Analytical Expressions for Steady-State Concentrations of Substrate and Oxidized and Reduced Mediator in an Amperometric Biosensor
Abstract
A mathematical model of modified enzyme-membrane electrode for steady-state condition is discussed. This model contains a nonlinear term related to enzyme kinetics reaction mechanism. The thickness dependence of an amperometric biosensor is presented both analytically and numerically where the biological layer is immobilized between a solid substrate and permeable electrode. The analytical expressions pertaining to the concentration of species and normalized current are obtained using the Adomian decomposition method (ADM). Simple and approximate polynomial expressions of concentrations of an oxidized mediator, substrate, and reduced mediator are derived for all possible values of parameters (Thiele modulus), BO (normalized surface concentration of oxidized mediator), and BS (normalized surface concentration of substrate). A comparison of the analytical approximation and numerical simulation is also presented. A good agreement between theoretical predictions and numerical results is observed.
1. Introduction
In recent years, polymer membranes are widely used as carriers for immobilization of enzymes [1]. They have been utilized in biomaterials, bioseparators, and biosensors [2]. The membranes provide an ideal support for the immobilization of the biocatalyst. Substrate partition at the membrane/fluid interphase can be used to improve the selectivity of the catalytic reaction towards the desired products [3]. Recently a new method for enzyme immobilization [4], based on a molecular recognition process, has been successfully used for the building of enzymatic biosensors and also of a chemically active membrane [5]. In the recent three decades, much effort has been devoted to the development of various biosensors involving biologically sensitive component and transformers—devices with many fields of applications [6]. Changes in membrane chemistry have been demonstrated by Robeson [7]. The better way of changing the membrane geometry aims to increase the membrane area per volume, thereby speeding the separation. This increased surface area has recently been identified as a high priority research need for membranes [8].
A two-substrate model for enzyme electrode has been devised experimentally [10, 11] where the nonlinear enzyme reaction was taken into account. This model was employed to describe the behaviour of a glucose oxidize (GOx) electrode [12, 13]. It has been found that the mediators could not totally replace the natural cosubstrate when both were present in the assay solution so that here a three-substrate model would be required. In these cases, a complex calibration curve of the enzyme electrode was observed [14, 15].
Inspite of extensive experimental investigations for the design of bio-sensor, only a few studies concerned the modeling or theoretical design of such system. Recently Loghambal and Rajendran [16] have described the theoretical model in an amperometric oxidase enzyme-membrane electrode. Numerical solutions [9, 17, 18] were reported to a novel enzyme electrode. In those papers the enzyme electrode was modeled numerically using shooting method [9, 18] and Runge-Kutta method [17]. But, in this paper, the same system is modeled analytically. However, to the best of our knowledge, till date no general analytical results for the concentration of oxidized mediator, substrate, and reduced mediator for all values of the parameters , BO, and BS have been reported [9]. The purpose of this communication is to derive the closed-form of analytical expressions [19, 20] of concentrations of substrate and oxidized and reduced mediator by solving the system of nonlinear reaction-diffusion equations using the Adomian decomposition method (ADM) [21]. The theoretical models of enzyme electrodes give information about the mechanism and kinetics operating in the biosensor. Thus, the information gained from this modeling can be useful in sensor design, optimization, and prediction of the electrode’s response.
2. Mathematical Formulation and Analysis of the Problems
2.1. Mathematical Formulation
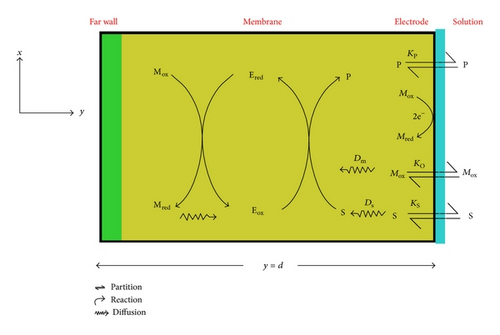
2.2. Normalized Form
3. Concentrations of Oxidized Mediator, Substrate, and Reduced Mediator under Steady-State Condition
3.1. Analytical Solution Using ADM
3.2. Numerical Simulation
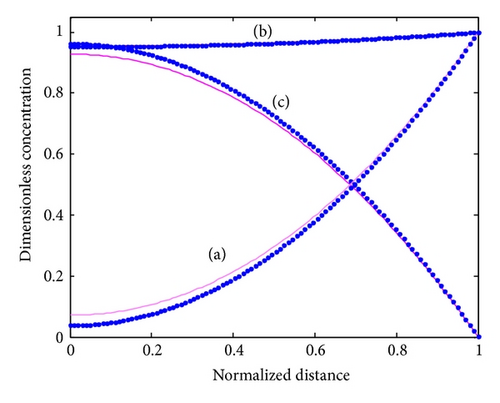
The nonlinear diffusion equations ((9a)–(9c)) for the boundary condition (10) are also solved numerically. We have used the function pdex4 in Scilab/Matlab numerical software to solve numerically the initial-boundary value problems for parabolic-elliptic partial differential equations. This numerical solution is compared with our analytical results in Figures 2 and 3 and Table 1. The average relative error between our analytical result (17) and the numerical result of oxidized mediator concentration FO is less than 0.3% for various values of . The normalized current I is compared with simulation result in Table 3 for various values of parameters . The average relative error is less than 3%. From the table it is also inferred that the value of the current increases when the thickness of the membrane increases.
