Synthesis of Novel Symmetrical and Unsymmetrical o-Phthalic Acid Diamides
Abstract
Phthalic anhydride was treated with secondary amines in acetic acid yielding 2-(diethyl (or) 4-alkylpiperazine or morpholine-1-carbonyl) benzoic acids. The latter were reacted, again, with secondary amines and arylamines by using the coupling reagent HATU and Et3N as a base in DMF giving the novel symmetrical o-phthalic acid diamides [o-R1R1NCOC6H4CONR1R1], unsymmetrical o-phthalic acid diamides [o-R1R1NCOC6H4CONR1R2], and primary amidic-secondary amidic containing unsymmetrical o-phthalic acid diamides [o-R1R1NCOC6H4CONHAr], respectively.
1. Introduction
Phthalic anhydride is used in the manufacture of dialkylphthalates [1, 2] which find application as plasticisers for polymers like polyvinyl chloride (PVC) and polyvinylacetate (PVA). It is used in the manufacture of phenolphthalein indicator [3, 4], anthraquinone [5] (a versatile, raw materials in the dye industry [6]), and metal phthalocyanines [7]. Phthalocyanine compounds are used in a variety of applications [7] in addition to their use as pigments, in paints [8] and in many types of dyestuffs [7]. Phthalic anhydride derivatives have been widely reported to possess beneficial pharmaceutical effects, like analgesic [9], anti-inflammatory [10] and antiviral effects [11].
Dunlap and Cummer reported [12] the preparation of symmetrical o-phthalic acid diamides [o-ArNHCOC6H4CONHAr] by the reaction of phthaloyl dichloride with two moles of aniline in ether at RT. Dann et al. reported [13] the preparation of symmetrical o-phthalic acid diamides by the reaction of phthaloyl dichloride with two moles of aniline in the presence of sodium fluoride in benzene under reflux for 1 h. de Toranzo and Brieux reported [14] the synthesis of unsymmetrical diamides [ArNHCOC6H4CONHAr1] by the reaction of phthalphenylisoimide with anilines in ether at RT. Reynolds reported [15] that unsymmetrical diamides [ArNHCOC6H4CONHAr1] can be made by the reaction of N-arylphthalamic acid with the sodium salt of o- or p-methylaniline in an atmosphere of nitrogen for 1 h at 75°C. However, these methods suffer from drawbacks such as long reaction times, excess use of organic solvents, harsh refluxing conditions, and preparation of difficult starting materials from phthalic anhydride by the reaction with primary amines in the presence of trifluoroacetic anhydride. Keeping these facts in mind, we wish to report our results on reactions of phthalic anhydride with primary and secondary amines using HATU as a coupling reagent. Probably, this appears to be the first ever case of facile preparation of symmetrical and unsymmetrical o-phthalic acid diamides. The use of HATU as a coupling agent has been reported in the literature for reactions such as amide bond formation in solid phase synthesis [16–18] and peptides synthesis [19]. However, its use in the preparation of diamides from phthalic anhydride with amines has probably not been reported so far.
2. Results and Discussion
Phthalic anhydride 1 was treated with the secondary amines 2a–2e in acetic acid at RT for 10–15 min resulting in the formation of 2-(diethyl (or) 4-alkylpiperazine (or) morpholine-1-carbonyl)benzoic acid 3a–3e. Reaction of 3a with the piperazine 2b in the presence of o-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluroniumhexafluorophosphate (HATU) and Et3N at 0–5°C for 40–45 min in DMF gave 5a. Alternatively, this compound was prepared by treating 2-(piperazine-1-carbonyl)benzoic acid 3b withdiethylamine 2a by HATU and Et3N at 0–5°C for 40–45 min in DMF to form N,N-diethyl-2-(piperazine-1-carbonyl)benzamide 5a. This reaction was examined by carrying out the condensation of 2-(diethylcarbonyl)benzoic acid 3a (1 mmol) with piperazine 2b (1 mmol) in the presence different coupling reagents (HATU, EDC.HCl/HOBt (1-hydroxybenzotriazole), DCC (N,N′-dicyclohexylcarbodiimide), HBTU and PTSA (4-methylbenzenesulfonic acid)) and tertiary bases (Et3N and DBU (2, 3, 4, 6, 7, 8, 9, 10-octahydropyrimido [1, 2-a] azepine) at different temperatures in DMF as a solvent (Table 1) with a view to study the generalisation of condensation between 3 and 2. However, coupling of 3a with 2b in the presence of HATU and Et3N at 0–5°C for 40–45 min in DMF was found to be the best method giving 5a in quality and yield (≥80%) (Table 1, entry 1).
