloud and the electron affinity EA = 0.21 eV.
| Compounds | μa (eV) |
ηb (eV) |
IPc (eV) |
EAd (eV) |
ωe (eV) |
|---|---|---|---|---|---|
| CH3–F | −3.14 | 5.97 | 9.10 | −2.83 | 0.83 |
| CH3–Cl | −4.09 | 3.97 | 8.06 | 0.12 | 2.10 |
| CH3–Br | −3.81 | 3.60 | 3.60 | 0.21 | 2.02 |
- μa : chemical potential.
- ηb: chemical hardness.
- IPc: Ionization potential.
- EAd: electron affinity.
- ωe: electrophilicity.
The electrophilic character is calculated with the largest stabilization energy when it is saturated by electrons from the external environment, with a value of ω: 0.83 eV to CH3–F, ω: 2.10 eV to CH3–Cl, and ω: 2.02 eV to CH3–Br. Shows that these global reactivity indexes are appropriate to study the chemical reactivity of inductive effect from a different way than that has been done in the chemical literature (binding energy). Figure 4 shows the trends of the global reactivity descriptors.
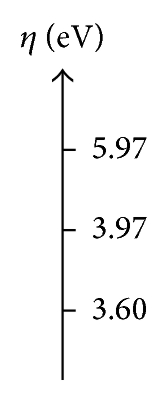
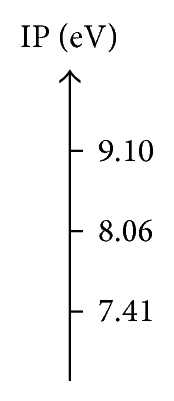
Figure 4 shows the same trend to the hardness (η) and the ionization potential (IP). According to the trends of overlap and Coulomb similarity indexes (see Figure 3), enabling the relationship of the trends in the quantum similarity descriptors of overlap and Coulomb with the chemical hardness (η) and the ionization potential (IP).
On the other hand, the trend in the electron affinity (EA) is in agreement with those found for the Euclidean distances of overlap and Coulomb and can be associated with a high electronic similarity determined by the differences in electronegativity in the molecular set and calculated by the Coulomb operator; see Table 2. In order to analyze the type of interactions present in C–X and C–H bonds the condensed Fukui functions were calculated as it shown in Table 8 to develop the topological model presented in this study.
 |
 |
 |
|||||||||
| Atoms | f+ | f− | ω+ | Atoms | f+ | f− | ω+ | Atoms | f+ | f− | ω+ |
| 1C | 0.38 | 0.12 | 0.32 | 1C | 0.53 | 0.03 | 1.11 | 1C | 0.45 | 0.02 | 0.91 |
| 2H | 0.20 | 0.14 | 0.17 | 2H | 0.01 | 0.00 | 0.02 | 2H | 0.02 | 0.00 | 0.04 |
| 3H | 0.20 | 0.04 | 0.17 | 3H | 0.01 | 0.02 | 0.02 | 3H | 0.02 | 0.02 | 0.04 |
| 4H | 0.20 | 0.04 | 0.17 | 4H | 0.01 | 0.02 | 0.02 | 4H | 0.02 | 0.02 | 0.04 |
| 5F | 0.02 | 0.66 | 0.02 | 5Cl | 0.42 | 0.92 | 0.88 | 5Br | 0.49 | 0.95 | 0.99 |
The values of the Fukui function of CH3–F on carbon atom are f+: 0.38 and f−: 0.66 (see Table 8), respectively. Is evident that the molecular polarization due to the delta of electronegativity (Table 5) together with the value of the Fukui function (f−: 0.66) represents the high susceptibility of attacks by electrophilic species. These values found are in agreement with the calculated for the polar character of the hydrogen atoms due to the fluoride in the C–H bond. To observe this type of interactions in Figure 5 the topological Fukui functions f+: 0.38 and f−: 0.66 are shown for the CH3–F, and also we can see the polar character of the C–H bonds and their contributions to the chemical reactivity due to the inductive effect.
