Sensitization of Perovskite Strontium Stannate SrSnO3 towards Visible-Light Absorption by Doping
Abstract
Perovskite strontium stannate SrSnO3 is a promising photocatalyst. However, its band gap is too large for efficient solar energy conversion. In order to sensitize SrSnO3 toward visible-light activities, the effects of doping with various selected cations and anions are investigated by using hybrid density functional calculations. Results show that doping can result in dopant level to conduction band transitions which lie lower in energy compared to the original band gap transition. Therefore, it is expected that doping SrSnO3 can induce visible-light absorption.
1. Introduction
Photocatalysis is an important technology for fuel production and environmental remediation from solar energy conversion [1–4]. Perovskite strontium stannate SrSnO3 has been reported to be a promising photocatalyst [5–7]. Due to its suitable valence band and conduction band edge positions, it is able to split water into hydrogen and oxygen. The main drawback of SrSnO3 is the large band gap of 4.1 eV [7] and therefore it can only absorb light in the ultraviolet region. A material for efficient solar energy conversion should be able to absorb the majority of the sunlight, namely, visible light. In order to sensitize wide gap materials to visible light, doping has been a common approach [8–10]. In this study, hybrid density functional theory calculations are carried out to investigate the effect of doping in SrSnO3. Cations Cr3+, Fe3+, and Rh3+ and anions N3−, N2−, and S2−, which have been reported to be effective dopants for sensitizing titanates to visible light, are considered [10, 11].
2. Computational Methods
All calculations are carried out based on density functional theory [12] with the range separated HSE06 hybrid functional [13]. Valence electrons are described by a plane wave basis set with the energy cutoff of 500 eV. The interactions between core and valence electrons are treated with the projector augmented wave (PAW) method [14]. The k-space is sampled with k-point spacing less than 0.03 Å−1. The lattice vectors and atomic positions in all cells are optimized until the force is converged to less than 0.03 eV-Å−1 at each atomic position. Vienna ab initio simulation package (VASP) [15] is used for all calculations.
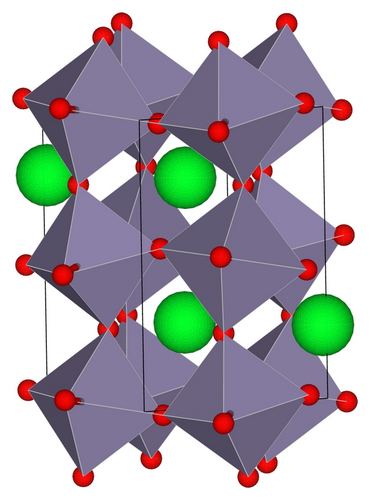
SrSnO3 adopts the orthorhombic perovskite structure with space group symmetry Pbnm [16], as shown in Figure 1. To model doped SrSnO3, a 2 × 1 × 2 SrSnO3 supercell, which contains 16 formula units (80 atoms), is constructed. Cation doping is simulated by substituting a dopant, Cr3+, Fe3+, or Rh3+, for one Sn4+, which corresponds to 6.25% of B-site cation impurity. To ensure the correct trivalent charge states of cation dopants, in each of the above cases La3+ is simultaneously substituted for Sr2+. Anion doping is modeled by substituting a dopant, N3−, N2−, or S2−, for one O2−, which corresponds to 2.08% of anion impurity. In the N3− case La3+ is simultaneously substituted for Sr2+.
3. Results and Discussions
The calculated lattice parameters for the orthorhombic perovskite SrSnO3 are a = 5.717 Å, b = 5.729 Å, and c = 8.084 Å, which are in good agreement with experimental values a = 5.709 Å, b = 5.703 Å, and c = 8.065 Å [17]. Figure 2 shows the density of states and band structure from our calculations. The calculated band gap of pristine SrSnO3 is 3.52 eV, smaller than experimentally reported optical gap 4.1 eV. The origin of this 0.5 eV discrepancy is not clear at the moment. It could be an underestimation from the functional used in calculations. It could be that the transition from the highest occupied state to the lowest occupied state is forbidden [18]. More detailed analysis is necessary to clarify this. The bottom of the conduction band is a mixture of Sn 5s states and O 2p states. Different from d state conduction bands, it is very dispersive and therefore electrons in this band should possess lower effective mass than in d bands. The top of valence band is comprised mainly of oxygen 2p states.
