Water Mediated Synthesis of N′-Arylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide Library
Abstract
A novel two-step synthesis of 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide has been developed. The library of N′-arylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide was generated by coupling of hydrazide to various aromatic and heterocyclic aldehydes in water media at ambient temperature with great flexibility regarding reaction time and yield.
1. Introduction
Derivatives of indazole and other pyrazole-containing condensed systems are attracting attention because of their biological activity and the possibilities of further conversions. Anti-inflammatory, analgesic, antipyretic, and antirheumatic activity has been reported for pyrazole derivatives [1–3]. One of these derivatives, 2-(1-phenyl-pyrazole-4-yl)propionic acid I (Figure 1), has been shown to be clinically active in the treatment of rheumatic disorders. In addition, it has been reported that pyrazole corticoids III, IV (Figure 1) are more active than parent corticoids. One of these derivatives, 17α,21-dihydroxy-20-oxopregn-4-eno[3,2-c]-2′-(4-fluorophenyl) pyrazole II (Figure 1) [4], has been used clinically as a topical anti-inflammatory agent. However, literature reveals that 4,5,6,7-tetrahydro-2H-indazole derivatives exhibit dopaminergic [5], anti-inflammatory [6], herbicidal [7], and antitumor [8] activity and cannabinoid modulators [9], as HMG-COA reductase inhibitors [10]. Hydrazide analogues also possess other biological activities like anticonvulsant [11], antidepressant [12], anti-inflammatory [13], antimalarial [14], antimycobacterial [15], anticancer [16], and antimicrobial [17–21] activities.
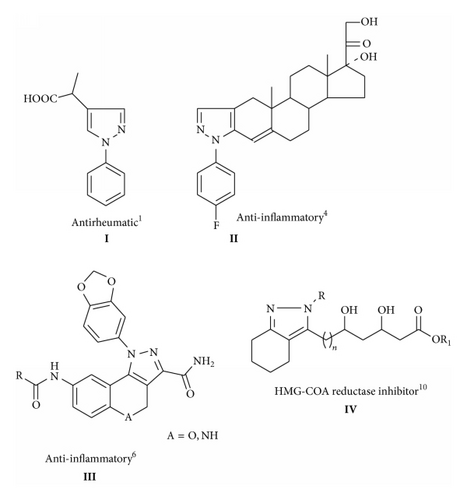
Our Continuous efforts for the synthesis of various novel heterocycles for biological interest using various catalyst and green approaches [22–26] and the remarkable pharmaceutical importance of fused hydrazide and pyrazole derivatives, prompted us to design and synthesize a scaffold 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide using water as a green solvent.
2. Materials and Methods
Melting points were determined on electrothermal apparatus using open capillaries and are uncorrected. Thin-layer chromatography was accomplished on 0.2 mm precoated plates of silica gel G60 F254 (Merck). Visualization was made with UV light (254 and 365 nm) or with an iodine vapor. IR spectra were recorded on a FTIR-8400 spectrophotometer using DRS prob. 1H NMR spectra were recorded on a Bruker AVANCE II (400 MHz) spectrometer in DMSO. Chemical shifts are expressed in δ ppm downfield from TMS as an internal standard. Mass spectra were determined using direct inlet probe on a GCMS-QP 2010 mass spectrometer (Shimadzu). Elemental analysis was performed on a Carlo-Erba EA 1108 elemental analyzer. All reagents were purchased from Fluka, Sigma Aldrich, Merck, and Rankem and used without further purification.