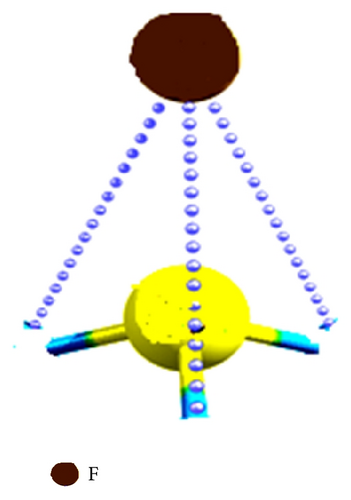

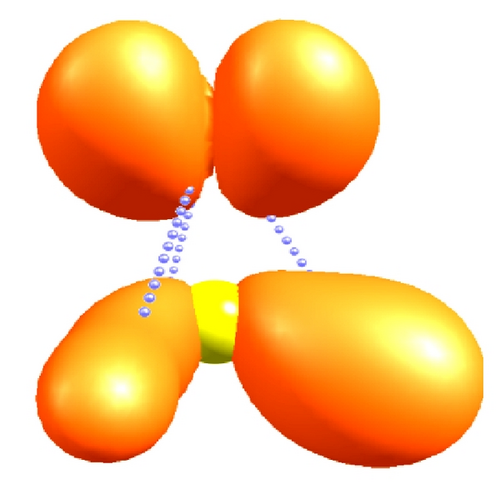
Figure 6 shows the nature of the polar interactions in the C–H bonds of CH3–Cl through the contour maps associated with the Fukui functions f+: 0.53 for the carbon atom and f+: 0.42 for the chloride atom, and these two sites are highly reactive to nucleophilic species, unlike to the CH3–F that only has a single reactive site f+: 0.38.
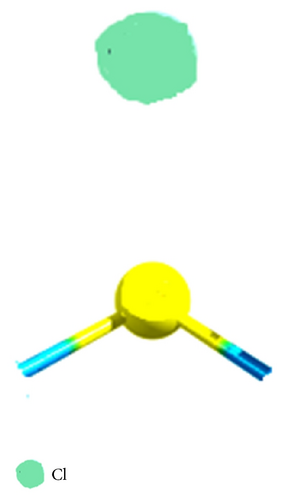
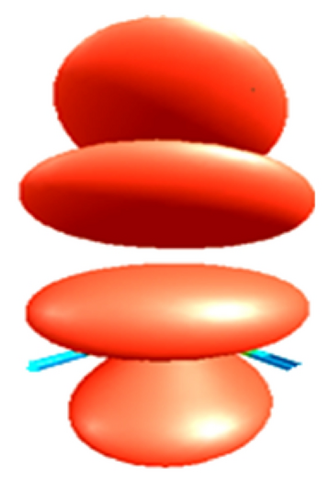
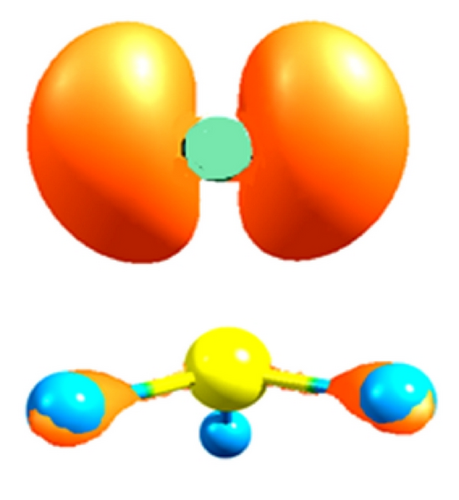
Finally, in Figure 7 the reactive sites f+: 0.45 for carbon and f+: 0.49 and f−: 0.95 for the bromide in the CH3–Br are shown (see Table 8). This value for the Fukui function f+ shows a lower inductive effect with respect to CH3–Cl and to the CH3–F, respectively, according to the electronegativity values.