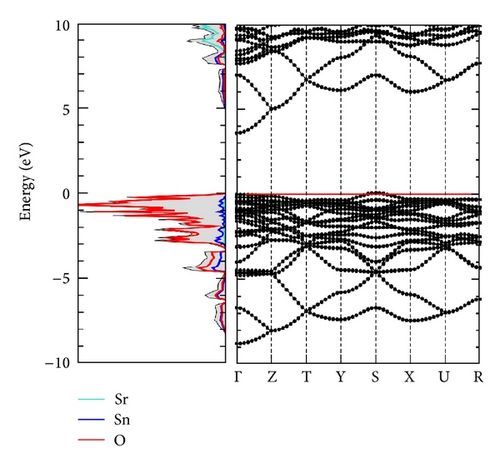
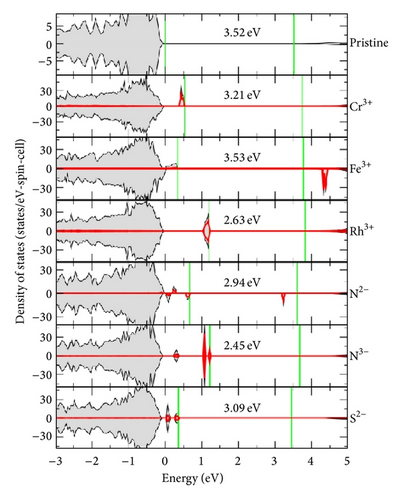
Figure 3 shows the calculated density of states of cation and anion doped SrSnO3. It is assumed that dopants do not influence the position of the SrSnO3 O 2p valence band and the top of O 2p valence band in each cell is aligned at 0 eV. In each case, dopant produces occupied states above the SrSnO3 valence band maximum (VBM). This indicates the emergence of dopant to conduction band minimum (CBM) transitions. However, in the cases of Cr3+, Fe3+, and Rh3+ doping, the position of CBM is also shifted up by about 0.3 eV compared to pristine SrSnO3. This is probably due to the s state interaction between Sn4+ ions, which form the lowest part of conduction band, being disturbed by the presence of cation impurity. Overall, the Cr3+ → CBM and Rh3+ → CBM transitions are 3.21 eV and 2.63 eV, respectively. The position of Fe3+ states above VBM is relatively shallow and therefore the Fe3+ → CBM transition 3.53 eV is even higher in energy than the pristine SrSnO3 band gap. In anion doping cases, the position of CBM is less affected. In the S2− doping case, the CBM is actually downshifted by about 0.1 eV. Overall, the transitions from N2−, N3−, and S2− to CBM are 2.94 eV, 2.45 eV, and 3.09 eV, respectively.
4. Conclusions
Through hybrid density functional calculations, it is shown that the doping of various cations and anions introduces occupied dopant states above the SrSnO3 O 2p valence band. Transitions from these dopant states to the SrSnO3 conduction band cost lower energies than the original band gap transition, apart from the Fe3+ case, and are hoped to contribute more towards visible-light photocatalytic activities. Among all considered cation dopants, Rh3+ gives rise to the deepest in gap states. The transition from Rh3+ to SrSnO3 conduction band is 0.89 eV lower than the band gap. Among all considered anion dopants, N3− appears to show the best result. The transition from N3− to SrSnO3 conduction band is 1.07 eV lower than the SrSnO3 band gap. Nevertheless, one should be cautioned that whether or not dopant associated localized states can really contribute to steady-state activities is a question [8, 19].
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work is partly supported by the Japan Science and Technology Agency (JST) Precursory Research for Embryonic Science and Technology (PRESTO) program and by the World Premier International Research Center Initiative on Materials Nanoarchitectonics (MANA), MEXT.




