A Promising Orange-Red Nanocrystalline Potassium Lanthanum Orthophosphate for White Light-Emitting Diodes
Abstract
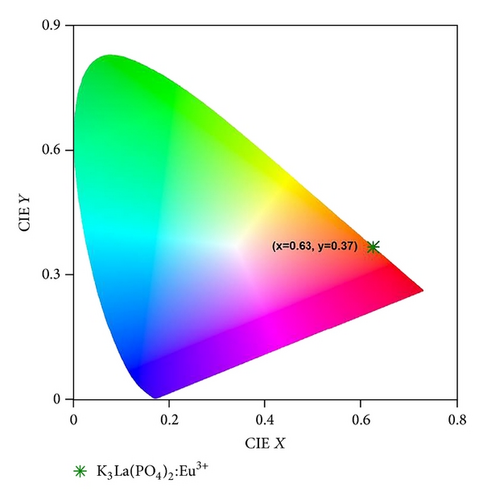
The spectral properties of the K3La(PO4)2:Eu3+ nanophosphors synthesized by the combustion method are reported. The phosphors were characterized by X-ray diffraction, transmission electron microscopy, and optical spectroscopy. The XRD pattern reveals a pure monoclinic phase of the K3La(PO4)2 with the average particle size of 30 nm in diameter. Under UV excitation, the phosphors exhibited several emission bands which were assigned to 4f-4f transitions of Eu3+ ions. The red shade of the Eu3+ ion with the CIE coordinates (x, y) as (0.63, 0.37) suggests that this material is a promising phosphor for near-UV InGaN-based LED lighting.
1. Introduction
In recent years, white light-emitting diodes (LEDs) have attracted more attention as possible replacement of the incandescent and fluorescent light sources due to their extraordinary characteristics such as high luminous efficiency, low cost synthesis, energy saving, long lifetime, reliability, mercury free, and environmental friendliness [1–4]. The rare earth doped phosphates are an important family of luminescent materials for solid state lighting applications as they possess excellent properties such as large band gap, moderate phonon energy, high thermal and chemical stability, exceptional optical damage threshold, and high absorption of in the VUV region [5–7]. Among the phosphates families, orthophosphate is considered to be an excellent host material to accommodate large amount of dopants due to its large band gap. Very often, orthophosphate crystallizes in the monoclinic phase with space group Pc/m or Pc/n [8].
The electronic transitions in the inner 4f orbitals, which are not influenced by the 5s and 5p electrons, are only found in the lanthanide. This leads to narrow and intense emission bands which are useful sources of the individual colors in multiphosphor devices. Therefore, in many commercial phosphors the blue (450 nm), green (545 nm), and red (610 nm) emissions of Eu2+, Tb3+, and Eu3+ have been widely used [9, 10]. Currently, there are several techniques used to synthesize these materials, like colloidal [11, 12], sonochemical [13], spray pyrolysis [14], and high temperature solid-state reactions [15, 16].
In the present paper, we report on the structure and photoluminescence (PL) properties of Eu3+ activated K3La(PO4)2 nanophosphors synthesized by the combustion technique.
2. Experimental
3. Results and Discussion
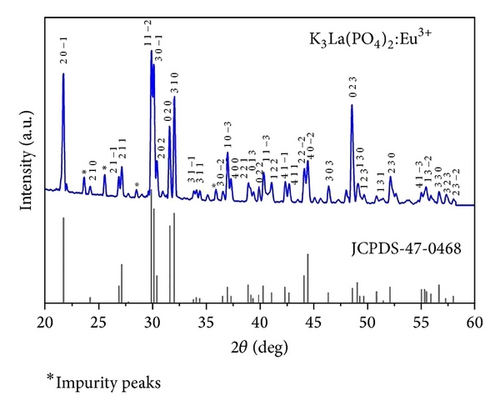
Figure 1 shows the X-ray diffraction (XRD) pattern of the annealed K3La(PO4)2:Eu3+ nanophosphor. The characteristic reflections confirm the monoclinic phase of the K3La(PO4)2 orthophosphates. The crystal structure of the host matrix was not affected by the addition of small amount of Eu3+ dopant ions. Since the ionic radii of Eu3+ () and La3+ () are approximately the same, the Eu3+ ions can therefore easily incorporate in La3+ sites in host lattice. The XRD pattern was compared with those of the standard JCPDS data (47-0468) and the peaks were indexed accordingly. The diffraction peaks at 23.7°, 28.5°, and 25.5° (in terms of 2θ) indicate that some impurity phases were present in the host. The average crystallite size calculated by the well-known Debye-Scherrer equation [17] was 30 nm.
The morphology of the K3La(PO4)2:Eu3+ nanophosphors was analyzed using TEM as shown in Figure 2. From the micrograph, the particles were mostly spheroidal and the average particle size was estimated to be in the range of ~28 ± 2 nm. This result is consistent with the XRD data. It is well known that the morphological characteristics of the combustion derived products are strongly dependent on the heat and gas generated during the reaction. Large amounts of gases generated during the combustion process are suitable for preparation of fine particles, while the heat released is an important factor for crystal growth. This type of porous network is typically the characteristic of combustion synthesized powders. The porous powders are highly friable which facilitates easy grinding to obtain finer particles. The evolution of gas with high pressure leads to the formation of pores along with the simultaneous growth of small particles near the pores [16].