| Entry | Coupling reagent | Tertiary base | Temp./°C | Time/min | 5a (%) |
|---|---|---|---|---|---|
| 1 | HATU | Et3N | 0–5 | 40–45 | 80 |
| 2 | HATU | Et3N | RT | 40–45 | 78 |
| 3 | HATU | DBU | 0–5 | 55–65 | 70 |
| 4 | HATU | DBU | RT | 50–55 | 70 |
| 5 | HATU | Et3N | 50–60 | 35–40 | 73 |
| 6 | DCC | — | 0–5 | 120–130 | 45 |
| 7 | DCC | — | RT | 100–110 | 48 |
| 8 | DCC/HOBt | — | 0–5 | 110–120 | 50 |
| 9 | DCC/HOBt | — | RT | 100–110 | 50 |
| 10 | DCC/HOBt | — | 50–60 | 55–60 | 72 |
| 11 | EDC·HCl/HOBt | Et3N | 0–5 | 80–85 | 70 |
| 12 | EDC·HCl/HOBt | Et3N | RT | 70–80 | 70 |
| 13 | EDC·HCl/HOBt | DBU | 0–5 | 70–80 | 60 |
| 14 | EDC·HCl/HOBt | DBU | RT | 70–75 | 65 |
| 15 | EDC·HCl/HOBt | Et3N | 50–60 | 65–70 | 72 |
| 16 | HBTU | Et3N | 0–5 | 70–75 | 70 |
| 17 | HBTU | Et3N | RT | 65–70 | 70 |
| 18 | HBTU | DBU | 0–5 | 70–80 | 65 |
| 19 | HBTU | DBU | RT | 65–75 | 65 |
| 20 | HBTU | Et3N | 50–60 | 50–55 | 70 |
| 21 | PTSA | — | 0–5 | 120–125 | — |
| 22 | PTSA | — | RT | 120–125 | — |
| 23 | PPA | — | 100 | 60–65 | 40 |
Using the above-stated optimised conditions, 3a–3e were condensed into 2a–2e using HATU and Et3N at 0–5°C for 40–65 min in DMF yielding 4a–4e and 5a–5j (Scheme 1) (Tables 2 and 3) in excellent yield. The structures of the products have been established on the basis of their spectral and analytical data. (Please see experimental section)
| Entry | Starting material used | Product obtained | Time (min) | Yield≠ | M.P (°C) |
|---|---|---|---|---|---|
| 1 | 2a | 3a | 10–12 | 85 | 145–148 |
| 2 | 2b | 3b | 12–15 | 85 | >220 |
| 3 | 2c | 3c | 15–18 | 83 | >220 |
| 4 | 2d | 3d | 15–18 | 80 | >220 |
| 5 | 2e | 3e | 15–20 | 81 | >220 |
- ≠Refers to yields of crude products only.
| Entry | Starting material used | Product obtained | Time (min) | Yield≠ | M.P (°C) | |
|---|---|---|---|---|---|---|
| 1 | 3a | 2a | 4a | 40–45 | 85 | >220 |
| 2 | 3b | 2b | 4b | 40–45 | 85 | >220 |
| 3 | 3c | 2c | 4c | 50–55 | 80 | >220 |
| 4 | 3d | 2d | 4d | 50–55 | 80 | >220 |
| 5 | 3e | 2e | 4e | 40–45 | 80 | >220 |
| 6 | 3a | 2b | 5a | 40–45 | 85 | >220 |
| 7 | 3a | 2c | 5b | 50–55 | 81 | 110–112 |
| 8 | 3a | 2d | 5c | 50–55 | 84 | 115–118 |
| 9 | 3a | 2e | 5d | 50–55 | 85 | 80–82 |
| 10 | 3b | 2a | 5a | 40–45 | 83 | >220 |
| 11 | 3b | 2c | 5e | 50–55 | 84 | >220 |
| 12 | 3b | 2d | 5f | 50–55 | 85 | >220 |
| 13 | 3b | 2e | 5g | 60–65 | 85 | 85–87 |
| 14 | 3c | 2a | 5b | 60–65 | 81 | 110–112 |
| 15 | 3c | 2b | 5e | 50–55 | 83 | >220 |
| 16 | 3c | 2d | 5h | 50–55 | 85 | >220 |
| 17 | 3c | 2e | 5i | 50–55 | 85 | 90–92 |
| 18 | 3d | 2a | 5c | 60–65 | 80 | 115–118 |
| 19 | 3d | 2b | 5f | 50–55 | 80 | >220 |
| 20 | 3d | 2c | 5h | 40–45 | 85 | >220 |
| 21 | 3d | 2e | 5j | 40–45 | 83 | >220 |
| 22 | 3e | 2a | 5d | 40–45 | 80 | 80–82 |
| 23 | 3e | 2b | 5g | 50–55 | 84 | 85–87 |
| 24 | 3e | 2c | 5i | 50–55 | 84 | 90–92 |
| 25 | 3e | 2d | 5j | 40–45 | 83 | >220 |
- ≠Refers to yields of crude products only.
