Growth and Characterization of Agar Gel Grown Brushite Crystals
Abstract
Brushite [CaHPO4·2H2O] or calcium hydrogen phosphate dihydrate (CHPD) also known as urinary crystal is a stable form of calcium phosphate. The brushite crystals were grown by single and double diffusion techniques in agar-agar gel at room temperature. Effects of different growth parameters were discussed in single diffusion and double diffusion techniques. Good quality star, needle, platy, rectangular, and prismatic shaped crystals in single diffusion and nuclei with dendritic growth were obtained in double diffusion. These grown nuclei were characterized by scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and thermogravimetric analysis (TGA). SEM has shown the different morphologies of crystals; FTIR has confirmed the presence of functional groups; crystalline nature was supported by XRD, whereas the TGA indicates total 24.68% loss in weight and formation of stable calcium pyrophosphate (Ca2P2O7) at 500°C.
1. Introduction
Calcium oxalates, phosphates, and their hydrates are very common in calcium renal stones. Some of the oxalates are found in either pure or in mixed form with phosphate and also reported with uric acid or ammonium urates [1, 2]. Calcium oxalate monohydrate [3, 4] and calcium oxalate dihydrate [5] are common constituents of calcium urinary crystal while hydroxypatite [6], carbonate apatite [7], and brushite [8–11] are in calcium phosphate crystals. Brushite [CaHPO4·2H2O] provides a medium to grow octacalcium phosphate [12] and hydroxypatite [13–16] urinary crystals, however used as a precursor to form apatite, which has an important application in bone formation [17, 18].
Gel method is the most versatile and simple technique for growing urinary crystals [19–21]. In this method, gel acts as an inert and viscous medium for the growth of these crystals [22, 23].
The growth inhibition study of brushite crystals are reported in silica gel by adding tamarind, tartaric acid, and citric acid [24, 25] and in the presence of sodium fluoride [26]. Lim et al. [27] have grown three-dimensional flower like brushite crystals from high internal phase emulsion processing route; whereas Kumar and Kalainathan [28] have grown in the presence of magnetic field and observed the effect on growth of these crystals. Parekh et al. [29] have also observed the growth inhibition and dissolution of urinary type micro-CHPD crystals. Most of the brushite crystals reported are grown in silica hydrogel; however the growth of these crystals is not reported in agar-agar gel.
In the present work, the brushite crystals were grown in agar-agar gel at ambient temperature and were characterized by scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and thermogravimetric analysis (TGA).
2. Experimental
In the present study, brushite crystals were grown by single and double diffusion techniques [30]. In this method, glass test tubes of size 15 cm in length and 1.8 cm in diameter and U-tube of size 25 cm in length and 2.5 cm in diameter were used as crystallization vessels. Agar gel (Himedia) solution was prepared by the mixing (0.25 to 1.0 gm) of agar powder in 100 mL double distilled water at boiling temperature. Potassium dihydrogen phosphate (KDP) (Merck) of concentration 0.5–1.0 M and calcium chloride (Qualigens) of concentration 0.5–1.0 M were used as reactants.
For single diffusion techniques, the agar solution was mixed with the desired concentration and appropriate volume of calcium chloride. After setting and aging of gel, an aqueous solution of KDP was poured over the gel. No precipitation was observed; however after 1–8 days, nucleation was seen at the interstitial and inside the gel.
The same method was repeated by reversing the reactants. But no nucleation was observed in this method.
In double diffusion, agar solution was poured in U tube up to appropriate height. After setting and aging of gel, an aqueous solution of KDP was poured in one limb, while in other limb an aqueous solution of calcium chloride was poured over the gel. As both the solutions diffused in the gel, nucleation was observed after 10 days. This nucleation was further increased and took two months for its complete growth. After two months, well-shaped brushite crystals were grown inside the gel as shown in Figure 2.
These grown crystals were harvested from test tubes and were characterized by, SEM, FTIR, XRD, and TGA.
3. Result and Discussion
3.1. Growth
In single diffusion technique, many researchers [9, 17, 31] have observed Liesegang ring phenomena in silica gel; however no Liesegang ring phenomena is observed in the present study. After diffusion of supernatant in the gel, heavy nucleation was observed. To control nucleation various parameters such as concentration of gel, concentration of reactant, aging of gel, and volume of reactants were changed, which can be seen in Figure 1, and thus optimum conditions are established as shown in Table 1. Figure 2 shows some good quality star, needle, and transparent prismatic platy shaped crystals grown in single diffusion.