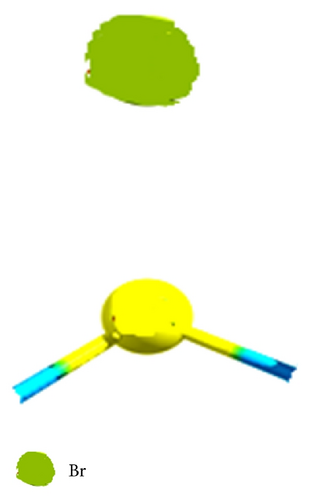
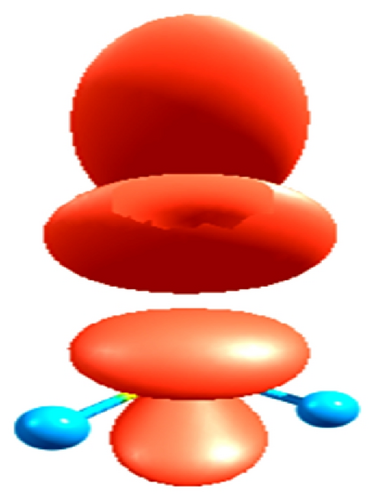
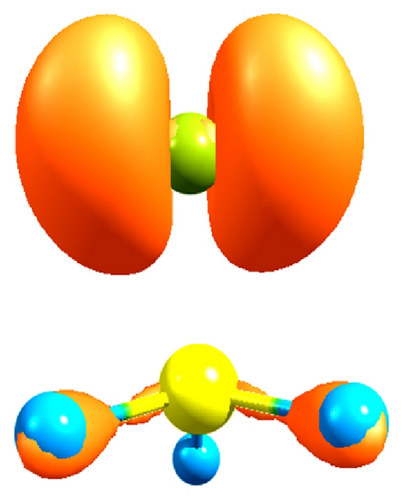
These values are quantified by the electrophilic character [81–83] produced by the inductive effect in the compounds studied, taking into account that when decrease the value of (ω+) increases the stabilization energy of atom most electronegative in the system when it is saturated by electrons from the external medium, and in this since, the CH3–F has the higher inductive effect; therefore, we can see the trend (CH3–F > CH3–Cl > CH3–Br) according to the trends of correlation reported in Figure 3, and these theoretical values are in agreement with experimental results [85–87].
From Figures 5–7, we can identify the polarization of covalent bonds and the prediction of reactivity sites susceptible to attack by electrophilic and nucleophilic species, allowing the analysis of the electronic density distribution in the molecular set (CH3–F, CH3–Cl, CH3–Br). Taking into account that the polarization can induce a dipole in a bond near to the substituent or in one located more away (inductive effect (I)), in this sense the Fukui topological (f−) can interact with the electron release (+I) and the Fukui topological (f+) with the electron-withdrawing (−I). Although this does not imply donation formal of a unit charge, considering that by convection is assumed as electron-withdrawing groups when are most stronger than the hydrogen atom and are designated as negative inductive effects (−I).
Unlike to those groups which have a less capacity of electron withdrawing (high electron release) with respect to hydrogen atom and are designated as positive inductive effect (+I) [88], showing new considerations among the electron flow in the inductive effect and the local chemical reactivity, using the description of molecular bond proposed in this study ((13)–(17)). In this sense we analyze the quantum similarity increasing steric and electronic effects.
5.3. Quantum Dissimilarity Analysis
In order to analyze the quantum dissimilarity increasing the steric and electronic effects for analyze the stability of the relations proposed are analyzed the dissimilarity indexes in a series of isopropane derivatives shown in Figure 8.
With the aim of characterizing the steric and electronic effect in Tables 9 and 10we calculate the overlap quantum dissimilarity.
| Compound | C3H8 | C3H8F | C3H8Cl | C3H8Br | C3H8I |
|---|---|---|---|---|---|
| C3H8 | 1.0000 | ||||
| C3H8F | 0.7427 | 1.0000 | |||
| C3H8Cl | 0.8895 | 0.8138 | 1.0000 | ||
| C3H8Br | 0.9025 | 0.7697 | 0.9887 | 1.0000 | |
| C3H8I | 0.9252 | 0.7318 | 0.9536 | 0.9800 | 1.0000 |
| Compound | C3H8 | C3H8F | C3H8Cl | C3H8Br | C3H8I |
|---|---|---|---|---|---|
| C3H8 | 0.0000 | ||||
| C3H8F | 1.5546 | 0.0000 | |||
| C3H8Cl | 0.7912 | 1.3483 | 0.0000 | ||
| C3H8Br | 0.7275 | 1.4751 | 0.2608 | 0.0000 | |
| C3H8I | 0.6190 | 1.5732 | 0.5214 | 0.3311 | 0.0000 |
In Table 9 we can see the structural effects on the atomic size, the higher overlap quantum dissimilarity values comparing C3H8I with C3H8F (0.7318), and the lowest values comparing C3H8Br with C3H8Cl (0.9887), forming the overlap quantum dissimilarity trend with respect to C3H8 (C3H8F (0.7427) > C3H8Cl (0.8895) > C3H8Br (0.9025) > C3H8I (0.9252)) in agreement with the trends shown in Figure 3.