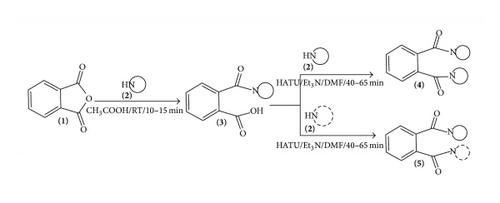
Condensation of 3a–3e with arylamines 6a–6e in the presence of HATU and Et3N at 0–5°C for 40–55 min in DMF gave primary amidic-secondary amidic containing unsymmetrical o-phthalic acid diamides [o-R1R1NCOC6H4CONHAr] 7a–7y (Scheme 2) (Table 4). The structures of the products have been established on the basis of their spectral and analytical data. An alternate protocol was attemptedto synthesize 7a–7y by treatment of 1 with aniline 6a in acetic acid at 0–5°C for 10–15 min which gave 2-(phenylcarbamoyl)benzoic acid 8a18 and subsequent reaction of 8a with diethylamine 2a in the presence of HATU and Et3N at RT for 20–25 min in DMF which resulted in the formation of 2-phenylisoindoline-1, 3-dione 9a18.
| Entry | Starting material used | Product obtained | Time (min) | Yield≠ | M.P (°C) | |
|---|---|---|---|---|---|---|
| 1 | 3a | 6a | 7a | 40–45 | 85 | 115–118 |
| 2 | 3b | 6a | 7b | 50–55 | 82 | 120–123 |
| 3 | 3c | 6a | 7c | 40–45 | 84 | 123–125 |
| 4 | 3d | 6a | 7d | 50–55 | 85 | >220 |
| 5 | 3e | 6a | 7e | 50–55 | 80 | 126–128 |
| 6 | 3a | 6b | 7f | 40–45 | 83 | 128–130 |
| 7 | 3b | 6b | 7g | 40–45 | 82 | 138–140 |
| 8 | 3c | 6b | 7h | 50–55 | 82 | 120–123 |
| 9 | 3d | 6b | 7i | 50–55 | 85 | >220 |
| 10 | 3e | 6b | 7j | 40–45 | 80 | 122–124 |
| 11 | 3a | 6c | 7k | 50–55 | 82 | 120–124 |
| 12 | 3b | 6c | 7l | 40–45 | 85 | 130–132 |
| 13 | 3c | 6c | 7m | 50–55 | 85 | 170–174 |
| 14 | 3d | 6c | 7n | 50–55 | 80 | >220 |
| 15 | 3e | 6c | 7o | 40–45 | 80 | 140–143 |
| 16 | 3a | 6d | 7p | 40–45 | 80 | 125–128 |
| 17 | 3b | 6d | 7q | 50–55 | 82 | 135–137 |
| 18 | 3c | 6d | 7r | 50–55 | 82 | 180–183 |
| 19 | 3d | 6d | 7s | 40–45 | 80 | >220 |
| 20 | 3e | 6d | 7t | 40–45 | 85 | 142–145 |
| 21 | 3a | 6e | 7u | 40–45 | 84 | 128–130 |
| 22 | 3b | 6e | 7v | 40–45 | 85 | 133–135 |
| 23 | 3c | 6e | 7w | 50–55 | 80 | 190–192 |
| 24 | 3d | 6e | 7x | 50–55 | 82 | >220 |
| 25 | 3e | 6e | 7y | 40–45 | 84 | 145–148 |
- ≠Refers to yields of crude products only.
