Hydrothermal Synthesis of Nitrogen-Doped Titanium Dioxide and Evaluation of Its Visible Light Photocatalytic Activity
Abstract
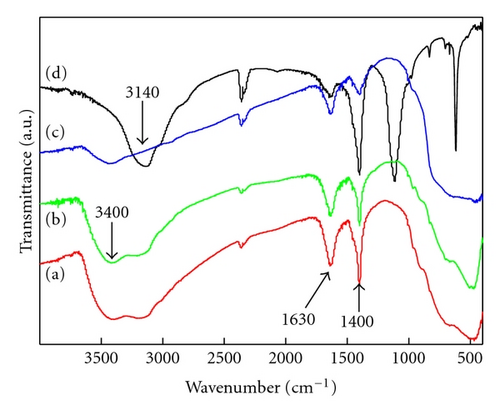
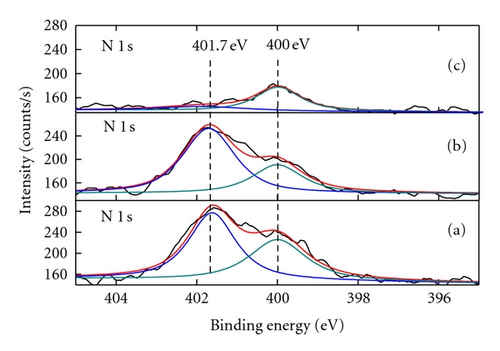
Nitrogen-doped titanium dioxide (N-doped TiO2) photocatalyst was synthesized from nanotube titanic acid (denoted as NTA; molecular formula H2Ti2O5 · H2O ) precursor via a hydrothermal route in ammonia solution. As-synthesized N-doped TiO2 catalysts were characterized by means of X-ray diffraction, transmission electron microscopy, diffuse reflectance spectrometry, X-ray photoelectron spectroscopy, electron spin resonance spectrometry and Fourier transform infrared spectrometry. It was found that nanotube ammonium titanate (NAT) was produced as an intermediate during the preparation of N-doped TiO2 from NTA, as evidenced by the N1s X-ray photoelectron spectroscopic peak of NH4 + at 401.7 eV. The catalyst showed much higher activities to the degradation of methylene blue and p-chlorophenol under visible light irradiation than Degussa P25. This could be attributed to the enhanced absorption of N-doped TiO2 in visible light region associated with the formation of single-electron-trapped oxygen vacancies and the inhibition of recombination of photo-generated electron-hole pair by doped nitrogen.
1. Introduction
Titania, as a currently known most effective photocatalyst, have been widely applied in purifying air and water, deodorization, and many other environmentally related fields [1]. However, TiO2 can only be induced by ultraviolet (UV), due to its large bandgap of ca. 3 eV [2]. As a result, TiO2 in practical application can only utilize a small fraction (~5%) of solar energy [3]. To increase the efficiency for solar energy transformation and utilization, many researchers have attempted to extend the absorption range of TiO2 from UV region to visible light (Vis in short) region by doping various cations [4–6] and anions [7, 8]. It has been found that N doping induces considerable visible light response of anatase TiO2; the mechanisms for the visible light response of N-doped TiO2, however, still remain disputable. About one decade ago, Asahi et al. reported a visible light active TiO2−x Nx film by sputtering a TiO2 target in the mixture of N2 and Ar (volume ratio 40% : 60%); and they suggested that the incorporation of dopant N atom into the crystal lattice of TiO2 via substituting Ti atom was indispensable for band-gap narrowing and photocatalytic activity generation [9]. In 2003, Irie et al. reported a TiO2−x Nx powder prepared by annealing anatase TiO2 under an ammonia flow; and they supposed that the isolated narrow band formed above the valence band of TiO2 was responsible for the visible light response of N-doped TiO2 [10]. Ihara et al. also prepared a visible light responsive photocatalyst by treating the hydrolysis product of Ti(SO4)2 in ammonia (a precursor for N doping); and they deduced that oxygen-deficient sites formed in grain boundaries gave rise to visible light activity of as-prepared N-doped product, while doped N at oxygen-deficient sites played a key role as a blocker for reoxidation [11]. Zhang et al. observed that the visible-light absorption of vacuum-dehydrated product of NTA was proportional to the intensity of electron spin resonance (ESR) signal [12]. We supposed in our previous researches that the visible light sensitization of N-doped TiO2 was due to the formation of single-electron-trapped oxygen vacancies (SETOVs), and doped-N played a role in preventing photogenerated electrons and holes from recombination [12, 13].
