Study of Complexation in Acetone-Chloroform Mixtures by Infrared Spectroscopy
Abstract
FTIR spectra of acetone-chloroform system with various component ratios were investigated within the spectral range 3950–4550 cm−1. Methods of multivariate curve resolution were applied to decompose the FTIR spectra into specific components of different composition. A method of decomposition based on structural model of solution which contains acetone, chloroform, and complex acetone/chloroform (1 : 1) was proposed. Results of both approaches are in good agreement within the range of measuring error.
1. Introduction
Acetone-chloroform mixture is a prominent example of a system with a pronounced negative deviation from ideal solution behavior. So it has been the subject of many theoretical and experimental researches [1–6]. The main problem is the structure and composition of associated species of these mixtures.
Infrared spectroscopy is powerful tool of investigation of liquid systems [7]. The IR spectra are very sensitive to structural changes caused by intermolecular interactions between components of solution. Multivariate regression can be used for the determination of quantitative information from IR spectra, such as number and concentration of species in the mixture [8].
The purpose of this work is quantitative analysis of acetone-chloroform mixture using model-free and model-based multivariate regression approaches.
2. Experiment
FTIR transmission spectra were measured with Thermo Scientific spectrometer Nicolet 6700 with a spectral resolution of 4 cm−1. Optical path length of the quartz cell was 1 mm. The heating of the sample almost did not occur during the measurements due to small value of absorption coefficient at excitation frequency. The temperature of liquid samples was 25 ± 0.2°C. The chloroform and acetone with purity 99.9% were used in this research. The concentration of components was changed from 0% to 100% (in volume %) with step 10%. Spectra recording was repeated 5 times for every sample. Thereafter the average spectra were calculated for every concentration and used in further analysis.
3. Theory
3.1. Multivariate Curve Resolution
There are many methods of decomposition experimental matrix D [12]. The one of most popular techniques is Multivariate Curve Resolution-Alternating Least Squares (MCR-ALS) [9]. MCR-ALS solves iteratively (2) by an alternating least squares algorithm which calculates concentration C and pure spectra ST matrices optimally fitting the experimental data matrix D. The algorithm comprises an iterative solving of two alternating least squares problems, that is, minimization of (2) over C for fixed S as well as minimization of (2) over S for fixed C. This optimization is carried out for a proposed number of components and using initial estimates of either C or ST.
3.2. Estimation of Band Boundaries of Feasible MCR Solutions
Complete resolution of a two-way data set without ambiguities is only possible in some favorable cases where selectivity [11] or local rank conditions [13] are present. When these resolution conditions are not present in the system, resolution without ambiguities is not possible even if constraints such as nonnegativity, unimodality, or closure are applied [14]. In these cases, instead of unique profiles, a range or band of feasible profiles fitting equally well the experimental data and fulfilling the physical and chemical constraints of the system has to be considered.
The method needs firstly the estimation of one of the feasible solutions within the range of all possible solutions, for instance, using alternating least squares with constraints [9]. Secondly, once this feasible solution is available, a nonlinear constrained optimization is initiated looking for the boundaries of the whole set of feasible solutions. For each component k we must solve problem (5). When the optimization is implemented as a minimization of the objective function as in (4), we will find the minimum band boundaries, whereas if the optimization is implemented as a maximization of the objective function with changed sign, we will find the maximum band boundaries functions. Implementation prior information about investigated system in form of equality and inequality constraints decreases the range of feasible solutions [15].
3.3. Model-Based Approach to Decomposition of Spectra
As it was mentioned above, multivariate curve resolution is model-free analysis and its solutions are not unique. Unlike this, model-based analysis gives unique solution and basic parameter of the process, that is, the rate constant and the equilibrium constant in equilibrium investigations [17]. Model-based approaches of fitting multivariate spectral data are based on mathematical relationships, which describe the measurements quantitatively. In chemical kinetics, the analysis is based on the kinetic model or reaction mechanism, which quantitatively describes the reactions and all concentrations in the solution under investigation. For equilibrium studies the analysis is based on the law of mass action [18].