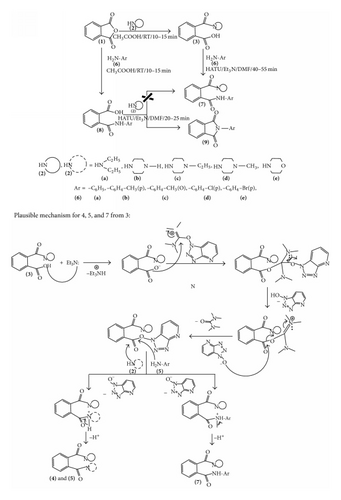
3. Conclusion
In conclusion, we have developed novel syntheses of symmetrical 4a–4e and unsymmetrical 5a–5j and 7a–7y o-phthalic acid diamides. This approach presents a simple and useful synthetic process which requires a few minutes of reaction time, easily available starting materials and straight forward and easy workup procedure. Probably, this appears to be the first ever case of facile preparation of symmetrical and unsymmetrical o-phthalic acid diamides. The use of HATU as a coupling agent has been reported in the literature for reactions such as amide bond formation in solid phase synthesis and peptides synthesis. However, its use in the preparation of diamides from phthalic anhydride with amines has probably not been reported so far. The overall yields of these compounds are very good.
4. Materials and Methods
Melting points are uncorrected and were determined in open capillary tubes in sulphuric acid bath, TLC was run on silica gel-G, visualization was done using iodine or UV light, and IR spectra were recorded using PerkinElmer 1000 instrument in KBr pellets. 1HNMR spectra were recorded in DMSO-d6 using TMS as internal standard using 400 MHz spectrometer. Mass spectra were recorded on Agilent-LCMS instrument under CI conditions and given by Q + 1 values only. Starting 1, 2, and 6 were obtained from commercial sources and used as such.
4.1. Preparation of 3a–3e
A mixture of 1a (10 mM), 2a–2e (10 Mm) and CH3COOH (20 mL) was stirred at RT for 10–20 min. A colourless solid separated out from reaction mixture which was filtered, washed with hexane (10 mL), and dried. The crude product was recrystallized from suitable solvent to obtain 3a–3e.
4.2. Preparation of 4a–4e & 5a–5j
A mixture of 3a–3e (10 mM), 2a–2e (10 mM) HATU (10 mM), Et3N (10 mM), and DMF (15 mL) was stirred at 0–5°C for 40–65 min. Then, ice-cold water (50 mL) was added to the reaction mixture. The separated solid was filtered, washed with water (10 mL), and dried. The product was recrystallized from a suitable solvent to obtain 4a–4e and 5a–5j.
4.3. Preparation of 7a–7y
A mixture of 3a–3e (10 mM), 6a–6e (10 mM), HATU (10 mM), Et3N (10 mM), and DMF (15 mL) was stirred at 0–5°C for 40–55 min. Then, ice-cold water (50 mL) was added to the reaction mixture. The separated solid was filtered, washed with water (10 mL), and dried. The product was recrystallized from a suitable solvent to obtain 7a–7y.
5. Supporting Information
3a: IR (KBr): 3100–3400 cm−1 (broad medium, –NH and –OH groups put together), 1720 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, storng, –CO– of amide group); 1H-NMR: δ 0.8 (t, 3H, –CH3), δ 1.2 (t, 3H, –CH3), δ 3.0 (q, 2H, –CH2), δ 3.6 (q, 2H, –CH2), 7.0–8.0 (m, 4H, Ar-H), 13.00 (s, 1H, –COOH, D2O exchangeable), 10.12 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.3, 41.4, 123.1, 128.5, 129.3, 129.5, 160.2, 165.5. Ms: m/z = 222 (M+. + 1).
3b: IR (KBr): 3100–3400 cm−1 (broad medium, –NH and –OH groups put together), 1725 cm−1 (sharp, strong, –CO– of acid group), 1670 cm−1 (sharp, storng, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 4H, Ar-H), 13.00 (s, 1H, –COOH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 49.4, 50.1, 51.4, 51.9, 124.1, 125.5, 127.3, 128.5, 161.2, 164.8. Ms: m/z = 235 (M+. + 1).