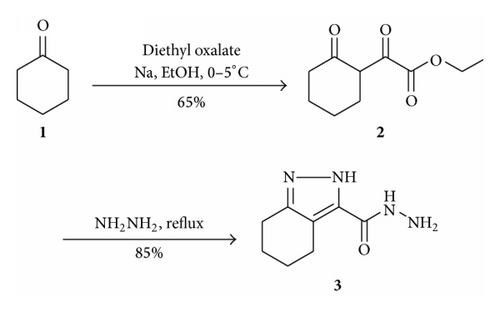
2.1. Synthesis of Ethyl 2-Oxo-2-(2-oxocyclohexyl)acetate 2
To the stirred solution of sodium ethoxide (13.6 g, 0.2 mol), a mixture of cyclohexanone (1, 19.6 g, 0.2 mol) and diethyl oxalate (29.2 g, 0.2 mol) was added drop wise below 5–10°C. Vigorous stirring is required to prevent complete solidification of the reaction mixture. When the addition is complete, the ice bath is retained for an hour, and then the mixture is stirred at room temperature for about six hours. The reaction mixture is then decomposed by careful addition of cold 15% sulfuric acid solution. During this neutralization the temperature of the mixture is maintained at about 5–10°C by means of an ice-salt bath. The ethyl 2-ketocyclohexylglyoxalate 2 has been extracted using chloroform as colorless oil after evaporation of solvent in vaccuo. The product was sufficient pure for further reaction Yield-25.7 g (65%).
2.2. Synthesis of 4,5,6,7-Tetrahydro-2H-indazole-3-carbohydrazide 3
Ethyl 2-oxo-2-(2-oxocyclohexyl) acetate (2, 19.8 g, 0.1 mol) was stirred at 5–10°C and 25 mL of 80% hydrazine hydrate was added drop wise. After the complete addition reaction mixture was allowed at room temperature and refluxed for 2 to 3 h in water bath, the reaction mixture was allowed to cool at room temperature and the precipitate obtained was filtered, dried, and recrystallized from ethanol to give a pure 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 3 as a white crystal in 85% yield, Mp—128–130°C. Light yellow solid, IR (KBr): 3450, 3390, 2750, 1785, 1575, 1247, 982, 750. 1H NMR (DMSO), 12.31 (S, 1H, NH), 10.65 (S, 1H, NH), 3.15–2.75 (m, 4H, CH2), 2.20 (s, 2H, NH2), 1.82–1.73 (m, 4H, CH2), MS (m/z): 180 (M+), Anal. Calcd for C8H12N4O: C, 53.32, H, 6.71, N, 31.09. Found: C, 53.10, H, 6.90, N, 30.25.
2.3. Synthesis of N′-Arylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 4a–t
A mixture of 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide (3, 1.8 g, 1 mmol) and appropriate aromatic aldehyde (1.5 mmol) was taken in 20 mL of water. The reaction mixture was stirred for 30 min at room temperature. The obtained solid was filtered and washed with saturated aqueous sodium bicarbonate, water, 1 N aq HCl, and brine subsequently to remove the unreacted aldehyde. The crystallization of obtained crude product from ethanol gives a pure N′-arylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 4a–t in good to excellent yield.
N′-Phenylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 4a. White solid, IR (KBr): 3626, 3593, 2889, 2947, 2852, 1666, 1554, 1492, 1448, 1261, 709, 630 cm−1, 1H NMR (DMSO), 12.36 (S, 1H, NH), 10.78 (S, 1H, NH), 8.34 (s, 1H, =CH), 7.76–7.67 (m, 2H, Ar), 7.40–7.37 (m, 3H, Ar), 3.15–2.78 (m, 4H, CH2), 1.81–1.75 (m, 4H, CH2), MS (m/z): 268 (M+), Anal. Calcd for C15H16N4O: C, 67.15, H, 6.01, N, 20.88. Found: C, 67.10, H, 6.12, N, 20.84.
3. Results and Discussion
The desired 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 3 was obtained starting from cyclohexanone 1 and diethyl oxalate followed by subsequent treatment with hydrazine hydrate, Scheme 1. In the first step the anion of starting compound cyclohexanone was generated with the help of sodium ethoxide in ethanol at 0–5°C and reacted with diethyl oxalate which resulted into ethyl 2-oxo-2-(2-oxocyclohexyl)acetate 2 [21]. When compound 2 was reacted with hydrazine hydrate in solvent medium like methanol, ethanol, dioxane, and so forth, it gave ethyl 4,5,6,7-tetrahydro-2H-indazole-3-carboxylate, while without solvent in excess hydrazine hydrate on reflux afforded 4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 3 in excellent yield (85%) as outlined in Scheme 1.