In the present research, we make use of a hydrothermal method to prepare N-doped TiO2 by selecting NTA as a precursor, hoping to harvest TiO2-based photocatalyst with high visible light activity while heat-treatment temperature is considerably lowered as compared with that for calcination of NTA in flowing ammonia [12]. This article reports the preparation and characterization of N-doped TiO2 photocatalyst, as well as its photocatalytic activity for the degradation of methylene blue (MB) and p-chlorophenol (4-CP) under visible light irradiation.
2. Experimental Section
2.1. Preparation of Samples
NTA was prepared using the method reported in literature [14]. N-doped TiO2 powders were prepared using a two-step protocol. Firstly, 1 g of NTA and 50 mL of ammonia solution (mass fraction 25%~28%) was added into a Teflon-lined stainless steel autoclave (75 mL) and airproofed and heated at 130, 160 and 210°C for 3 h in an oven. Then, the autoclave was cooled in air down to room temperature after the reaction was completed, followed by filtration, drying at 60°C in vacuum (−0.1 MPa) overnight, and grounding to yield samples denoted as N-NTA-130, N-NTA-160, and N-NTA-210, respectively. Sample H-NTA-210 for a comparative study was also prepared in the same manners except that distilled water was used to replace ammonia solution.
2.2. Characterization
X-ray diffraction (XRD) patterns were measured with a Philips X’Pert Pro X-ray diffractometer (Cu Kα radiation, 2θ range 7~90°, scan step size 0.04°, scanning interval 0.5 s, generator voltage 40 kV, tube current 40 mA). A JEM-100CX transmission electron microscope (TEM; JEOL Ltd., Japan) was performed at an accelerating voltage of 100 kV to observe the morphology and microstructure of various as-prepared photocatalysts. Diffuse reflection spectra (DRS) were obtained with a UV-Vis spectrophotometer (UV-3010; Shimadzu, Japan; reference: BaSO4). An Axis Ultra X-ray photoelectron spectroscope (XPS; Kratos, England) equipped with a multichannel detector was performed to analyze the chemical states of various photocatalysts, where Al Kα radiation (hν = 1486.6 eV) was used as the excitation source. All the binding energies were referenced to the C1s peak at 284.8 eV of surface adventitious carbon. Electron spin resonance (ESR) spectra were collected with a Brüker ESP 300E apparatus (reference: diphenyl-picryl hydrazide (DPPH), g = 2.0036). Fourier transform infrared (FTIR) spectra were recorded with a Nicolet 360 spectrometer (Nicolet Company, USA).
2.3. Evaluation of Visible Light Photocatalytic Activity
The photocatalytic activities of various photocatalysts were evaluated by monitoring the degradation of MB and 4-CP under the irradiation of a 500 W xenon lamp as the light source (I420 = 2.1 mW/cm2) with which a filter and a water cell were attached to eliminate UV (λ < 420 nm) and infrared light. Into a quartz reactor (140 mL) charged with 100 mL of the aqueous solution of MB (10 mg/L) or 4-CP (0.15 mM) was added 100 mg of to-be-tested photocatalyst powder. The adsorption equilibrium of aqueous MB or 4-CP on the photocatalyt was reached in the reactor prior to irradiation. The concentration of MB was monitored with a visible light spectrometer (722, Shanghai Jingke Instrument Plant, China) at 664 nm, and that of 4-CP was measured with an UV-Vis spectrometer (752, Shanghai Oppler Instrument Plant, China) at 225 nm.