In Table 10 we found the higher Euclidean distance of overlap between C3H8I and C3H8F (1.5732) and the lowest is between C3H8Br and C3H8Cl (0.2608), in agreement with the values of Table 9. With the aim of analyzing the electronic effects on the quantum dissimilarity the MQS of Coulomb are shown in Table 11.
| Compound | C3H8 | C3H8F | C3H8Cl | C3H8Br | C3H8I |
|---|---|---|---|---|---|
| C3H8 | 1.0000 | ||||
| C3H8F | 0.9468 | 1.0000 | |||
| C3H8Cl | 0.9631 | 0.9801 | 1.0000 | ||
| C3H8Br | 0.9643 | 0.9730 | 0.9990 | 1.0000 | |
| C3H8I | 0.9675 | 0.9640 | 0.9947 | 0.9980 | 1.0000 |
In Table 11 we can see the higher electronic quantum dissimilarity value between C3H8F and C3H8 (0.9468). However, the lowest is between C3H8Br and C3H8Cl (0.9990). Also the electronic quantum dissimilarity trend with respect to C3H8 C3H8F (0.9468) > C3H8Cl (0.9631) > C3H8Br (0.9643) > C3H8I (0.9675) is in agreement with the outcomes of Table 9 and Figure 3. This result is consistent with the Euclidean distances of Coulomb (Table 12).
| Compounds | C3H8 | C3H8F | C3H8Cl | C3H8Br | C3H8I |
|---|---|---|---|---|---|
| C3H8 | 0.0000 | ||||
| C3H8F | 5.4638 | 0.0000 | |||
| C3H8Cl | 4.3025 | 2.9845 | 0.0000 | ||
| C3H8Br | 4.1482 | 3.4601 | 0.6427 | 0.0000 | |
| C3H8I | 3.8690 | 3.9867 | 1.4585 | 0.8895 | 0.0000 |
In Table 12 the higher Euclidean distance of Coulomb is between C3H8F and C3H8 (5.4638), and the lowest is between C3H8Br and C3H8Cl (0.6427), in agreement with the results of Table 11. This result shows the electronegativity character and the atomic size on the steric and electronic effects related to the inductive effects and extrapolating the result of Figure 3.
6. Conclusions
We present a topological analysis on the inductive effect in CH3–F, CH3–Cl, and CH3–Br alkyl halides taken as an example; using local molecular quantum similarity and chemical reactivity in DFT context, this quantification was performed through scales of quantitative convergence using descriptors based in MQSI of overlap and Coulomb (see Figure 3), presenting a good alternative relationship and the chemical bonds, and it also explains the polar character associated with the C–H bonds from the point of view of the global and local chemical reactivity, in a different way than that has been done in the chemical literature (dipole moments and energy binding); in addition these results are consistent with the effects produced by halogen substituents according to experimental results.
The alignment method used is the Topo-Geometrical Superposition Algorithm (TGSA), allowing calculating appropriate measures of the asymmetry in the central carbon atom of the alkyl halides. This TGSA method is a straightforward procedure to cope with the problem of relative orientation of the molecules to evaluate MQS; also this work shows the Hirschfield partitioning of the electronic density in the proposed model of covalent bond (see (13)–(17)) can describe and relate the inductive effect in the molecular set (CH3–F, CH3–Cl, and CH3–Br).
The reactivity global indexes can characterize the reactivity of the compounds tested, the higher value of the chemical potential (μ: −3.14 eV) to the CH3–F, with the greater hardness (η: 5.97 eV) and the most stable the CH3–Cl with a chemical potential (μ: −4.09 eV) and (η: 3.97 eV) (see Table 7). The characterization of the reactivity sites calculated by the condensed Fukui functions (see Table 8) helped identify areas susceptible to attack by electrophilic and nucleophilic species, associating topological Fukui functions (f−) with the electron-release (+I) and the topological Fukui (f+) with the electron-withdrawing (−I).
Moreover, through this model we can be demonstrated the origin of the polar interactions of the C–H bond (see Figures 5–7) that can be applied to a much wider range of compounds such as CH3–NH2, CH3–(CH2)n–NH2, CH3–OH, CH3–(CH2)n–OH, CH3–SH, and CH3–(CH2)n–SH, with (n) large, due to the nature of the TGSA alignment method and in general systems where the inductive effect is marked by the high delta in electronegativity and polarizability, considering that the characterization of inductive effect is an area of growing interest in organic physical chemistry.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Alejandro Morales-Bayuelo thanks the Universidad Nacional Andres Bello (Santiago-Chile) for a Ph.D. fellowship (CONICYT (63100003)). Ricardo Vivas-Reyes is indebted to the Universidad de Cartagena (Cartagena, Colombia), for continuous support to the research group.




