Systematic Literature Review of the Epidemiology of Nongenetic Forms of Hypogonadism in Adult Males
Abstract
This study summarizes the literature on the prevalence, incidence, and proportion of patients receiving treatment for male hypogonadism and a systematic literature search was performed for articles published in the last 20 years. Of the 97 studies identified, 96 examined the prevalence, 2 examined the incidence, and 4 examined the proportion of males with hypogonadism patients receiving treatment. Based on studies conducted in Europe and USA, the prevalence of hypogonadism in the general population ranged from 2.1% to 12.8% of middle-aged to older men, with an estimated incidence of 12 new cases per 1,000 person-years. Prevalence was higher among patients with comorbid conditions, such as type 2 diabetes mellitus and obesity. Approximately 10–12% of men with hypogonadism were receiving testosterone treatment. This literature review suggests that there is potentially a significant burden of hypogonadism in the general population. Burden seems to increase with age and in the presence of certain disease conditions. Data suggests that many hypogonadal men who may benefit from testosterone replacement are not receiving treatment. This may be the result of underdiagnosis of the disease, lack of awareness by patients or physicians, irregularities surrounding the diagnostic criteria, and deficiency of long-term safety studies.
1. Introduction
Hypogonadism in men has been defined as a clinical syndrome resulting from failure of the testis to produce physiological levels of testosterone (androgen deficiency) and a normal number of spermatozoa, due to disruption of one or more levels of the hypothalamic-pituitary-testicular axis [1]. A diagnosis of hypogonadism is typically based on the signs and symptoms associated with low T, followed by biochemical confirmation of low testosterone (T) [1]. The most widely accepted parameter used to establish hypogonadism is the measurement of serum total testosterone (TT) [1]; however, cut-off values used to indicate hypogonadism have not been clearly defined and vary across studies. Recent clinical practice guidelines published by the Endocrine Society have reported that the average TT threshold, at which the likelihood of most symptoms associated with hypogonadism increases, corresponds to the lower limit of the normal range for young men, that is, approximately 300 ng/dL (10.4 nmol/liter) [1]. Correspondingly, a common threshold used in the literature to indicate hypogonadism is serum TT <300 ng/dL (<10.4 nmol/L); however, cut-off values of <200 ng/dL (6.94 nmol/L) to <350 ng/dL (<12 nmol/L) are not uncommon. These thresholds are sometimes combined with requisite signs and symptoms, but not always. The result is a range of operational definitions of hypogonadism used in the literature, varying in degree of stringency and potentially leading to variations in estimates of prevalence of the disease.
The literature on the epidemiology of hypogonadism is confounded by considerable heterogeneity across studies, varying not only by operational definition but also by patient populations, study design, time frame of study, and geography. Estimates of prevalence, incidence, and treatment rates are influenced by all of these factors. The aim of this systematic review of the epidemiology of hypogonadism was to determine the prevalence and incidence of hypogonadism, as well as the proportion of males with hypogonadism receiving treatment of hypogonadism, while accounting for differences in patient and study characteristics and operational definition of hypogonadism used.
2. Methods
2.1. Eligibility Criteria
Eligible studies were defined in this systematic review as original studies that assessed the prevalence, incidence, or treatment rates of adult males (≥18 years of age) with hypogonadism, acquired or idiopathic. Studies on genetic forms of hypogonadism (i.e., Klinefelter’s syndrome) and secondary publications of the same results were not included. Observational studies with sample sizes >30 were included in this review; treatment and intervention studies were excluded.
2.2. Search Methods for Identification of Studies
Three pharmacologic and biomedical literature databases were searched for this systematic literature review: PubMed/MEDLINE database, Cochrane Library Database, and EMBASE. Search strings composed of medical subject heading (MeSH) terms, from the controlled vocabulary indexing hierarchy and/or in-text terms in title and/or abstract fields, were used (Table 1). Citations were limited to English language articles, published during a 20-year period (January 01, 1992, to August 31, 2012). The reference list of included publications was searched for relevant articles.
| Search | Search string [field] | Hits |
|---|---|---|
| 1 | Search “hypogonadism/epidemiology” [mesh] | 345 |
| 2 | Search hypogonadism [title] OR testosterone deficiency* [title] OR “testosterone/deficiency” [mesh] OR testoid deficiency* [title] OR androgen deficiency* [title] OR testosterone replacement therapy* [title] | 3173 |
| 3 | Search epidemiology* [title/abstract] OR incidence [title/abstract] OR prevalence [title/abstract] OR rate [title/abstract] OR rates [title/abstract] OR treatment rate [title/abstract] OR treatment rates [title/abstract] | 2380843 |
| 4 | Search (“epidemiology” [mesh]) OR “incidence” [mesh] OR “prevalence” [mesh]) | 318545 |
| 5 | Search #3 OR #4 | 2472821 |
| 6 | Search #2 AND #5 | 331 |
| 7 | Search #1 OR #6 | 603 |
| 8 | Search #7 filters: publication date from 1992/01/01 to 2012/8/31; humans; English; male; adult: 19+ years | 298 |
| Non-English articles | 21 |
2.3. Data Extraction
We abstracted key data elements using a standardized abstraction tool, including type of research design, country, study period, sample size, operational definition of hypogonadism used, study population characteristics, and outcomes. One author screened the search results against the predefined inclusion and exclusion criteria. Titles were evaluated, abstracts were reviewed, and potentially relevant full-text articles were evaluated for relevance. A quality check was conducted on 20% of the abstracts by a second reviewer.
We categorized studies based on population of interest: population-based studies were those with large sample sizes (>1 thousand) and minimal age restrictions and conducted in a specific geographical location; community-based studies were those with relatively smaller sample sizes (i.e., hundreds) and more restricted age ranges and conducted in a specific geographical location; primary care-based studies were those that recruited patients from a general primary care or medical clinic seeking medical care; screening-based studies recruited healthy individuals from a general health screening clinic. Primary care-based studies and screening-based studies were classified together as they were conducted in the same setting. Finally, clinical condition-based studies were those that examined prevalence of hypogonadism in patient populations with specific clinical conditions. We also separately summarized prevalence of hypogonadism by age for studies that provided these data.