If we know structural model of mixture and values of components concentrations before mixing, we can estimate the matrix of concentrations. We could get elements of matrix C by solving (8) at fixed value of Kc.
4. Results and Discussion
4.1. IR Measurements
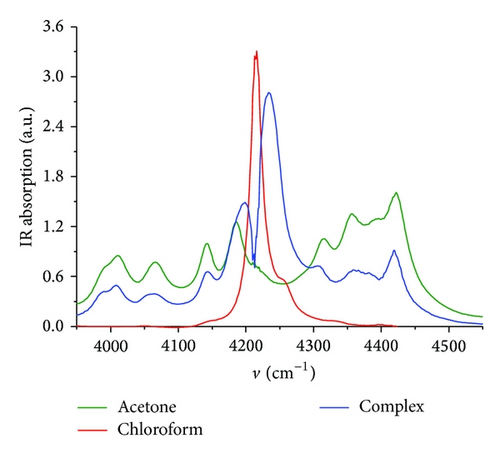
FTIR transmission spectra (Figure 1) were detected in spectral range 3950–4550 cm−1. This spectral range was chosen due to the weak absorption of overtones and composite frequencies in Near-IR region. Weak absorption value allows using the liquid cell with a relatively large value of the optical beam path (1 mm). Measurements in Mid-IR region require to use the optical beam path around 10–25 μm. Therefore, this range is not preferred for the quantitative analysis. However, the Near-IR range allows carrying out reproducible concentration analysis with relative accuracy of at least 1%.
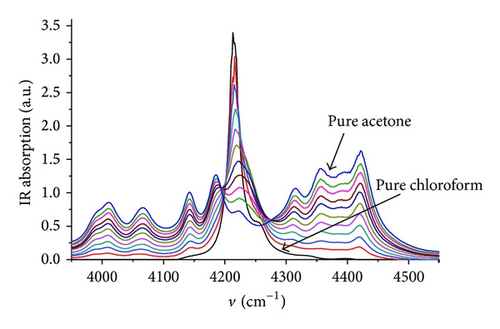
4.2. MCR-ALS Analysis
Our approach is based on a three-component MCR-ALS analysis of the FTIR spectra of water-methanol solutions. During ALS optimization nonnegativity, unimodality, and closure constraints were applied. We found that three components are required to obtain a good fit to the data (accuracy better than 1%). The “pure” components were identified as “free acetone (C3H6O)” and “free chloroform (CHCl3),” and the third component as “acetone-chloroform complex.” The graphical user interface (GUI) in the MATLAB environment developed by Jaumot et al. [20] was used for determination of concentration and spectral matrixes.
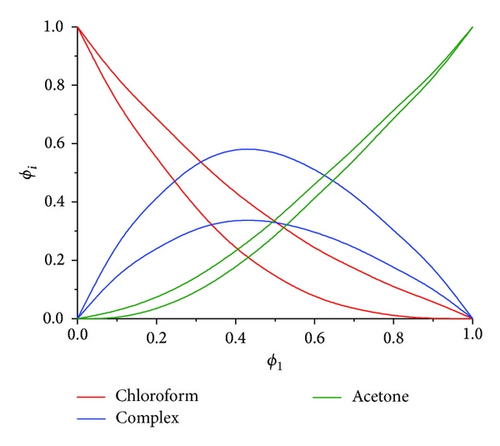
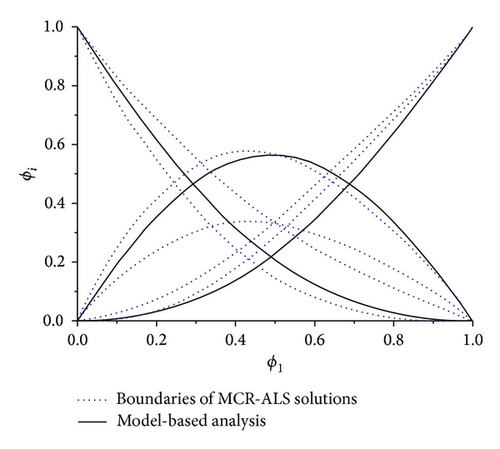
We used MCR-BANDS GUI [16] to decrease rotational ambiguity of obtained solutions; in other words it was used to solve nonlinear constrained optimization problem (5). MCR-ALS solutions were used as initial estimation and nonnegativity, unimodality, and closure constraints were also applied. The results on the resolved FTIR spectra (ST) and the concentrations (C) (band boundaries of feasible solutions) are shown in Figure 2. ϕ1 is volume fraction of acetone before mixing; ϕi, i = A, B, and AB, is volume fraction of species in the mixture.