3c: IR (KBr): 3100–3400 cm−1 (broad medium, –NH and –OH groups put together), 1730 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, storng, –CO– of amide group); 1H-NMR: δ 1.2 (t, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 4H, Ar-H), 13.2 (s, 1H, –COOH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.5, 49.4, 50.1, 50.9, 51.9, 52.2, 123.1, 124.5, 125.3, 127.5, 163.2, 164.8. Ms: m/z = 263 (M+. + 1).
3d: IR (KBr): 3100–3400 cm−1 (broad medium, –NH and –OH groups put together), 1720 cm−1 (sharp, strong, –CO– of acid group), 1660 cm−1 (sharp, storng, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 4H, Ar-H), 13.1 (s, 1H, –COOH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 39.4, 55.1, 55.9, 55.9, 55.2, 126.1, 127.5, 128.3, 129.5, 160.2, 165.8. Ms: m/z = 249 (M+. + 1).
3e: IR (KBr): 3100–3400 cm−1 (broad medium, –NH and –OH groups put together), 1720 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, storng, –CO– of amide group); 1H-NMR: δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 4H, Ar-H), 13.1 (s, 1H, –COOH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 50.2, 50.6, 71.9, 72.2, 125.1, 125.5, 126.3, 127.5, 162.2, 164.8. Ms: m/z = 236 (M+. + 1).
4a: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.2 (t, 6H, –CH3, –CH3), δ 1.4 (t, 6H, –CH3, –CH3), δ 2.8 (q, 4H, –CH2, –CH2) δ 3.2 (q, 4H, –CH2, –CH2), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 12.9, 14.1, 31.6, 36.7, 39.1, 43.5, 125.3, 126.5, 127.1, 129.3, 163.6, 169.6. Ms: m/z = 277 (M+. + 1).
4b: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 2H, –NH, –NH), δ 3.0 (t, 8H, Four –CH2 groups), δ 3.4 (t, 8H, Four –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 47.4, 48.6, 49.7, 50.9, 51.5, 52.4, 125.3, 125.5, 127.1, 128.6, 164.3, 167.6. Ms: m/z = 303 (M+. + 1).
4c: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1660 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.3 (t, 6H, –CH3, –CH3), δ 2.8 (q, 4H, –CH2, –CH2), δ 3.2 (t, 8H, Four –CH2 groups), δ 3.2 (t, 8H, Four –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 12.3, 16.1, 46.4, 47.6, 48.7, 49.1, 50.5, 51.4, 123.3, 124.5, 128.1, 128.9, 164.6, 168.6. Ms: m/z = 359 (M+. + 1).
4d: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1670 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.4 (t, 6H, –CH3, –CH3), δ 3.0 (t, 8H, Four –CH2 groups), δ 3.2 (t, 8H, Four –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 32.3, 33.4, 48.4, 48.9, 49.7, 50.5, 52.4, 55.3, 125.3, 125.6, 128.3, 129.9, 165.2, 169.3. Ms: m/z = 331 (M+. + 1).
4e: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1675 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 3.0 (t, 8H, Four –CH2 groups), δ 3.2 (t, 8H, Four –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 49.3, 49.9, 50.7, 52.8, 55.9, 56.8, 125.4, 126.3, 127.4, 128.9, 164.2, 167.3. Ms: m/z = 305 (M+. + 1).
5a: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of amide group), 1660 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0–1.2 (t, 6H, –CH3, –CH3), δ 2.2 (s, 1H, –NH), δ 3.0 (t, 4H, –CH2, –CH2), δ 3.2 (t, 4H, –CH2, –CH2), δ 3.4 (q, 2H, –CH2), δ 3.6 (q, 2H, –CH2, –CH2), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 12.9, 31.7, 43.5, 44.6, 51.1, 52.5, 123.1, 124.3, 128.3, 129.6, 161.7, 165.5. Ms: m/z = 290 (M+. + 1).