3. Results
3.1. Literature Search Results
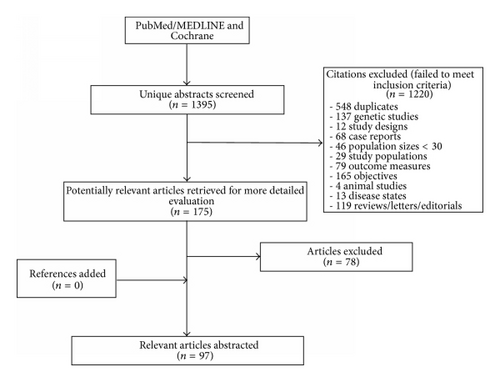
The literature search identified 1,395 articles that were reviewed for inclusion. After review of titles and abstracts, we evaluated 175 full-text articles and identified 97 articles that met our eligibility criteria: 96 reported on the prevalence of hypogonadism, 2 on the incidence, and 4 on treatment proportions (not mutually exclusive). The PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow chart can be found in Figure 1.
3.2. Prevalence of Hypogonadism
Among the 96 identified prevalence studies, 7 were population- (n = 4) [2–5] or community- (n = 3) [6–8] based studies and 7 were primary care or health screening studies [9–15]. Ten examined age-related changes for hypogonadism [2, 3, 8, 9, 12, 13, 16–19] and seventy-eight were clinical condition-based studies [20–97]. Studies varied considerably in geographic location, patient characteristics, sample sizes, and operational definition for hypogonadism. These studies are described in Tables 2 and 3.
| Author, year, and study name | Country and years of data collected | Study design | N | Age, mean (SD), and range | Definition of hypogonadism used | Reported prevalence (%) |
|---|---|---|---|---|---|---|
| Population-based studies | ||||||
| Haring et al., 2010 [5] (SHIP) | Germany, 1997–2001 | Population-based | 1490 | 50.65 (15.41), NR | TT < 300 ng/dL | 12.8 |
| Araujo et al., 2007 [3] (BACH) | USA, 2002–2005 | Population-based | 1475 | 47.3 (12.5), NR |
|
5.6 |
| Araujo et al., 2004 [2] (MMAS) | USA, 1987–1997 | Population-based | 1691 | NR (NR), 40–70** |
|
6 |
| Tajar et al., 2012 [4] (EMAS) | 8 European countries, 2003–2005 | Population-based††† | 2966 | 59.15 (10.82), 40–79 |
|
2.1 |
| Community-based studies | ||||||
| Ponholzer et al., 2010 [6] | Austria, 2000–2002 | Community-based | 247 | 75.8 (0.4), 75–78 | TT < 350 ng/dL | 31.2 |
| Khoo et al., 2008 [7] | Malaysia, NR | Community-based | 351 | 58 (7), 50–93 | TT < 317 ng/dL | 19.1 |
|
6 | |||||
| Wong et al., 2006 [8] | China (Hong Kong), 2003-2004 | Community-based | 252 | 54 (NR), 45–64 |
|
9.52 |
| Primary care and screening studies | ||||||
| Mulligan et al., 2006 [13] (HIM) | USA, 2003-2004 | Primary care patients | 2162 | 60.5 (10.33), 45–96 | TT < 300 ng/dL | 38.7 |
| Schneider et al., 2009 (DETECT) [15] | Germany, 2003 | Primary care patients | 2719 | 58.7 (13.4), NR | TT < 300 ng/dL | 19.3 |
| Nardozza et al., 2011 [14] | Brazil, 2009 | Health screening | 1623 | 57 (NR), 24–87 | TT < 300 ng/dL | 19.8 |
| Goel et al., 2009 [11] | India, 2006 | Health Screening†††† | 157 | 53.1 (NR), 40–60; | TT < 300 ng/dL | 24.2 |
|
20.4 | |||||
| Liu et al., 2009 [12] | Taiwan, 2007-2008 | Health screening | 734 | 57.4 (6.7), 43–87 | TT < 300 ng/dL | 24.1 |
|
12 | |||||
| Blümel et al., 2009 [9] | Chile, NR | Primary care patients | 96 | 55.1 (12.0), 40–85 |
|
28.1 |
| Di Luigi et al., 2010 [10] | Italy | Health screening | 183 | 61.9 (7.5), 50–75 | TT < 230 ng/dL | 12 |
- TT: 200 ng/dL = 6.94 nmol/L; 230 ng/dL = 8 nmol/L; 300 ng/dL = 10.4 nmol/L; 317 ng/dL = 11 nmol/L; 350 ng/dL = 12 nmol/L; FT: 5 ng/dL = 0.17 nmol/L; 8.9 ng/dL = 0.3092 nmoL/L.
- ADAM: androgen deficiency in the aging male; BACH: Boston Area Community Health; BT: bioavailable testosterone; cFT: calculated free testosterone; DETECT: diabetes cardiovascular risk-evaluation: targets and essential data for commitment of treatment; EMAS: European Male Aging Study; HIM: hypogonadism in males; MMAS: Massachusetts Male Aging Study; NR = not reported; SHIP = Study of Health in Pomerania; TT = total testosterone.
- *Decreased frequency of morning erections, sexual thoughts, and erectile dysfunction.
- **Participants were equally divided between men in their 40s, 50s, and 60s with a similar mean age to the BACH data.
- ***Positive answer to items 1 or 7, or any 3 other questions on the ADAM questionnaire.
- †Specific symptoms include low libido, erective dysfunction, or osteoporosis; the nonspecific symptoms include sleep disturbance, depressed mood, lethargy, or low physical performance.
- ††Symptoms include loss of libido, erectile dysfunction, depression, lethargy, inability to concentrate, sleep disturbance, irritability, and depressed mood.
- †††4.4% of original population were excluded which included men already receiving testosterone replacement therapy.