| Condition | Single diffusion | Double diffusion |
|---|---|---|
| % of gel | 0.5 | 1.0 |
| Con. of calcium chloride | 1 M | 1 M |
| Con. of KDP | 1 M | 1 M |
| Gel setting period | 24 hrs | 24 hrs |
| Gel aging | 48 hrs | 48 hrs |
| Period of growth | 60 days | 80 days |
| Temperature | Room temp. | Room temp. |
| Quality | Transparent | Opaque |
| Shape | star, needle, platy, rectangular, and prismatic shaped | Dendritic shaped |




In double diffusion technique, nucleation was started after diffusion of both reactants in gel. This growth can be seen in Figure 3, and their optimum conditions are shown in Table 1. Interesting phenomenon was observed in case of crystals grown in double diffusion. After formation of crystals, the growth was again started from edges and found in dendratic shape. Figure 4(a) shows the 20X image of dendratic shaped brushite crystals in double diffusion. Figure 4(b) shows that a bulk nucleus is surrounded by several tiny nuclei at the interstitial, but not at the centre. This one can be easily understood by considering free energy changes associated with the formation of nuclei, and association of several tiny nuclei at the edges may be due to the reason that the surface molecules are less well bound to their neighbors than are those in bulk [32].



3.2. Characterization
3.2.1. Scanning Electron Microscopy
The morphology of the crystals can be observed by SEM as shown in Figures 5(a) and 5(b). These figures show that the crystals are platy shaped and crowded by making some angles with c-axis. In Figure 5(a), microrectangular plate shape placed over the other morphology can be seen. Whereas Figure 5(b) shows that rods are inclined and crowded. The reason of formation of platy shape may be due to the growth of crystals in two dimensions.


3.2.2. Fourier Transform Infrared Spectroscopy
Figure 6 shows the recorded FTIR spectrum of brushite crystal.
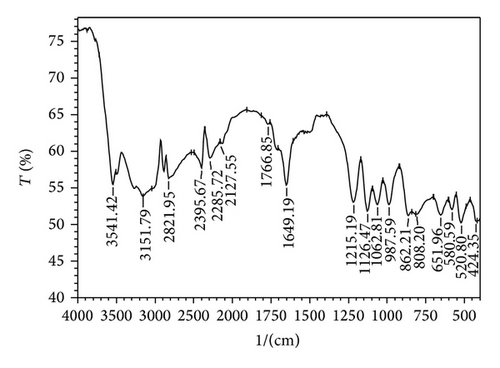
The absorption peaks at 3541.42 cm−1 [33, 34] and 3151.79 cm−1 [9] are attributed to O–H stretching. The peak at 1649.19 cm−1 [35] is assigned to that of O–H bending, whereas the weak absorptions at 2395.67 and 1766.85 are associated to [9], while stretching vibrations associated with P=O were observed at 1215.19, 1126.47, and 1062.81 cm−1 [17]. The P–O–P asymmetric stretching vibrations give rise to absorptions at 987.59, 862.21, and 808.20 cm−1 [17]. Two bands at 580.59 and 520.80 cm−1 are due to acid phosphate (H–O–) P=O bond vibration [17, 36]. FTIR spectroscopy confirmed the growth of brushite crystals due to the presence of water of crystallization, P=O bond, and O–H bond [9]. The assigned vibrations with observed wave numbers are given in Table 2.
| Sr. no. | Observed wave number in cm−1 | Bonds/vibrational assignments |
|---|---|---|
| 1 | 3541.42 | O–H stretching of water |
| 2 | 3151.79 | O–H stretching of water |
| 3 | 2395.67 | Weak absorption |
| 4 | 1766.85 | Weak absorption |
| 5 | 1649.19 | H–O–H symmetric bending vibrations |
| 6 | 1215.19 | PO4 P=O associated stretching vibrations |
| 7 | 1126.47 | PO4 bond, P=O stretching vibrations |
| 8 | 1062.81 | PO4 bond, P=O stretching vibrations |
| 9 | 987.59 | P–O–P asymmetric stretching bond |
| 10 | 862.21 | P–O–P asymmetric stretching bond |
| 11 | 808.20 | P–O–P asymmetric stretching bond |
| 12 | 580.59 | (H–O–) P=O bond acid phosphate |
| 13 | 520.80 | (H–O–) P=O bond acid phosphate |
3.2.3. X-Ray Diffraction
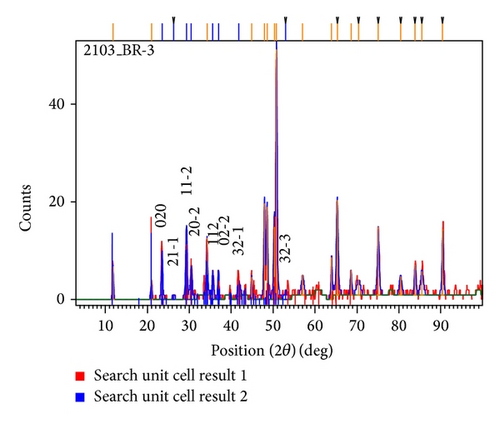
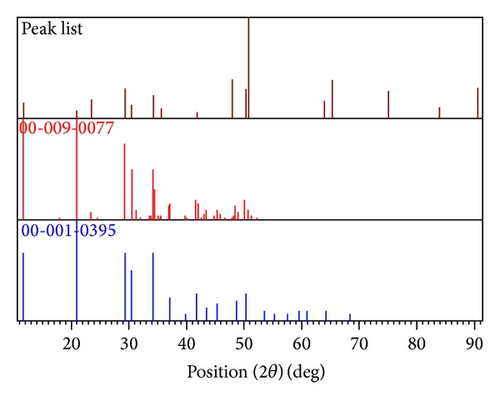
The X-ray diffractogram of the gel grown brushite crystal is shown in Figure 7. The crystalline phases and d-values obtained from the XRD pattern have been compared with those in the JCPDS data. The XRD pattern of Figure 7 matched with the JCPDS files [37, 38], indicating that the sample consisted of brushite crystalline. Table 3 gives the position, d-values, and matching peaks of the brushite crystals. Figure 8 shows plot identified phases of brushite crystal.