3. Results and Discussion
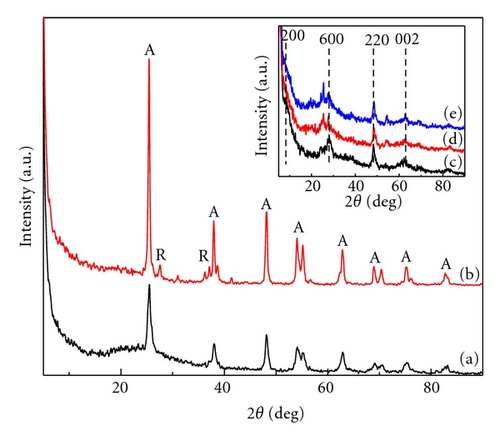
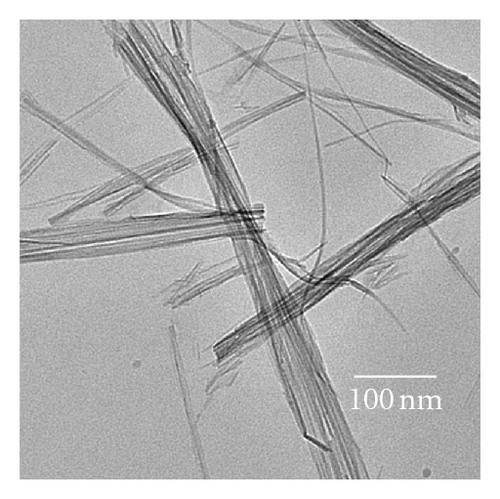
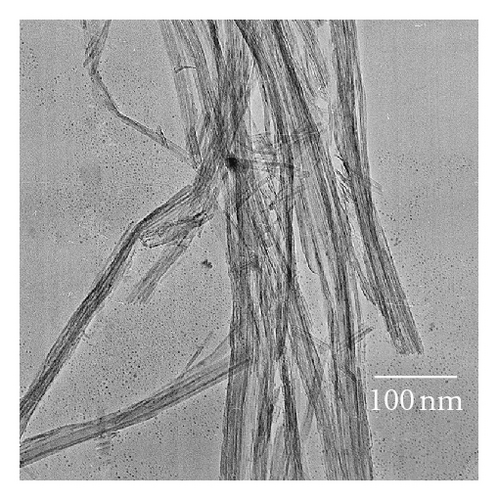
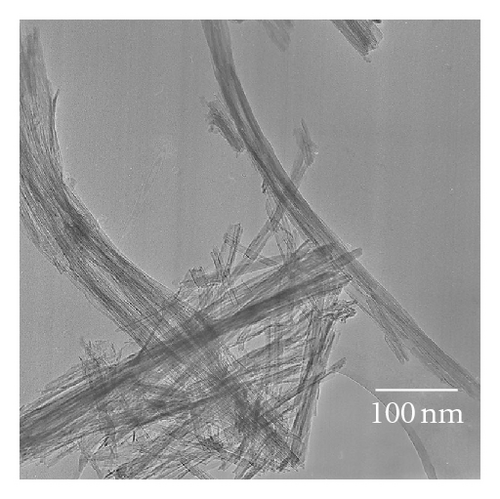
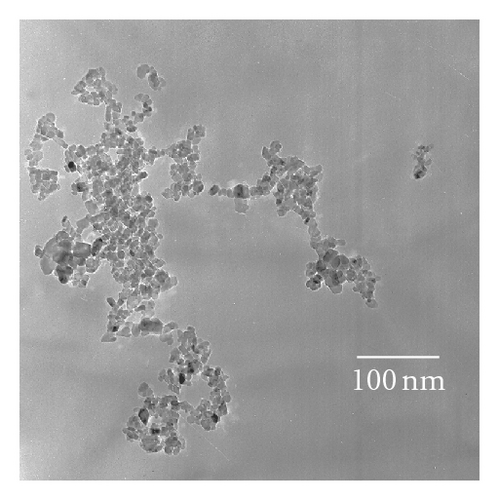
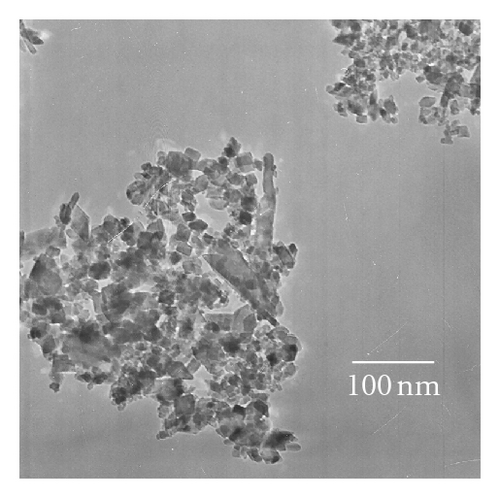
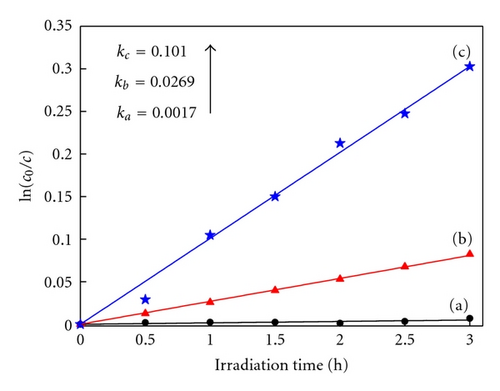
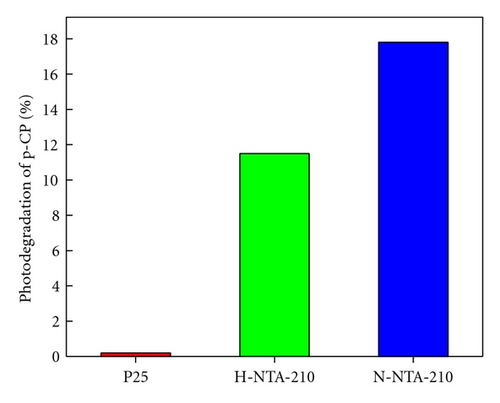
Photocatalytic tests indicate that samples NTA, N-NTA-130, and N-NTA-160 have no photocatalytic activity for the discoloration of MB; but sample N-NTA-210 is able to effectively catalyze the discoloration reaction of MB, following the first-order reaction kinetics model (the kinetic constants are ranked as k(N-NTA-210) > k(H-NTA-210) > k(P25), see Figure 5. In the meantime, NTA, N-NTA-130, and N-NTA-160 are inert to the degradation of colorless organic pollutant 4-CP under visible light irradiation; but H-NTA-210 and N-NTA-210 can well accelerate the degradation of 4-CP under the same conditions, and N-NTA-210 possesses better photocatalytic activity than H-NTA-210 (Figure 6).