- ††††Study subjects were healthy hospital employees volunteered for a health check-up.
| Author, year, and study name | Country and years of data collected | Study population | N | Definition of hypogonadism used | Prevalence (%) among patients with disease of interest |
|---|---|---|---|---|---|
| Type 2 diabetes mellitus (T2DM) | |||||
| Kapoor et al., 2007 [56] | UK/not specified | Primary care retinopathy Screening service and medical clinic | 355 men with T2DM |
|
|
| Biswas et al., 2012 [25] | UK/not specified | Medical clinic |
|
TT < 11.9 nM | 45 |
| Chen et al., 2006 [29] | Australia/NZ/1992 to 2000 | Population-based |
|
TT < 8.0 nmol/L | 14.3 |
| Corona et al., 2006 [36] | Italy/not specified | Medical clinic |
|
TT < 10.4 nmol/L | 24.5 |
| Anderson et al., 2012 [23] | UK/January 2008–June 2009 | Medical clinic |
|
|
|
| Talukder et al., 2010 [87] | India/May 2008–April 2009 | Medical clinic |
|
Symptoms/signs of hypogonadism and TT ≤ 12 nmol/L | 34.7 |
| Dhindsa et al., 2004 [40] | US/not specified | Medical clinic |
|
TT < 300 ng/dL | 33 |
| Grossmann et al., 2008 [48] | Australia/not specified | Medical clinic |
|
TT < 10 nmol/L | 43 |
| Grossmann et al., 2009 [47] | Australia/2005 | Medical clinic | 464 men with T2DM | TT < 10 nmol/L | 43 |
| La Vignera et al., 2009 [61] | Italy/not specified | Medical clinic | 110 men with T2DM | TT < 231 ng/mL | 10 |
| Ogbera et al., 2011 [71] | Nigeria/December 2009 to May 2010 | Medical clinic | 203 men with T2DM | TT < 8 nmol/L | 36 |
| Hackett et al., 2009 [50] | UK/September 2007 and May 2008 | Medical clinic | 488 men with T2DM | TT < 8 nmol/LTT 8–12 nmol/L |
|
| Ganesh et al., 2009 [45] | India/not specified | Medical clinic | 100 men with T2DM | cFT < 64.8 pg/mL (0.225 nmol/L) | 15 |
| T2DM + obesity or metabolic syndrome (MetS) | |||||
| Ogbera 2011 [70] | Nigeria/December 2009 to May 2010 | Medical clinic |
|
T2DM + TT < 12 nmol/L | 36 |
| T2DM + MetS | 43 | ||||
| T2DM + MetS + low TT | 47 | ||||
| Dhindsa et al., 2010 [39] | US/not specified | Medical clinic | 1849 | FT < 300 ng/dL or 10.4 nmol/L: | |
| Obesity + T2DM (n = 398) | Obesity and T2DM | 51 | |||
| Obesity (n = 398) | Obese | 31 | |||
| Obesity | |||||
| Hofstra et al., 2008 [54] | The Netherlands/not specified | Medical clinic | 160 obese males | TT < 11 nmol/L | 57.7 |
| Pellitero et al., 2012 [74] | Spain/not specified | Clinical research center | 33 obese males | TT < 300 ng/dL | 78.8 |
| TT < 200 ng/dL + FT <65 pg/mL | 100 | ||||
| TT < 300 but >200 ng/dL | 23.1 | ||||
| FT low and TT > 300 ng/dL | 3.85 | ||||
| Allan et al., 2006 [22] | Australia/May 2001–February 2003 | Community-based | 223 | TT < 8 nmol/L plus symptoms | |
| Obese (n = 99) | Obese | 15 | |||
| Nonobese (n = 124) | Nonobese | 3 | |||
| MetS | |||||
| Laaksonen et al., 2005 [62] | Finland/1987–2001 | Population-based | 854 men with MetS | TT < 11 nmol/L | 10.7 |
| Singh et al., 2011 [83] | India/not specified | Medical clinic | 95 men with MetS | FT < 0.225 nmol/L | 30.2 |
| Caldas et al., 2009 [27] | Brazil/January 2003 to March 2007 | Medical clinic | 80 men with MetS | TT < 300 ng/dL | 30.0 |
| Cardiac disease | |||||
| Jankowska et al., 2006 [55] | Poland/October 2001 to November 2002 | Medical clinic |
|
Serum TT and FT levels at or below the 10th percentile of healthy peers | 13–39 (by age) |
| Wang et al., 2010 [94] | China/March 1, 2005, to May 31, 2007 | Medical clinic | 175 men with CHF | Serum levels at or below the 10th percentile of healthy peers | 21.7 |
| Serra et al., 2012 [84] | Italy/not specified | Medical clinic | 52 male heart transplantation recipients | TT < 10.4 nmol/L | 34.6 |
| Florvaag et al., 2012 [44] | Germany/not specified | Medical clinic | 137 male heart failure patients | Free testosterone (age-related cut-off values) | 39 |
| Malkin et al., 2010 [67] | UK/June 2000 and June 2002 | Medical clinic | 930 men with coronary disease | TT < 8.1 nmol/L | 16.9 |
| Wehr et al., 2010 [95] | Germany/1997 to 2000 | Medical clinic | 2299 men who were routinely referred for coronary angiography | T < 11.3 nmol/L | 18 |
| Lerchbaum et al., 2012 [65] | Germany/1997–2000 | Medical clinic | 2069 men with coronary disease | TT < 11.3 nM | 18.4 |
| Erectile dysfunction (ED) | |||||
| Köhler et al., 2008 [59] | US/1987 to 2002 | Chart review | 2794 men presenting for ED evaluation | TT < 300 ng/dL | 23 |
| Bunch et al., 2002 [26] | US/1996 to 1999 | Chart review | 501 men with ED | TT < 300 ng/dL | 44 |
| Corona et al., 2009 [33] | Italy/not specified | Chart review | 2302 men with ED | TT < 12 nmol/L + symptoms | 28.1 |
| Arafa et al., 2012 [24] | Egypt/not specified | Medical clinic | 212 men T2DM + ED | TT < 300 ng/dL + symptoms | 41 |
| Guay et al., 2010 [49] | US/July 1995 to July 1997 | Chart review | 990 with men ED | 10.4 nmol/L + symptoms | 36.3 |
| Makhlouf et al., 2008 [66] | US/July 2001 to June 2003 | Medical clinic | 157 men with ED | TT < 300 mg/dL | 36 |
| Corona et al., 2007 [35] | Italy/not specified | Medical clinic | 1134 men with ED | TT < 10.4 nmol/L | 16.9 |
| Corona et al., 2010 [37] | Italy/2000 to 2007 | Medical clinic | 1687 men with ED | TT 230–300 ng/dL no symptoms | 13.3 |
| Corona et al., 2009 [34] | Italy/January 2001 to April 2008 | Medical clinic | 1093 men with ED | ||
| Somani et al., 2010 [85] | UK/March 2007–August 2008 | Medical clinic | 124 men with ED | Low TT (not further defined) | 23.4 |
| Corona et al., 2010 [32] | Italy/January 2001–April 2009 | Medical chart review | 3712 men with sexual dysfunction | TT <8 nmol/L + symptoms | 7.5 |