5b: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of amide group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0–1.2 (t, 6H, –CH3, –CH3), δ 1.4 (t, 3H, –CH3), δ 2.2 (q, 2H, –CH2), δ 3.0 (t, 4H, –CH2, –CH2), δ 3.2 (t, 4H, –CH2, –CH2), δ 3.4 (q, 2H, –CH2), δ 3.6 (q, 2H, –CH2, –CH2), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 12.9, 13.6, 31.5, 44.5, 45.6, 48.6, 54.1, 55.5, 123.6, 125.3, 128.3, 129.6, 162.7, 165.4. Ms: m/z = 318 (M+. + 1).
5c: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1695 cm−1 (sharp, strong, –CO– of amide group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0–1.2 (t, 6H, –CH3, –CH3), δ 1.8 (t, 3H, –CH3), δ 3.0 (t, 4H, –CH2, –CH2), δ 3.2 (t, 4H, –CH2, –CH2), δ 3.4 (q, 2H, –CH2), δ 3.6 (q, 2H, –CH2, –CH2), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 13.9, 32.7, 41.5, 44.6, 45.5, 53.1, 54.5, 122.3, 125.3, 129.2, 129.9, 164.7, 166.4. Ms: m/z = 304 (M+. + 1).
5d: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of amide group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0–1.2 (t, 6H, –CH3, –CH3), δ 3.0 (t, 4H, –CH2, –CH2), δ 3.2 (t, 4H, –CH2, –CH2), δ 3.4 (q, 2H, –CH2), δ 3.6 (q, 2H, –CH2, –CH2), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 13.2, 33.4, 44.3, 45.2, 50.1, 52.4, 124.2, 123.5, 125.4, 125.7, 160.3, 165.6. Ms: m/z = 291 (M+. + 1).
5e: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of amide group), 1665 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.4 (t, 3H, –CH3), δ 2.2 (q, 2H, –CH2), δ 2.2 (s, 1H, –NH), δ 2.8–3.6 (t, 16H, eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 12.4, 29.4, 30.2, 31.7, 33.4, 34.3, 42.5, 43.6, 50.2, 51.2, 124.1, 125.2, 127.3, 129.3, 160.3, 164.2. Ms: m/z = 331 (M+. + 1).
5f: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of amide group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.2 (s, 1H, –NH), δ 2.8–3.6 (t, 16H, eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 28.4, 31.4, 32.5, 33.3, 35.4, 41.4, 42.4, 51.4, 52.3, 125.2, 126.1, 128.3, 129.2, 161.6, 165.1. Ms: m/z = 317 (M+. + 1).
5g: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of amide group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 2.8–3.6 (t, 16H, eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 30.1, 31.3, 33.5, 34.2, 42.6, 43.8, 50.1, 51.4, 123.2, 125.1, 128.2, 129.3, 160.3, 165.3. Ms: m/z = 304 (M+. + 1).
5h: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of amide group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.4 (t, 3H, –CH3), δ 1.8 (t, 3H, –CH3), δ 2.2 (q, 2H, –CH2), δ 2.8–3.6 (t, 16H, eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 13.2, 28.6, 29.5, 32.2, 33.7, 34.5, 35.4, 43.5, 44.5, 53.5, 54.6, 125.5, 126.4, 126.9, 129.5, 161.3, 165.9. Ms: m/z = 345 (M+. + 1).
5i: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of amide group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.4 (t, 3H, –CH3), δ 2.2 (q, 2H, –CH2), δ 2.8–3.6 (t, 16H, eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 13.2, 29.4, 31.2, 32.2, 33.2, 34.5, 42.7, 44.8, 50.9, 51.9, 124.3, 125.9, 127.8, 129.0, 160.7, 168.9. Ms: m/z = 332 (M+. + 1).
5j: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1670 cm−1 (sharp, strong, –CO– of amide group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.8–3.6 (t, 16H, Eight –CH2 groups), 7.0–8.0 (m, 4H, Ar-H). 13C NMR (DMSO-d6, 400 MHz): 28.4, 33.2, 34.7, 35.4, 39.3, 44.5, 47.6, 53.2, 55.2, 127.1, 128.2, 129.3, 129.9, 163.3, 169.4. Ms: m/z = 318 (M+. + 1).
7a: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1645 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0 (t, 6H, –CH3, –CH3), δ 3.2 (q, 2H, –CH2), δ 3.4 (q, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.2, 13.4, 37.9, 42.5, 123.4, 126.2, 127.8, 128.1, 128.5, 130.3, 134.1, 137.4, 139.1, 165.8, 169.2. Ms: m/z = 297 (M+. + 1).