| Pos. (2θ) (deg) | Height (cts) | FWHM (2θ) (deg) | d-spacing (Å) | Rel. int. (%) | Matched by |
|---|---|---|---|---|---|
| 11.7472 | 7.91 | 0.2952 | 7.52726 | 15.48 | 001-0395 [37]; 009-0077 [38] |
| 20.9844 | 4.13 | 0.2952 | 4.23006 | 8.07 | 001-0395 [37]; 009-0077 [38] |
| 23.5421 | 9.55 | 0.2952 | 3.77594 | 18.69 | 009-0077 [38] |
| 29.3470 | 15.26 | 0.3444 | 3.04092 | 29.85 | 001-0395 [37]; 009-0077 [38] |
| 30.4833 | 7.03 | 0.3936 | 2.93012 | 13.75 | 001-0395 [37]; 009-0077 [38] |
| 34.2373 | 11.61 | 0.2952 | 2.61693 | 22.72 | 001-0395 [37]; 009-0077 [38] |
| 35.6589 | 4.96 | 0.5904 | 2.51580 | 9.70 | 009-0077 [38] |
| 41.8101 | 3.15 | 0.5904 | 2.15879 | 6.17 | 001-0395 [37]; 009-0077 [38] |
| 47.9518 | 19.64 | 0.2952 | 1.89564 | 38.43 | 009-0077 [38] |
| 50.3254 | 14.82 | 0.2952 | 1.81166 | 28.99 | 001-0395 [37]; 009-0077 [38] |
| 50.8203 | 51.11 | 0.2952 | 1.79517 | 100.00 | 009-0077 [38] |
| 63.9728 | 8.74 | 0.2952 | 1.45417 | 17.10 | 001-0395 [37] |
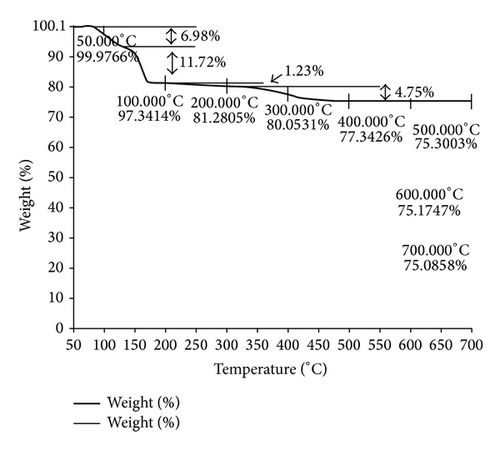
3.2.4. Thermogravimetry
4. Conclusions
The brushite crystals were grown by single and double diffusion techniques using agar-agar gel as a medium of growth. Different morphologies, namely, star, platy, rectangular and prismatic shapes in single diffusion and dendratic growth in double diffusion were obtained. The presence of various functional groups is confirmed by FTIR spectroscopy. X-ray diffraction spectra have confirmed the crystalline nature. The proposed chemical formula and crystal structure for the grown sample are in good agreement with the results obtained from TGA studies.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors are thankful to the Principal of Shri V. S. Naik Arts, Commerce and Science College, Raver (M. S.), for providing laboratory facilities, thankful to Dr. P. V. Dalal, Shri V. S. Naik College, Raver, for fruitful discussion, and thankful to the Director of SICART (Gujarat) for providing characterization facilities.