| Tan et al., 2011 [88] | Multiethnic Asia/Pacific/2003 | Medical chart review |
|
TT < 10.4 nmol/L | 20.4 |
| Hamidi et al., 2012 [52] | Iran/November 2009 to August 2010 | Medical clinic | 241 men with ED | FT < 5 ng/dL | 36.5 |
| HIV | |||||
| Klein et al., 2005 [58] | US/not specified | Community-based | 502 (had or at risk of HIV) | TT < 300 ng/dL | 45.6 |
| Crum-Cianflone et al., 2007 [38] | US/September 2004 to May 2005 | HIV medical clinic | 300 HIV+ | TT < 300 ng/dL | 17 |
| Wahlstrom et al., 2000 [93] | US/not specified | Observational study | 20 HIV+ | T ≤ 410 ng/dL | 25 |
| Collazos et al., 2009 [31] | Spain/not specified | Medical chart review | 188 HIV+ | T < 2.6 ng/mL | 3.7 |
| Wunder et al., 2007 [96] | Switzerland/not specified | Cohort study | 139 HIV+ | FT below the age-adjusted normal limits | 70 |
| Wagner et al., 1995 [92] | US/not specified | Study setting (baseline data) | 234 HIV+ | TT ≤ 400 ng/dL | 38 |
| Rietschel et al., 2000 [78] | US/November 1997–April 1999 | Cohort study | 90 HIV+ | Low TT/FT | 19 |
| Respiratory disease | |||||
| Laghi et al., 2005 [64] | US/not specified | Cohort study | 101 men with COPD | FT < 50 pg/mL | 37.6 |
| Kirbas et al., 2007 [57] | Turkey/November 2004 to July 2006 | Medical clinic | 96 men with obstructive sleep apnea | TT < 2.62 ng/mL | 19.8 |
| Halabi et al., 2011 [51] | US/not specified | Medical clinic | 104 men with COPD | FT < 35 pg/mL for patients <70 years; FT < 30 pg/mL for patients ≥70 years | 33.6 |
| Depression | |||||
| Hintikka et al., 2009 [53] | Finland/1998, 1999, 2001, and 2005 | Population-based |
|
FT < 160 pmol/L plus BDI scores: | |
| +depressive symptoms | 28.8 | ||||
| −depressive symptoms | 10.5 | ||||
| McIntyre et al., 2006 [68] | Canada/not specified | Medical clinic | 94 men with and without depression | TT < 12.14 nmol/L: | |
| Men with depression | 34 | ||||
| Men without depression | 6 | ||||
| Cancer | |||||
| Tromp et al., 2011 [91] | The Netherlands/2008 | Medical clinics | 565 male 5-year survivors of childhood cancer | T < 11.0 nmol/L | 12.4 |
| Lackner et al., 2007 [63] | Austria/not specified | Medical clinic | 68 men with testicular cancer | T < 3.0 ng/mL + symptoms | 33.8 |
| Pühse et al., 2011 [75] | Germany/2004 | Medical clinic | 376 men with testicular germ-cell cancer (TGCC) | TT 2.84 ng/mL or 9.85 nmol/L | ~25 |
| Fleishman et al., 2010 [43] | US/July 2007 through July 2009 | Medical clinic | 428 male patients with nontestosterone-related cancer | TT < 300 ng/dL | 48 |
| Salonia et al., 2011 [80] | Italy/June 2006 and June 2008 | Medical clinic | 673 prostate cancer patients | TT < 3 ng/mL | 21.4% |
| Greenfield et al., 2007 [46] | US/not specified | Medical clinic | 389 cancer survivors | TT < 10 nmol/L | 13.6 |
| Rajagopal et al., 2004 [76] | US/not specified | Chart review | 40 cancer survivors | <345 ng/dL | |
| All | 65 | ||||
| On opioids | 90 | ||||
| Not on opioids | 40 | ||||
| Schatzl et al., 2001 [82] | Austria/January 1999–April 2000 | Medical clinic | 156 men with prostate cancer | TT <3.0 ng/mL | 33.3 |
| Romerius et al., 2009 [79] | Sweden/2004–2006 | Medical clinic | 292 men with history of childhood treatment of cancer or brain tumor | T < 10 nmol/L | 23 |
| Rheumatoid arthritis (RA) | |||||
| Tengstrand et al., 2002 [89] | Sweden/not specified | Medical clinic | 104 patients with RA |
|
31.7 |
| Osteoporosis | |||||
| Fink et al., 2006 [42] | US/March 2000 to April 2002 | Medical clinics | 3674 men with osteoporosis | TT < 200 ng/dL | 6.9 |
| Clapauch et al., 2008 [30] | Brazil/January 2005 | Medical clinic | 216 men with osteoporosis | cFT < 6.5 ng/dL + symptoms | 25 |
| Chronic kidney disease (CKD) | |||||
| Yilmaz et al., 2011 [97] | Turkey/March 2006 to June 2010 | Medical clinic | 239 men with CKD | TT 10 nmol/L | 33 |
| Carrero et al., 2011 [28] | Sweden/June 1999 and October 2007 | Medical clinics | 260 men with ESRD | TT < 10 nmol/L | 44 |
| Kyriazis et al., 2011 [60] | Greece/October 2005 and December 2006 | Medical clinic | 111 male hemodialysis patients | TT < 8 nmol/L | 49 |
| Albaaj et al., 2006 [21] | UK/not specified | Medical clinic | 214 male patients with CKD or ESRD | TT < 10 nmol/L | 26.2 |
| Myopathies | |||||
| Travison et al., 2008 [90] | Canada/3-year study | Medical clinic | 59 men with myopathies | 10–30 nmol/L | 54 |
| Durga et al., 2011 [41] | US/July 2006 to April 2007 | Medical clinic | 60 men with spinal cord injuries | TT < 325 ng/dL | 43.3 |
| Okun et al., 2002 [73] | US/not specified | Medical clinic | 68 men with PD | FT < 70 pg/mL | 35 |
| Abbasi et al., 1994 [20] | US/not specified | Medical clinic | 102 men with poststroke hemiplegia | TT < 2.5 percentile of the healthy old men | 17 |
| Miscellaneous diseases | |||||
| Schatzl et al., 2000 [81] | Austria/October 1998 to May 1999 | Medical clinic | 312 men with lower urinary tract symptoms | T < 3 ng/mL | 22.1 |
| Rhoden et al., 2005 [77] | Brazil/January 2000 to December 2001 | Medical clinic | 746 urological patients | TT < 13.87 nmol/L | 24.5 |
| Taddesse et al., 2012 [86] | US/September 21 to December 21, 2010 | Medical clinic | 34 men with sickle cell | T ≤ 250 ng/dL | 24 |
| Okun et al., 2004 [72] | US/not specified | Chart review | 118 men with PD + AD |
|
|
| Moreno et al., 2009 [69] | US/January 2006 to January 2008 | Medical chart review | 121 men with Peyronie′s disease | TT < 300 ng/dL | 29.5 |
- CHD: chronic heart failure; CKD: chronic kidney disease; ED: erectile dysfunction; ESRD: end stage renal disease; MetS: metabolic syndrome; RA: rheumatoid arthritis; T2DM: type 2 diabetes mellitus; PD: Parkinson’s disease; AD: Alzheimer’s disease.