| χ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d = 0.7878 μM) | (d = 7.878 μM) | (d = 39.39 μM) | (d = 78.80 μM) | (d = 118.17 μM) | |||||||||||
| (17) | Num | Error % | (17) | Num | Error % | (17) | Num | Error % | (17) | Num | Error % | (17) | Num | Error % | |
| 0 | 0.9999 | 1 | 0.01 | 0.9955 | 0.9955 | 0.00 | 0.8889 | 0.8889 | 0.00 | 0.5801 | 0.5773 | 0.46 | 0.1895 | 0.1908 | 0.69 |
| 0.2 | 0.9999 | 1 | 0.01 | 0.9957 | 0.9957 | 0.00 | 0.8933 | 0.8933 | 0.00 | 0.5965 | 0.5939 | 0.43 | 0.2197 | 0.2200 | 0.14 |
| 0.4 | 0.9999 | 1 | 0.01 | 0.9962 | 0.9962 | 0.00 | 0.9066 | 0.9066 | 0.00 | 0.6462 | 0.6440 | 0.34 | 0.3115 | 0.3101 | 0.45 |
| 0.6 | 0.9999 | 1 | 0.01 | 0.9971 | 0.9971 | 0.00 | 0.9288 | 0.9288 | 0.00 | 0.7295 | 0.7279 | 0.22 | 0.4686 | 0.4664 | 0.47 |
| 0.8 | 0.9999 | 1 | 0.01 | 0.9984 | 0.9984 | 0.00 | 0.9599 | 0.9599 | 0.00 | 0.9484 | 0.8471 | 0.85 | 0.6962 | 0.6947 | 0.21 |
| 1 | 1 | 1 | 0.0 | 1 | 1 | 0.00 | 1 | 1 | 0.00 | 1 | 1 | 0.00 | 1 | 1 | 0.00 |
| Average deviation | 0.01 | Average deviation | 0.00 | Average deviation | 0.00 | Average deviation | 0.38 | Average deviation | 0.33 | ||||||
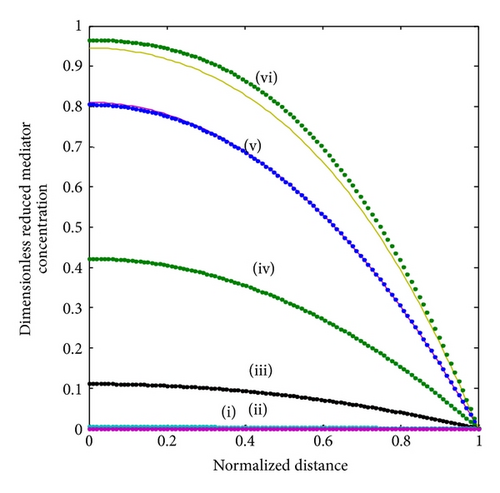
4. Results and Discussion
Equations (17), (13), and (14) represent the new closed and simple approximate analytical expressions of the normalized concentrations of oxidized mediator FO, substrate FS, and reduced mediator FR, respectively, for all values of parameters , BO, and BS. The current response is given in (18). All possible numerical values of the dimensionless parameters used by Gooding and Hall [9] and in this work are given in Table 2.