7b: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1645 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 49.3, 50.3, 51.5, 51.3, 124.3, 124.5, 125.5, 126.4, 127.3, 128.5, 129.5, 129.9, 131.5, 163.2, 164.5. Ms: m/z = 310 (M+. + 1).
7c: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of acid group), 1645 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.2 (t, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.6, 44.5, 47.3, 48.5, 50.5, 51.3, 120.3, 122.7, 125.4, 126.3, 127.7, 128.3, 129.2, 130.9, 131.4, 160.6, 164.9. Ms: m/z = 338 (M+. + 1).
7d: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 34.5, 46.4, 47.2, 48.6, 50.2, 121.2, 123.4, 123.9, 124.6, 125.6, 126.3, 128.2, 131.9, 132.4, 164.2, 169.2. Ms: m/z = 324 (M+. + 1).
7e: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1660 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 50.3, 51.3, 71.5, 72.3, 120.3, 121.2, 124.2, 125.6, 128.1, 128.9, 129.1, 129.9, 131.4, 165.1, 166.3. Ms: m/z = 311 (M+. + 1).
7f: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1644 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0 (t, 6H, –CH3, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.2 (q, 2H, –CH2), δ 3.4 (q, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.6, 13.8, 19.4, 39.9, 43.6, 123.6, 124.6, 125.2, 126.6, 127.6, 129.8, 131.2, 133.5, 136.5, 166.7, 168.3. Ms: m/z = 311 (M+. + 1).
7g: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 20.5, 44.5, 49.3, 50.5, 51.5, 120.2, 122.4, 123.4, 124.5, 125.3, 126.6, 128.5, 130.1, 133.5, 163.7, 166.6. Ms: m/z = 324 (M+. + 1).
7h: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1695 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.2 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.2, 19.3, 44.2, 47.6, 48.3, 51.2, 52.2, 119.3, 121.3, 124.2, 124.9, 126.2, 127.1, 130.4, 131.3, 132.3, 161.1, 165.1. Ms: m/z = 352 (M+. + 1).
7i: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 18.4, 35.4, 45.4, 46.3, 47.3, 51.3, 120.1, 122.2, 122.6, 124.5, 124.7, 127.4, 128.6, 131.0, 132.8, 164.6, 169.9. Ms: m/z = 338 (M+. + 1).
7j: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 17.9, 51.2, 52.0, 70.2, 72.4, 121.2, 122.4, 125.3, 126.0, 126.9, 127.0, 129.6, 130.1, 131.5, 160.4, 166.7. Ms: m/z = 325 (M+. + 1).
7k: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of acid group), 1645 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.1 (t, 6H, –CH3, –CH3), δ 2.6 (s, 3H, –CH3), δ 3.2 (q, 2H, –CH2), δ 3.4 (q, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.4, 13.5, 19.6, 40.9, 43.5, 124.6, 125.6, 126.7, 128.6, 129.4, 130.6, 131.2, 136.5, 135.5, 166.9, 168.7. Ms: m/z = 311 (M+. + 1).
7l: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.4 (s, 1H, –NH), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 19.5, 43.4, 45.5, 52.5, 53.4, 119.3, 123.4, 123.9, 124.9, 125.5, 127.6, 128.7, 130.2, 132.5, 161.3, 166.5. Ms: m/z = 324 (M+. + 1).
7m: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1695 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.4 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 11.23, 18.2, 42.1, 43.4, 45.2, 50.8, 51.3, 119.5, 120.4, 124.5, 126.9, 127.4, 126.1, 130.6, 131.3, 132.6, 160.1, 165.6. Ms: m/z = 352 (M+. + 1).
7n: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1683 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 18.3, 36.3, 43.5, 45.2, 46.2, 50.2, 121.2, 121.1, 122.4, 124.4, 125.6, 126.3, 127.2, 130.0, 131.4, 162.6, 166.8. Ms: m/z = 338 (M+. + 1).
7o: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1685 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 18.5, 50.1, 51.6, 73.5, 74.3, 119.2, 120.2, 121.2, 122.1, 123.4, 124.1, 125.3, 127.3, 128.3, 163.5, 167.4. Ms: m/z = 325 (M+. + 1).