3.2.1. Population-Based Studies
The prevalence of hypogonadism in the 4 population-based studies ranged from 2.1% to 12.8% [2–5] (Table 2). Sample sizes in these studies ranged from 1,490 to 2,966 participants and the mean (SD) ages of the study participants ranged from 47.3 (12.5) to 59.15 (10.8) years. Mean age was not reported in one of the studies [3]; however, the sample was equally divided among men in their 40s, 50s, and 60s [3]. The studies were conducted in Europe (Belgium, Estonia, Hungary, Italy, Poland, Spain, Sweden, and UK) and USA.
The operational definition of hypogonadism used across studies varied considerably: the Study of Health in Pomerania (SHIP, 1997–2001) (Germany) [5] used the most liberal definition of hypogonadism (TT < 300 ng/dL, no symptom criteria) and reported the highest prevalence at 12.8%; the European Male Aging Study (EMAS, 2003–2005) [4] used the most stringent definition of symptomatic hypogonadism (TT < 317 ng/dL plus 3 specific symptoms of decreased frequency of morning erections, sexual thoughts, and erectile dysfunction) and reported the lowest prevalence at 2.1%. The Boston Area Community Health Survey (BACH, 2002–2005) [3] and the Massachusetts Male Aging Study (MMAS, 1987–1989) [2], both USA based, used similar operational definitions of symptomatic hypogonadism (TT cut-offs of <300 ng/dL plus ≥1 specific symptom or ≥2 nonspecific symptoms (BACH) and either TT <200 ng/dL plus ≥3 symptoms or TT 200–400 ng/dL plus FT <8.91 ng/dL plus ≥3 symptoms (see Table 2)) with reported prevalence of 5.6% (BACH) and 6.0% (MMAS), respectively.
3.2.2. Community-Based Studies
The prevalence of hypogonadism among the 3 community-based studies ranged from 9.5% to 31.2% (Table 2) [6–8]. All studies were cross-sectional and were conducted in Austria, China (Hong Kong), and Malaysia. The sample sizes ranged from 247 to 351 men, and the mean (SD) ages of men varied from 54 (NR) to 75.8 (0.4) years. The operational definition of hypogonadism varied considerably, with TT cut-offs ranging from <200 to <400, with and without low FT, and with and without symptoms. The highest prevalence (31.2%) was reported in a study of men with a mean age of 75.8 years [6].
3.2.3. Primary Care/Screening-Based Studies
Seven cross-sectional studies, conducted in Brazil, Chile, India, Italy, Taiwan, and USA, examined the prevalence of hypogonadism in a primary care or screening-based settings [9–15] (Table 2). Prevalence was higher than that observed in the general population surveys, ranging from 12% [10, 12] to 38.7% [13], with the majority around 20% [11, 12, 14, 15]. The sample sizes ranged from 96 to 2,719 men and the mean (SD) age of the participants in the studies ranged from 53.1 (NR) to 61.9 (7.5) years. Six of the studies used low TT to define hypogonadism [10–15] and one study used low bioavailable T and symptoms [9]. Using TT <300 ng/dL as a marker, prevalence was 19.3% [15], 19.8% [14], 24.1% [12], 24.2% [11], and 38.7% [13]. One study used a stricter TT threshold (TT < 230 ng/dL) with a lower resulting prevalence of 12% [10]. Adding symptoms as a criterion, the prevalence decreased from 24.1% to 12% [12] and from 24.2% to 20.4% [11].
3.2.4. Clinical Condition-Based Studies
A total of 78 studies were identified and assessed prevalence of hypogonadism among patients with specific medical conditions (Table 3). The prevalence varied considerably by medical condition. Hypogonadism occurred commonly among patients with type 2 diabetes mellitus (T2DM). Thirteen studies conducted in the UK, Australia, India, Italy, and USA showed the prevalence of hypogonadism in patients with T2DM ranging from 4.4% to above 45% [23, 25, 29, 36, 40, 45, 47, 48, 50, 56, 61, 71, 87] varying based on the definition of hypogonadism used: prevalence ranged from 4.4% to 36% using TT <8 mmol [23, 29, 45, 50, 56, 61, 71], from 24.5 to 43% using TT <10.4 mmol [36, 40, 47, 48], and from 34.7% to 45% using TT <12 mmol [25, 87]. Hypogonadism was also common among obese patients with prevalence ranging from 15% to 78.8% [22, 54, 74] and among patients with MetS, ranging from 30 to 35% [27, 62, 83]. When combined with obesity or metabolic syndrome (MetS), prevalence estimates among patients with T2DM were higher (51% [39] and 43% [70] for patients with T2DM and obesity and MetS, resp.).