| Parameters | Numerical values | |
|---|---|---|
| Gooding and Hall [9] | This work | |
| 10–400 | 0.01–400 | |
| BO = [MedOX] b/βO | 0.00195 | 0-1 |
| BS = [S] b/βS | 0.018, 0.035, 0.07, 0.14 | 0-1 |
| μS = DM[MedOX] b/DS[S] b | 0.94736 | 0.05, 0.5 |
| Normalized current I | Error % | ||
|---|---|---|---|
| Analytical | Numerical | ||
| 1 | 0.009 | 0.009 | 0 |
| 25 | 0.223 | 0.223 | 0 |
| 50 | 0.441 | 0.441 | 0 |
| 75 | 0.655 | 0.652 | 0.5 |
| 100 | 0.864 | 0.838 | 3 |
4.1. Concentration Profiles
The concentration profiles for all the three species are shown in Figure 2. The condition that the oxidized mediator FO (17) is consumed by the enzyme reaction, as the mediator moves inwards from the electrode interface, is established in Figure 2. We understand that when the substrate concentration FS (13) is changed slightly across the matrix, the oxidized mediator is limiting the reaction under these conditions rather than the substrate itself. The reduced mediator concentration FR (14) has the reciprocal variation as expected from (9d). The maximum reduced mediator concentration is noticed at the same position in the enzyme layer, as the oxidized species becomes completely consumed.
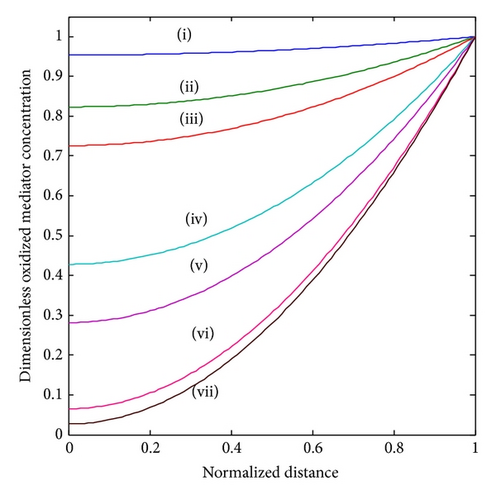
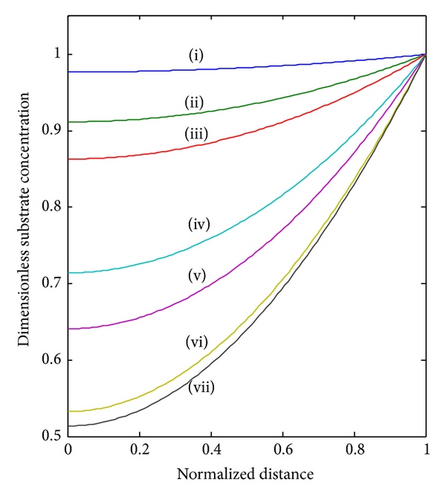
4.2. Variations in the Thiele Modulus
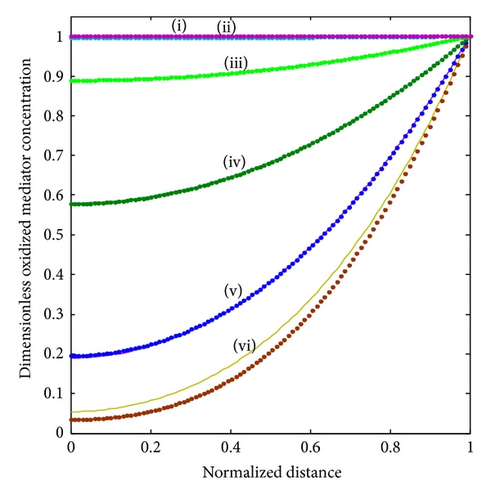
The Thiele modulus essentially compares the reaction and diffusion in the enzyme layer. We examine the rise and downfall of concentration profiles in two cases. (a) When is less than 1, the kinetics dominate and the uptake of oxygen and substrate is kinetically controlled. The overall kinetics are governed by the total amount of active enzyme. (b) The response is under diffusion control, when the Thiele modulus is large , which is observed at high catalytic activity and great membrane thickness or low diffusion coefficient values.
In Figure 3, under the consideration of lower and the sensor under kinetic control, the concentration profile varies slightly from nonenzyme linked oxygen. As increases, the oxidized mediator concentration is consumed in the enzyme reaction. Therefore, the profile deviates more from the linearity. Furthermore, all the oxygen within the polymer matrix is consumed well before reaching the electrode. Thus, the concentration gradient nears the electrode, and hence the flux of oxygen at the electrode surface decreases. We observe that, for a given layer and hence value of , the concentration profiles are also altered according to the bulk concentrations of the substrate and cosubstrate. Thus, with increasing [S] b the concentration profile deviates from the response where no oxygen is consumed. Thus, an increased substrate concentration FS causes a decrease in the flux to the electrode. The concentration of the reduced mediator FR increases in direct proportion to the thickness of the enzyme layer or the amount of enzyme immobilized in the matrix.