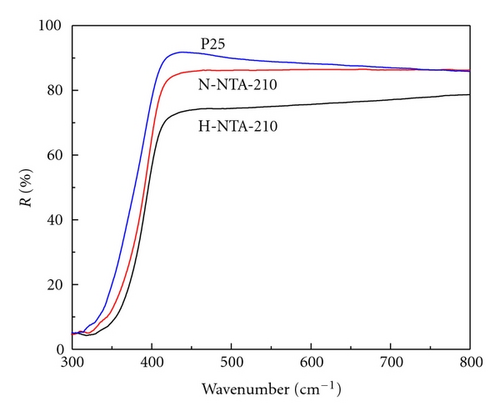
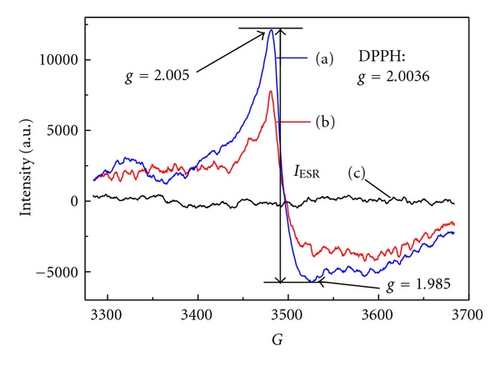
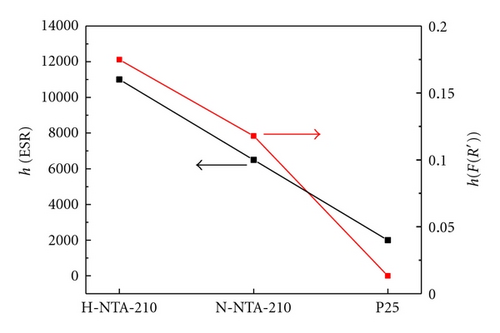
It is well known that photocatalytic activity is dependent on light absorption efficiency and separation efficiency of photogenerated electrons and holes. Figure 7 shows the UV-Vis spectra of various photocatalysts. Precursor NTA does not show any absorption in visible light region, but samples H-NTA-210 and N-NTA-210 both show broadened absorptions at λ > 400 nm, which corresponds to their enhanced ESR signals at g = 2.003 (Figure 8) and accounts for better visible photocatalytic activity of N-NTA-210 as compared with Degussa P25. Besides, the present research once again provides evidence to the supposition that SETOV density in TiO2 lattice is proportional to the absorption intensity of the photocatalyst in visible light region (Figure 9) [12, 21–23].
4. Summary
A simple and mild hydrothermal method has been established to prepare nanoscale N-doped TiO2 photocatalyst with good visible light catalytic activity, where heat treatment of precursor NTA in ammonia solution at 210°C for 3 h facilitates structural transformation from orthorhombic NTA to N-doped TiO2. Heat treatment of the same precursor at lowered temperature of 130°C and 160°C gives rise to intermediate NAT deserving further research, a potential precursor for fabricating novel N-doped TiO2. As-prepared N-NTA-210 possesses better photocatalytic performance than Degussa P25 for the degradation of MB and 4-CP under visible light irradiation, which is attributed to the formation of a large number of SETOV on the surface of N-doped TiO2 and the occupation of interstitial sites in the crystal lattice of TiO2 by dopant N as Ti–O–N.
Acknowledgments
The authors thank the National Natural Science Foundation of China (no. 20973054) for financial support.