Prevalence of hypogonadism in patients with cardiac disease [44, 55, 65, 67, 84, 94, 95], erectile dysfunction [24, 26, 32–35, 37, 49, 52, 59, 66, 85, 88], HIV [31, 38, 58, 78, 92, 93, 96], respiratory disease [51, 57, 64], depression [53, 68], cancer [43, 46, 63, 75, 76, 79, 80, 82, 91], rheumatoid arthritis [89], osteoporosis [30, 42], chronic kidney disease [21, 28, 60, 97], sickle cell disease [86], myopathies [20, 41, 52, 73, 90], urological diseases [77, 81], Peyronie’s disease [69], and Alzheimer’s and Parkinson’s disease [72] is detailed in Table 3.
3.2.5. Prevalence of Hypogonadism by Age
A total of 10 studies, conducted in Chile, China, Europe, Italy, and USA, examined changes in prevalence of hypogonadism with age (Table 4) [2, 3, 8, 9, 12, 13, 16–19]. Sample sizes ranged from 96 to 2,162 and mean (SD) ages ranged from 47.3 (12.5) to 61.9 (7.5) years. Five of the studies were population-based[2, 3, 17–19], one was community-based [8], and 4 were primary care-/screening-based [9, 12, 13, 16]; one was longitudinal [17] and 9 were cross-sectional [2, 3, 8, 9, 12, 13, 16, 18, 19].
| Author and year (study name) | Country and years of data collected | Study design | N | Age, mean (SD), and range | Definition of hypogonadism used | Prevalence, per age group, years (%) | |
|---|---|---|---|---|---|---|---|
| Harman et al., 2001 (BLSA) [17] | USA, 40-year period | Population-based | 890 | 53.8 (16.0), NR |
|
20–29 | <5 |
| 30–39 | <5 | ||||||
| 40–49 | <10 | ||||||
| 50–59 | 12 | ||||||
| 60–69 | 19 | ||||||
| 70–79 | 28 | ||||||
| >80 | 49a | ||||||
| Wu et al., 2010 [19] (EMAS) |
|
Population-based | 2966 | 59.15 (10.82), 40–79 |
|
40–49 | 0.1 |
| 50–59 | 0.6 | ||||||
| 60–69 | 3.2 | ||||||
| 70–79 | 5.1b | ||||||
| Araujo et al., 2007 [3] (BACH) | USA, 2002–2005 | Population-based | 1475 | 47.3 (12.5) |
|
30–39 | 1.1 |
| 40–49 | 4.1 | ||||||
| 50–59 | 8.1 | ||||||
| 60–69 | 9.1 | ||||||
| 70–79 | 22.1c | ||||||
| Araujo et al., 2004 [2] (MMAS) | USA, 1987–1997 | Population-based | 1691 | NR (NR), 40–70 |
|
40–49 | 4.1 |
| 50–59 | 4.5 | ||||||
| 60–70 | 9.4d | ||||||
| Li et al., 2005 [18] | China, 2002-2003 | Population-based study | 1080 | NR (NR), 20–>70 | cFT < 0.3 nmol/L | 40–49 | 13.0 |
| 50–59 | 31.8 | ||||||
| 60–69 | 30.1 | ||||||
| >70 | 46.7e | ||||||
| Wong et al., 2006 [8] | China, 2003-2004 | Community-based | 252 | 54 (NR), 45–64 |
|
45–49 | 8.2 |
| 50–54 | 6.3 | ||||||
| 55–59 | 10 | ||||||
| 60–65 | 16.7 f | ||||||
| Mulligan et al., 2006 [13] (HIM) | USA, 2003-2004 | Primary care patients | 2162 | 60.5 (10.33), 45–96 | TT < 300 ng/dL | 45–54 | 34 |
| 55–64 | 40.2 | ||||||
| 65–74 | 39.9 | ||||||
| 75–84 | 45.5 | ||||||
| 85+ | 50 | ||||||
| 50–54 | 6.33 | ||||||
| 55–59 | 10 | ||||||
| 60–64 | 16.67g | ||||||
| Liu et al., 2009 [12] | Taiwan, 2007-2008 | Health screening clinic in a medical center | 734 | 57.4 (6.7), 43–87 | TT < 300 ng/dL | 40–49 | 16.5 |
| 50–59 | 23 | ||||||
| 60–69 | 28.9 | ||||||
| ≥70 | 37.2h | ||||||
| Di Luigi et al., 2010 [16] | Italy, NR | Health screening clinic* | 183 | 61.9 ± 7.5 years (range 50–75). | TT < 230 ng/dL | 50–59 | 5.4 |
| 60–69 | 10 | ||||||
| 70–79 | 27.5i | ||||||
| Blümel et al., 2009 [9] | Chile, NR | Primary care-based (health services center) | 96 | 55.1 ± 12.0 years (range 40–85 years) |
|
40–54 | 17.9 |
| 55–69 | 29.2 | ||||||
| ≥70 | 66.7j | ||||||
- TT: 200 ng/dL = 6.94 nmol/L; 230 ng/dL = 8 nmol/L; 300 ng/dL = 10.4 nmol/L; 317 ng/dL = 11 nmol/L; 325 ng/dL = 11.3 nmol/L; 350 ng/dL = 12 nmol/L; FT: 5 ng/dL = 0.17 nmol/L; 8.9 ng/dL = 0.3092 nmoL/L.
- ADAM: androgen deficiency in the aging male; BACH: Boston Area Community Health; BT: bioavailable testosterone; cFT: calculated free testosterone; EMAS: European Male Aging Study; HIM: hypogonadism in males; MMAS: Massachusetts Male Aging Study; NR: not reported; SHIP: Study of Health in Pomerania; TT = total testosterone.
- aNo statistical test done; bno statistical test done; call pairwise comparisons, P < 0.05 over 70 versus all other age groups; dP values for trend <0.001; echi square P < 0.000; fnot significant; gno statistical test done; hP < 0.019 for trend; iP < 0.01 versus age 50–59 and age 60–69; jP value for trend P < 0.0004.
- †Specific symptoms include low libido, erective dysfunction, or osteoporosis; the nonspecific symptoms include sleep disturbance, depressed mood, lethargy, or low physical performance.