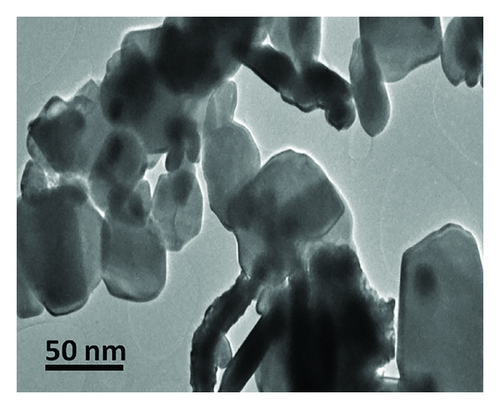
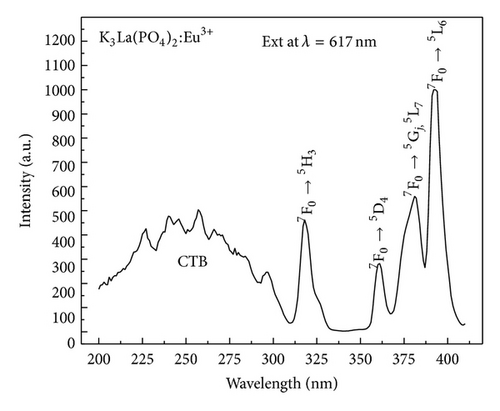
Figure 3 shows the photoluminescence excitation spectrum of the K3La(PO4)2:Eu3+ phosphor. The spectrum has a broad band ranging from 200 to 310 nm with a set of sharp lines protruding from the band. The broad band is attributed to charge transfer (CTB) from O2− → Eu3+, while the sharp lines at wavelengths of 318, 360, 382, and 392 nm are assigned to (7F0→ 5H3, 5D4, 5Gj, 5L7, 5L6) intraconfigurational 4f-4f transitions of Eu3+ ion.
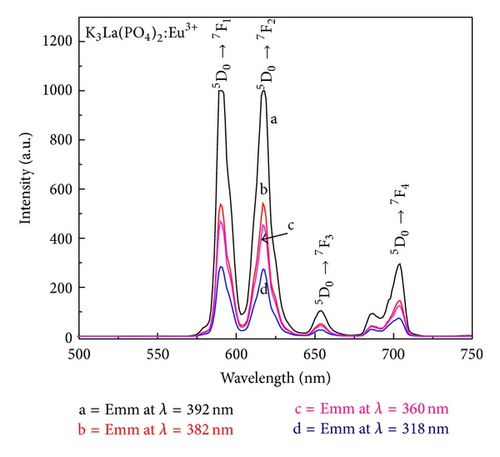
The PL emission spectra at different excitation wavelengths are presented in Figure 4 and it is clear that the peak positions and shapes of the spectra did not change for all the excitation wavelengths. The emission spectrum consists of four typical emission peaks at 591, 617, 653, and 703 nm, which results from the intraconfigurational 4f-4f characteristic transitions (5D0 → 7Fj, j = 1, 2, 3, 4) of the Eu3+ ion, respectively. The emission line at 591 nm is due to magnetic dipole transition 5D0→7F1 which is insensitive to the site symmetry, whereas the emission at 617 nm is due to the electric dipole transition of 5D0 → 7F2 and it is induced by the lack of inversion symmetry at the Eu3+ site. Generally, the forced electric dipole transitions arise from the lack of centre of symmetry and it is much stronger than that of the 5D0 → 7F1 transition. Also, the 5D0 → 7F2 transition is dependent upon the local symmetry, while the 5D0 → 7F1 emission is related to the local symmetry due to insensitivity to the site symmetry. Therefore, in the present case, the Eu3+ ion occupies a symmetric and a nonsymmetric site almost equally, as both the peak intensities of Eu3+ ion due to 5D0 → 7F2 and 5D0 → 7F1 transitions are the same [18, 19]. The CIE 1931 color space chromaticity coordinates were calculated and are presented in the CIE diagram in Figure 5. The diagram was constructed using the Commission International de L’Eclairage (CIE) coordinate calculator. The calculated CIE coordinates of our materials were (0.63, 0.37) which correspond to the shade of orange-red emission of the Eu3+ ion. Bright orange-red light-emitting diodes may be fabricated by the combination of the synthesized K3La(PO4)2:Eu3+ with 392 nm emitting InGaN-based chips.
4. Conclusions
K3La(PO4)2:Eu3+ nanophosphors were synthesized by the combustion method and its luminescence properties were investigated under UV excitation. The emission spectrum consists of two prominent peaks at 591 and 617 nm which corresponds to magnetic-dipole transitions (5D0 → 7F1) and electric-dipole transitions (5D0 → 7F2) of the Eu3+ ion. The CIE chromaticity coordinates were calculated to be (x = 0.63, y = 0.37) which corresponds to the orange-red shade. The maximum emission from 392 nm excitation makes this material a potential candidate for the near-UV-light-emitting diode applications.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
A. K. Bedyal would like to thank Inter University Accelerator Centre, New Delhi, for the financial support in the form of fellowship under UFR Project code—MS/30505. Vinay Kumar is grateful to the Department of Science and Technology (DST), Government of India, for funding the Fast Track Project reference no. SR/FTP/PS-043/2011 under DST Young Scientist Scheme.