4.3. Model-Based Analysis
We can obtain volume fraction of AB complex by solving (17) with known values of K and ϕ1, and thus we can obtain volume fraction of species A and B using (16). Matrix C can be obtained by changing values of ϕ1.
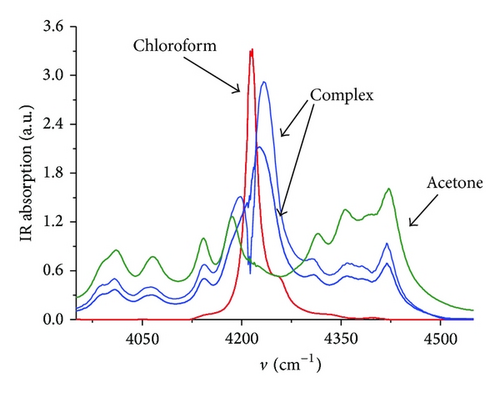
We solved (11) numerically with optimization parameter K. The optimal value is K = 6.76. In Figure 3 concentration and spectral profiles at this value of equilibrium constant are shown.
4.4. Comparison
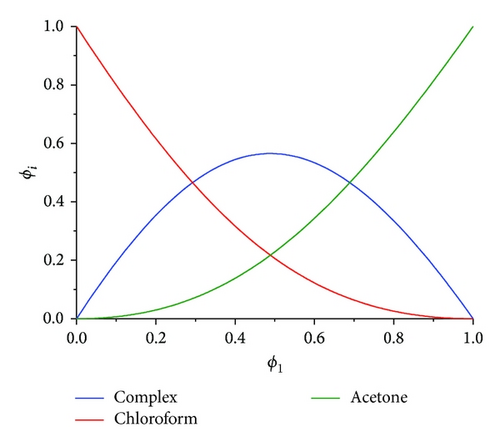
We compared the resolved results by model-based analysis concentration profiles with those obtained by MCR-ALS. The result of comparison is shown in Figure 4. The results of both approaches are in good agreement within the range of measuring error. The concentration profile of complex obtained by MCR-ALS is skew. It may be explained by existence of small amount of AB2 complex at low concentration of acetone. Because MCR-ALS analysis is model-free we do not know what type (or types) of complex forms this concentration profile. Thus complex profile in model-free analysis may be considered as integral profile of all complexes which are existent in mixture.
5. Conclusions
The analysis of FTIR spectra at different acetone concentrations in acetone-chloroform system using MCR-ALS method was carried out. It can give very necessary information about complex formation in this mixture. Three-component model of the mixture was chosen for the analysis. MCR-BANDS technique was used to obtain the band boundaries of MCR-ALS solutions.
The most difficult aspect of model-based approach is the determination of correct model. The process of fitting several models and comparing the results can be tedious. Three-component model of the mixture was used successfully for model-based approach to decomposition of spectra. Despite simplifying model usage, the concentration profiles obtained by model-free and model-based approaches are in good agreement within the range of measuring error. AB2 complex forming will be subject of our further investigation.
Both approaches should be used in decomposition liquid mixture vibrational spectra. The model-free analysis can be invaluable in supporting the model choice.
Conflict of Interests
The authors declare that there is no conflict of interests.