- ††Symptoms include loss of libido, erectile dysfunction, depression, lethargy, inability to concentrate, sleep disturbance, irritability, and depressed mood.
- #Specific signs/symptoms are low libido, erectile dysfunction, or osteoporosis.
- *To evaluate risks associated with participation in sports in a group of healthy athletic men.
- **Positive answer to items 1 or 7, or any 3 other questions on the ADAM questionnaire.
The prevalence of hypogonadism increased with each increasing age category [2, 3, 8, 9, 12, 13, 16–19], with the rare exception within certain age categories [3, 8] (Table 4). Three of the studies reported significant P values for trend [2, 9, 12], 3 reported significant differences between older and younger age categories [3, 9, 18], 2 studies did not conduct statistical analysis for differences in prevalence by age [17, 19], and one study was nonsignificant [8]. One cross-sectional study [13] reported a 17% (95% CI, 1.08–1.27) increase in risk of hypogonadism, for every 10-year increase in age. Prevalence ranged from approximately 0.1% to 16.5% among men aged 40–49, from 0.6% to 31.8% among men aged 50–59, from 3.2% to 30.1% among men aged 60–69, up to 66.7% among men of age ≥70 years [2, 3, 12, 17–19], and 49% in the study that assessed the 80+ age category [17] (not all studies used the same age cut-offs).
3.3. Incidence of Hypogonadism
In two population-based studies (SHIP [5] and BACH [2]), in which incidence was examined, the reported incidence was 12.3 cases per 1,000 person-years in the US and 11.7 cases per 1000 person-years in Germany.
3.4. Treatment Rates for Hypogonadism
The proportion of patients receiving treatment was defined as the percentage of men with hypogonadism who were receiving testosterone therapy. Only 4 studies were identified and reported treatment proportions: one population-based [98], one primary care-based [13], and 2 clinical condition-based studies [38, 79]. The population-based (BACH) and clinical-based studies (HIM) reported that 10% [98] and 12% [13] of patients were receiving treatment for hypogonadism, respectively. Among the clinical condition-based studies, 12.1% of 144 childhood cancer survivors [79] and 38% of 296 men with HIV were on treatment for hypogonadism [38].
4. Discussion
In this systematic review, we identified 97 studies that met our inclusion/exclusion criteria for assessing the epidemiology of hypogonadism. The prevalence of hypogonadism was high and varied according to the operational definition of hypogonadism used and the population studied. The prevalence of hypogonadism in the population-based, community-based, and primary care- or screening-based studies ranged from 2.1 to 12.8% [9–15], from 9.5 to 31.2% [9–18], and from 12 to 38.7% [9–15], respectively. The prevalence was higher among clinical-based populations with specific conditions. For example, among patients with T2DM, obesity, and MetS, the prevalence ranged from 4.4 to >50% [23, 25, 29, 36, 40, 45, 47, 48, 50, 56, 61, 71, 87], from 15 to 78.8% [22, 54, 74], and from 30 to 35% [27, 62, 83], respectively.
In most instances, studies showed a linear relationship between prevalence and the degree of liberalness in the operational definition of hypogonadism [4, 5, 7, 12]. For instance, Haring et al. [5] reported prevalence estimates below TT levels of 8.0, 8.7, 10.4, and 12.0 nmol/L, which were 3.4%, 4.5%, 12.8%, and 21.6%. The added criteria of symptoms typically ensued a lower prevalence due to increased stringency of diagnosis. Among the population- and community-based studies, those that used a more stringent definition of hypogonadism, which included symptoms and low T, reported the lowest prevalence, ranging from 2.1% to 9.52% [2–4, 7, 8], while those using a more liberal definition of hypogonadism (low T only) reported higher prevalence, ranging from 19.1% to 31.2% [5–7]. The association between definition of hypogonadism and prevalence was also apparent among the primary care-/health screening-based studies (12–39%).
Prevalence also varied by age. Hypogonadism was consistently found to increase with age for studies reporting data by age (REF), with one study citing an increase of 17% (95% CI, 1.08–1.27) in risk of hypogonadism, for every 10-year increase in age [13]. One study that had a considerably older cohort (mean age of 75.8 years) [6] reported the highest prevalence (31.2%) among community-based studies. It was estimated in a 2007 publication that there were 4.7 million American men aging 30–79 years with symptomatic androgen deficiency [3]. It was projected that there will be as many as 6.5 million American men aging 30–79 years with symptomatic hypogonadism by 2025, an increase of 38% from the year 2000 population estimates, due to an aging population [3].
Despite the significant prevalence of hypogonadism, data suggest that the vast majority of hypogonadal men are not receiving treatment for this condition [13, 98]. Reasons for this low treatment proportion have not been definitively determined; however, contributing factors may include inadequate knowledge base among physicians regarding hypogonadism and uncertainty regarding diagnostic criteria. The main indication for testosterone replacement therapy is sexual dysfunction with symptoms of reduced libido and/or erectile dysfunction. This may not be an issue to some men and therefore they are not treated or subjects may decline therapy. Also, individuals may not be able to afford therapy in countries where people have to pay for their prescriptions and healthcare. Furthermore, with the lack of long-term placebo-controlled safety studies, some clinicians may have concerns especially in the older man in regard to risks of prostate cancer and cardiovascular disease, even though there is limited evidence from current published studies or meta-analyses. The only study which did show a possible relationship between testosterone replacement therapy and cardiovascular events was the TOM trial [99]. However, this study involved the administration of larger testosterone doses than those used in normal clinical practice and was not sufficiently powered to detect an increase in cardiovascular events. A similar trial where routine doses of testosterone were used did not detect any increase in cardiovascular events [100].
Results of this review highlight the fact that there is no standardized definition used in the hypogonadism epidemiologic research. The hypogonadism literature reports wide-ranging prevalence based not only on the different populations or subpopulations studied, but also on the use of diverse biochemical cut points or varied choices of symptoms. The actual prevalence of disease that a clinician will treat in his or her office may in fact be higher than what we found in this review, as clinicians may use a more liberal definition of hypogonadism to base their treatment decisions on.
The studies in this review represented a wide geographic distribution with samples from USA, Europe, Asia, and South America. The prevalence of hypogonadism may vary by geographical location, as recently demonstrated in a large (n = 5003) international cohort of older men (>65 years of age) showing important geographical differences in concentrations of T [101]. However, differences in patient populations and operational definitions used across studies made it difficult to interpret the findings based on geography in the current review.