The reaction of various hydrazides 3 with various aromatic aldehydes at room temperature in water media led to the formation of a series of new indazole derivatives 4a–t as demonstrated in Scheme 2. An excess of the aldehydes was used to achieve a high conversion. It is noteworthy that a maximum conversion of hydrazide 3 to 4a–t was achieved within 25–30 minutes by stirring at ambient temperature. Each coupling reaction was worked up by filtration and washing of solid with saturated aqueous sodium bicarbonate, water, 1 N aq HCl, and brine. Subsequent purification of each compound by crystallization in ethanol delivered pure N′-arylmethylene-4,5,6,7-tetrahydro-2H-indazole-3-carbohydrazide 4a–t with 85–95% yield.
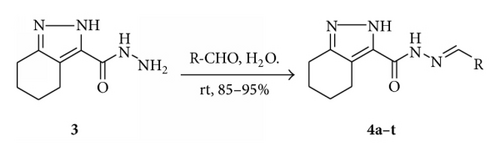
Concerning the functionalized indazoles 4a–t, we found no significant electronic effects caused by electron-withdrawing or electron-donating groups on the aryl ring of the hydrazone, though yields were variable. Also, the coupling of heterocyclic aldehydes to hydrazide works well without affecting reaction time and yield.
All the compounds were characterized by IR, mass, 1H NMR spectroscopy, and elemental analysis to confirm the compound identity, which is consistent with the proposed molecular structures. As per 1H NMR spectral study, the number of protons and their chemical shifts were found to support the proposed structures. Methylene protons of cyclohexane ring were observed between 1.7 to 2.9 δ ppm. Amide proton was observed at 10.7–11.0 δ ppm as a singlet, while cyclic NH proton of indazole ring was observed at very downfield with 12.34–12.85 δ ppm value as a singlet. The ethylenic proton was shown as a singlet around 8.3–8.8 δ ppm. Aromatic protons were observed between 6.8 to 7.8 δ ppm with characteristic splitting according to the substitution. In mass spectral study, molecular ion peak was observed in agreement with molecular weight of respective compound. As per IR spectral study, the presence of functional groups such as secondary amine, amide, and aromatic ring system was confirmed on the basis of its characteristic absorption range. The physicochemical data of synthesized compounds are presented in Table 1.
| Entry | R | Yielda (%) | Mp °C |
|---|---|---|---|
| 4a | Ph | 95 | 230–232 |
| 4b | 4-OCH3 Ph | 91 | 216–218 |
| 4c | 4-CH3 Ph | 86 | 208–210 |
| 4d | 3,4-diOCH3 Ph | 86 | 220–222 |
| 4e | 2,5-diOCH3 Ph | 89 | 212–214 |
| 4f | 3-Br Ph | 95 | 214–216 |
| 4g | 4-OH Ph | 91 | 219-220 |
| 4h | 2-OH Ph | 85 | 226–228 |
| 4i | 4-N(CH3)2 Ph | 85 | 208–210 |
| 4j | 3-Cl Ph | 90 | 212–214 |
| 4k | 4-Cl Ph | 90 | 225-226 |
| 4l | 2-Cl Ph | 90 | 216–218 |
| 4m | 4-F Ph | 86 | 226–228 |
| 4n | 4-NO2 Ph | 80 | 217-218 |
| 4o | 3-NO2 Ph | 80 | 204–206 |
| 4p | 3-Pyridyl | 92 | 228–230 |
| 4q | 2-Furyl | 88 | 214–216 |
| 4r | 1-Napthyl | 92 | 227–229 |
| 4s | 3-OH Ph | 93 | 214–216 |
| 4t | 2-OCH3 Ph | 87 | 223-224 |
- aIsolated yield after purification.
4. Conclusion
In summary, a new 4,5,6,7-tetrahydro-2H-indazole carbohydrazide has been developed and utilized for the synthesis of corresponding arylmethylene hydrazone in water media at ambient temperature. The methodology shows the great flexibility regarding reaction time, yield, and green solvent. In principle, the strategy should be applicable in the generation of hydrazone library.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Authors are thankful for the facilities and grants given under UGC-SAP for Department of Research Support (DRS) and Department of Science and Technology (DST), New Delhi, also thankful for Fund for Improvement of Science and Technology (FIST) and Department of Chemistry, Saurashtra University, for providing laboratory facilities.