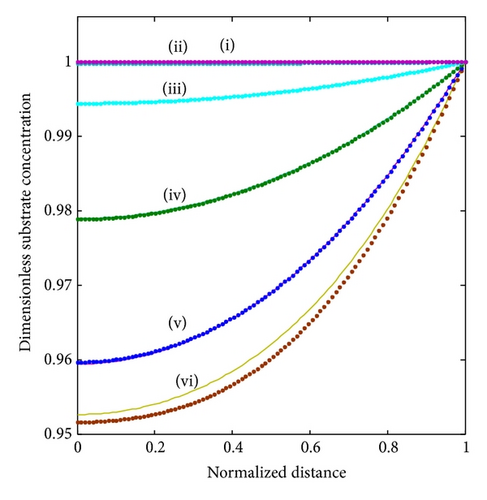
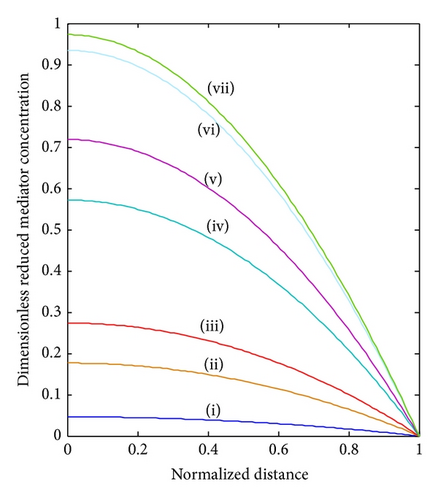
In Figure 4, the concentrations of the oxidized mediator, substrate, and reduced mediator are shown for different substrate concentrations in the bulk electrolyte. In the absence of substrate (BS ≤ 0.001), a straight line is observed for the mediator profile. With increasing substrate concentration in the bulk electrolyte, the profile of reduced mediator bends upward as the generation of reduced mediator by the enzyme reaction increases. The profiles for oxidized mediator and substrate bend downwards.
4.3. Current Response
The parameter of the greatest interest in an amperometric biosensor is the current. For a particular membrane, the variation in can be achieved practically, by varying the thickness of the membrane d or the loading of the enzyme [ET]. As can be seen in (8), the thickness appears as a squared term and thus small changes will have a much more pronounced effect on the response of the enzyme electrode than the enzyme loading. The variation in current for various values of d and hence is shown in Figure 5. It is evident from the figure that the current I (18) increases when increases. Furthermore, when BO and BS are greater than 0.5, all the curves reach the steady-state value. Electroanalytical systems for high spatial resolution scanning and fabrication of micronano sensors need the three-dimensional study of current response.
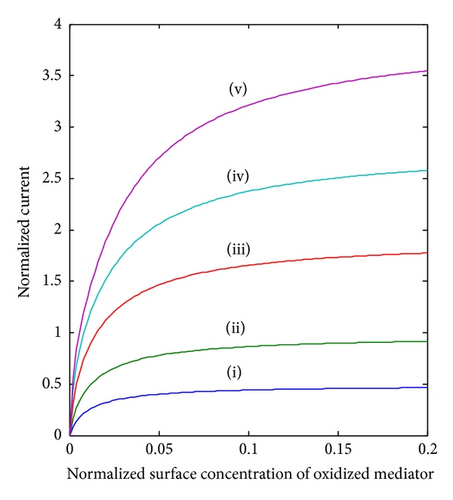
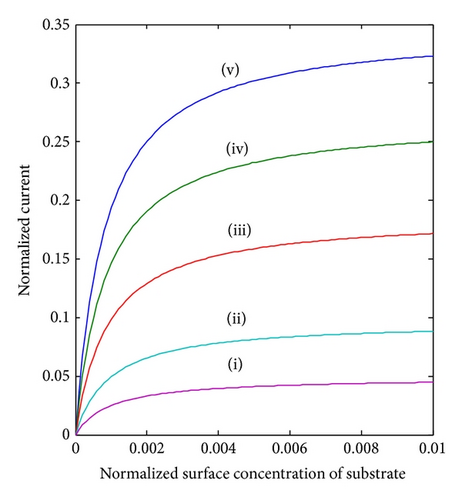
5. Conclusions
This paper presents a theoretical treatment of an oxidized enzyme-membrane electrode in an amperometric biosensor. In this paper, we have solved the nonlinear differential equations both analytically and numerically. The approximate analytical expressions for the steady-state concentrations of oxidised mediator, substrate, and reduced mediator for all values of parameters , BO, and BS at the enzyme-membrane electrode geometry are obtained using the Adomian decomposition method. A satisfactory agreement with the numerical result is noted. The analytical results obtained in this paper can be used to analyze the effect of different parameters such as membrane thickness, type of buffer in the external solution and enzyme loading in the membrane. This theoretical result is also useful for the optimization of the biosensor.