We can expect approximately 12 new cases of hypogonadism per 1,000 person-years [2, 5] and approximately 481,000 new cases of hypogonadism per year in US men aged 40–69 years [2]. It has been projected that there will be as many as 6.5 million US men aging 30–79 years with symptomatic AD by 2025 [3], an increase of 38% from the year 2000 population estimates, due to an aging population. While there is no conclusive evidence from this review that the prevalence of hypogonadism in the general population is increasing over time, there is evidence that the prevalence of hypogonadism does increase with age and with certain comorbidities such as T2DM and obesity, all of which are increasing in the general population. It follows that there may well be an increasing proportion of older men who will benefit from treatment for hypogonadism. As the proportion of the world’s population in the older ages continues to increase, the importance of studying, diagnosing, and treating male hypogonadism will also rise.
The mainstay treatment of low testosterone is testosterone replacement therapy (TRT), although there is indication that modification of diet and exercise regimes have the ability to improve testosterone levels [99]. Despite the significant prevalence of hypogonadism, self-reported data suggest that the vast majority of hypogonadal men are not receiving TRT treatment for this condition [13, 98]. Reasons for this low treatment proportion have not been definitively determined; however, contributing factors may include inadequate knowledge base among physicians regarding hypogonadism and uncertainty regarding diagnostic criteria. The main indication for TRT is sexual dysfunction, which, for some men, may not affect their daily lives, and therefore they choose not to be treated. Also, individuals may not be able to afford therapy in countries where people have to pay for their prescriptions and healthcare.
Another impeding factor for prescribing TRT may be a concern over the potential risk of cardiovascular disease and prostate cancer. The safety of TRT lacks examination in large, prospective, and adequately powered controlled trials [100]. Smaller studies have been published, some suggesting an association between testosterone replacement therapy and cardiovascular adverse events [100]. The TOM trial was terminated early due to an increased frequency of cardiovascular events in the men treated with testosterone [102]. However, this study involved the administration of larger testosterone doses than those used in normal clinical practice and was not sufficiently powered to detect an increase in cardiovascular events. A similar trial, which used routine doses of testosterone, did not detect any increase in cardiovascular events [103]. To address the question of safety and in lieu of long term, prospective safety data, the Endocrine Society Task Force on Testosterone Use in Adult Men commissioned a meta-analysis of randomized and observational studies with long-term follow-up in testosterone therapy in 2010. The analyses of 51 studies found no significant effect of TRT on mortality, prostate, or cardiovascular outcomes [100]. More recently, a retrospective database study of 8709 male veterans who underwent coronary angiography between 2005 and 2011 reported an increased risk (absolute risk increase: 5.8% (95% CI: −1.4% to 13.1%)) of experiencing an adverse cardiovascular event (all-cause mortality, MI, or ischemic stroke) among TRT versus nontreated men at 3 years after coronary angiography [104]. Although this study garnered media attention, it is limited by serious methodological concerns which put the results in question.
4.1. Limitations
A general limitation in hypogonadism research is the lack of a standardized operational definition of hypogonadism. Recently, professional societies have proposed operational definitions which include criteria of specific signs and symptoms plus biochemical low TT, typically defined as TT <300 ng/dL (<10.4 nmol/L) [1]. However, the literature is confounded by numerous studies using a variety of different definitions: some include sign and symptom criteria and others do not; biochemical TT thresholds used range from <8 nmol/L to <12 nmol/L, others use an FT criterion and no TT (as men with normal TT may be quite symptomatic in clinical due to low FT), and others use a TT and FT criterion. The varying definitions used make comparisons between studies difficult.
The proportions of patients receiving T therapy in the population were obtained from one population-based study and one primary care-based study, both of which reported on the number of participants who were receiving T therapy. Medication use, including use of T therapy, was assessed by patient self-report in the studies. Self-report of medication use is subjective and is at risk of both reporting bias and measurement bias. A more accurate measurement of treatment proportions could be obtained from chart review or commercial claims database study and may be an interesting objective for a future study.
As with all systematic reviews, publication bias, which can lead to bias if only positive studies or studies with a statistically significant difference are published, is an issue in this review. Another limitation of this review is that a quality appraisal of the individual studies was not conducted. Although methodological aspects of the individual studies, which may have influenced outcomes or comparisons between studies, were considered and commented on during the assessment of the evidence, a systematic quality assessment of the studies was not carried out.
5. Conclusions
This literature review suggests that there is potentially a significant burden of hypogonadism in the general population. The burden of hypogonadism increases with age and in the presence of certain clinical conditions, such as T2DM and obesity. Data suggested that the vast majority of hypogonadal men in the general population are not receiving treatment. This may be the result of underdiagnosis of the disease, due to lack of awareness by patients and/or physicians and the irregularities surrounding the diagnostic criteria.
Disclosure
The authors met criteria for authorship as recommended by the International Committee of Medical Journal Editors and are fully responsible for all content and editorial decisions and were involved in all stages of paper development. This original paper has not been previously presented in whole by the authors. A portion of the information has been presented as a poster entitled “The prevalence, incidence and treatment rates of hypogonadism in men across geographies: a systematic review of the literature” at the ISPOR, May 18–22, 2013, New Orleans, LA.
Conflict of Interests
Victoria Zarotsky and Wendy Carman are employees of Optum and were contracted by Merck & Co., Inc., to conduct this study. Donna Coffin is employee of Boolean Research Consulting Services who was contracted by Optum. Ming-Yi Huang is a Postdoctoral Fellow funded by Merck & Co., Inc. Puneet K. Singhal is employed by Merck & Co., Inc. T. Hugh Jones has received research grants from BayerHealthcare, UK, and honoraria for educational lectures and/or advisory boards from BayerHealthcare, Clarus, Lilly, Merck, and Prostrakan. T. Hugh Jones and Abraham Morgentaler have served as Consultants to Merck & Co., Inc., for this study.
Acknowledgments
This study was conducted by Optum located in Eden Prairie, Minnesota, USA, and was funded by Merck & Co., Inc., West Point, PA. This study was supported by Merck & Co., Inc.