7p: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1670 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0 (t, 6H, –CH3, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.2 (q, 2H, –CH2), δ 3.4 (q, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.4, 14.4, 32.9, 40.5, 121.4, 125.8, 126.8, 129.1, 130.3, 131.4, 134.6, 138.4, 139.9, 163.6, 169.9. Ms: m/z = 331 (M+. + 1).
7q: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1684 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 44.5, 44.9, 53.2, 54.2, 119.6, 120.5, 121.9, 122.9, 127.5, 128.5, 129.6, 130.2, 131.3, 162.4, 164.2. Ms: m/z = 344 (M+. + 1).
7r: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1650 cm−1 (sharp, strong, –CO– of acid group), 1640 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.2 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.8, 44.2, 45.4, 46.2, 51.8, 52.4, 119.6, 121.5, 124.5, 125.6, 127.3, 125.6, 130.2, 132.4, 135.7, 160.7, 165.9. Ms: m/z = 372 (M+. + 1).
7s: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1640 cm−1 (sharp, strong, –CO– of acid group), 1635 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 38.3, 45.6, 46.2, 47.5, 50.6, 123.2, 126.6, 127.3, 128.3, 129.1, 129.9, 130.2, 131.4, 132.4, 160.3, 166.4. Ms: m/z = 358 (M+. + 1).
7t: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 8H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 48.1, 50.6, 72.5, 73.1, 119.5, 120.6, 122.3, 122.9, 123.5, 124.3, 125.7, 126.3, 129.3, 165.7, 169.4. Ms: m/z = 345 (M+. + 1).
7u: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1680 cm−1 (sharp, strong, –CO– of acid group), 1650 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.0 (t, 6H, –CH3, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.2 (q, 2H, –CH2), δ 3.4 (q, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 10.6 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.5, 15.4, 37.9, 43.5, 125.4, 126.5, 126.8, 129.6, 131.2, 131.3, 135.6, 138.6, 139.3, 164.6, 169.8. Ms: m/z = 375 (M+. + 1).
7v: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1687 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.2 (s, 1H, –NH), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 41.5, 45.9, 52.3, 54.5, 118.4, 121.3, 125.9, 126.9, 127.7, 128.4, 129.7, 131.4, 133.5, 165.4, 167.7. Ms: m/z = 388 (M+. + 1).
7w: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1681 cm−1 (sharp, strong, –CO– of acid group), 1655 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.2 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 2.6 (q, 2H, –CH2), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 10.4 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 12.4, 43.5, 45.7, 47.3, 52.9, 53.4, 118.4, 121.6, 124.7, 125.5, 128.3, 129.5, 130.5, 131.3, 135.4, 164.5, 165.9. Ms: m/z = 416 (M+. + 1).
7x: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1690 cm−1 (sharp, strong, –CO– of acid group), 1658 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 1.6 (t, 3H, –CH3), δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 39.2, 46.4, 47.3, 48.4, 49.5, 121.2, 124.3, 124.9, 125.7, 126.4, 128.3, 130.6, 131.1, 131.3, 166.1, 169.3. Ms: m/z = 402 (M+. + 1).
7y: IR (KBr): 3100–3400 cm−1 (broad medium, –NH–), 1675 cm−1 (sharp, strong, –CO– of acid group), 1635 cm−1 (sharp, strong, –CO– of amide group); 1H-NMR: δ 2.4 (s, 3H, –CH3), δ 3.0 (t, 2H, –CH2), δ 3.2 (t, 2H, –CH2), δ 3.4 (t, 2H, –CH2), δ 3.8 (t, 2H, –CH2), 7.0–8.0 (m, 9H, Ar-H), 13.00 (s, 1H, –NH, D2O exchangeable). 13C NMR (DMSO-d6, 400 MHz): 48.9, 51.4, 73.5, 74.5, 119.6, 121.4, 122.5, 123.8, 124.7, 125.7, 126.4, 127.3, 129.3, 165.8, 169.3. Ms: m/z = 389 (M+. + 1).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
The authors are thankful to the authorities of Jawaharlal Nehru Technological University Hyderabad for providing laboratory facilities and for financial support to two of the authors (Padam Praveen Kumar and Yervala Dathu Reddy).