Symbols
-
- [ET]:
-
- Total enzyme concentration (mM)
-
- [EOX]:
-
- Enzyme concentration of the oxygen (mM)
-
- [ES]:
-
- Enzyme concentration of the substrate (mM)
-
- [Ered]:
-
- Reduced enzyme concentration (mM)
-
- [MedOX]:
-
- Concentration of oxidized mediator at any position in the enzyme layer (mM)
-
- [Medred]:
-
- Concentration of reduced mediator at any position in the enzyme layer (mole cm−3)
-
- DM:
-
- Diffusion coefficient of oxidized mediator (cm2s−1)
-
- DS:
-
- Diffusion coefficient of substrate (cm2s−1)
-
- d:
-
- Thickness of the planar matrix (cm)
-
- [MedOX] b:
-
- Oxidized mediator concentration at the enzyme layer ∣electrode boundary (mM)
-
- [MedOX] ∞:
-
- Oxidized mediator concentration in bulk solution (mM)
-
- [S]:
-
- Concentration of substrate at any position in the enzyme layer (mM)
-
- [S] b:
-
- Substrate concentration at the enzyme layer|electrode boundary (mM)
-
- [S] ∞:
-
- Substrate concentration in bulk solution (mM)
-
- k1, k3, k4:
-
- Rate constants (M−1s−1)
-
- k−1, k2:
-
- Rate constants (s−1).
Greek Symbols
-
- :
-
- Thiele modulus for the oxidized mediator (none).
Subscripts
-
- OX:
-
- Oxidized
-
- T:
-
- Total
-
- red:
-
- Reduced
-
- O:
-
- Oxygen
-
- S:
-
- Substrate
-
- P:
-
- Product
-
- R:
-
- Reduced
-
- b:
-
- Boundary
-
- ∞:
-
- Bulk.
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (CSIR no.: 01(2442)/10/EMR-II), Government of India. The authors also thank Mr. M. S. Meenakshisundaram, Secretary, The Madura College Board, Dr. R. Murali, the Principal, and Professor S. Thigarajan, HOD, Department of Mathematics, The Madura College, Madurai, Tamil Nadu, India, for their constant encouragement. The author S. Loghambal is very thankful to the Manonmaniam Sundaranar University and V V college of Engineering, Tirunelveli, for allowing the research work.
Appendices
A. Basic Concept of the Adomian Decomposition Method (ADM)
B. Analytical Expression of Concentrations of Oxidized Mediator Using the Adomian Decomposition Method
C. Scilab/Matlab Program
-
function pdex4
-
m = 0;
-
x = ;
-
t = ;
-
sol = pdepe(m,@pdex4pde,@pdex4ic,@pdex4bc,x,t);
-
u1 = sol(:,:,1);
-
u2 = sol(:,:,2);
-
u3 = sol(:,:,3);
-
figure
-
plot(x,u1(end,:))
-
xlabel(‘Distance x’)
-
ylabel(‘u1(x,2)’)
-
figure
-
plot(x,u2(end,:))
-
xlabel(‘Distance x’)
-
ylabel(‘u2(x,2)’)
-
figure
-
plot(x,u3(end,:))
-
xlabel(‘Distance x’)
-
ylabel(‘u3(x,2)’)
-
% –––––––––––––––––––––––––––––––
-
function [c, f, s] = pdex4pde(x,t,u,DuDx)
-
Bs = 0.01;
-
Bo = 0.1;
-
A = 10;
-
us = 0.05;
-
up = 1;
-
c = [1; 1; 1];
-
f = [1; 1; 1].* DuDx;
-
F1 =-A ∧2*(u(1)*u(2)/(u(1)/Bs+u(2)/Bo+(u(1)*u(2))));
-
F2 =-us*A ∧2*(u(1)*u(2)/(u(1)/Bs+u(2)/Bo+(u(1)*u(2))));
-
F3 =up*A ∧2*(u(1)*u(2)/(u(1)/Bs+u(2)/Bo+(u(1)*u(2))));
-
s = [F1; F2; F3];
-
% –––––––––––––––––––––––––––––––
-
function u0 = pdex4ic(x);
-
u0 = [1; 0; 1];
-
% –––––––––––––––––––––––––––––––
-
function [pl,ql,pr,qr] = pdex4bc(xl,ul,xr,ur,t)
-
pl = [0; 0; 0];
-
ql = [1; 1; 1];
-
pr = [ur(1)-1; ur(2)-1; ur(3)];
-
qr = [0; 0; 0];




