Interferon Alpha in Systemic Lupus Erythematosus
Abstract
The pleiotropic cytokine interferon alpha is involved in multiple aspects of lupus etiology and pathogenesis. Interferon alpha is important under normal circumstances for antiviral responses and immune activation. However, heightened levels of serum interferon alpha and expression of interferon response genes are common in lupus patients. Lupus-associated autoantibodies can drive the production of interferon alpha and heightened levels of interferon interfere with immune regulation. Several genes in the pathways leading to interferon production or signaling are associated with risk for lupus. Clinical and cellular manifestations of excess interferon alpha in lupus combined with the genetic risk factors associated with interferon make this cytokine a rare bridge between genetic risk and phenotypic effects. Interferon alpha influences the clinical picture of lupus and may represent a therapeutic target. This paper provides an overview of the cellular, genetic, and clinical aspects of interferon alpha in lupus.
1. Introduction
In systemic lupus erythematosus, a finely tuned system of cells and signals is dysregulated, and the balance between tolerance and autoimmunity is disrupted. Cytokines, as a fundamental mechanism through which the immune system is kept in balance, play an important role in the etiology and pathogenesis of lupus. An example of an important cytokine involved in lupus etiology and pathogenesis is interferon alpha (IFNα).
IFNα is a pleiotropic cytokine that can affect multiple cell types involved in lupus. Several genes in the interferon pathway are associated with risk for lupus, suggesting a role for this pathway in etiology. Additionally, increased IFNα levels and expression of IFN response genes are often found in lupus. IFNα may affect the clinical manifestations of lupus and is a promising target for therapeutic interventions.
2. Cellular Aspects of IFNα in Lupus
Interferon alpha (IFNα) is a key molecule in immune regulation. It is produced by multiple cell types in response to viral infection. Plasmacytoid dendritic cells have a special role in the production of IFNα and are the main sources of serum interferon [1]. IFNα has the potential to dramatically influence the development, progression, and pathogenesis of SLE as it can influence the function and activation state of most major immune cell subsets and function as a bridge between innate and adaptive immunity.
2.1. Toll-Like Receptors and Interferon
One of the principal mechanisms through which IFNα is produced is through Toll-like receptor (TLR) signaling [2, 3]. TLR7 recognizes single-stranded RNA, culminating in interferon regulatory factor (IRF) 5 and IRF7 activation [4] and production of IFN [5–7]. Excessive TLR 7 signaling produces lupus-like autoimmunity in male Yaa mice, where an extra copy of the TLR7 gene is present on the Y chromosome [8–10]. The autoimmune phenotype conferred by the Yaa genotype is dependent on IFN α, and addition of IFNα can partially duplicate the Yaa phenotype [11]. Additionally, knocking out the IRF7 gene or inhibiting its action with pharmacologic agents inhibits antibody production against RNA-containing nuclear components [12], suggesting that TLR7 is essential for this type of autoantibody production.
A characteristic of many cases of lupus is the production of antibodies against RNA-containing protein complexes such as Sm, nRNP, Ro, and La. In fact, antibodies against the spliceosomal protein Sm are so specific for lupus that they are used as a diagnostic criterion. The RNA found in these complexes is capable of promoting the production of IFNα through the stimulation of TLR7 [3, 13] (Figure 1).
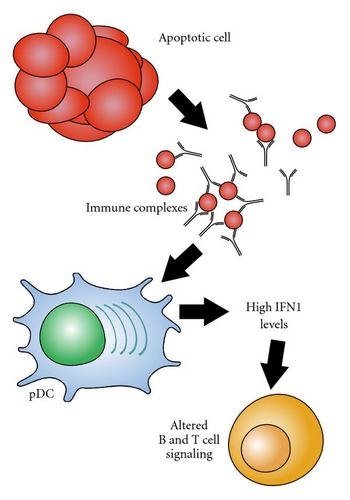
Because TLR7 is located in the endosomes, RNA-containing complexes must access the interior of the cell before they are able to act as activators. Autoantibodies specific for these lupus-associated riboproteins can bind with antigens derived from apoptotic cells and form antibody-protein-RNA complexes. The Fc portions of the immune complexes are recognized and internalized by cells with Fc receptors, providing a route of entry for RNA to reach TLR7, resulting in interferon alpha production [3, 14]. This process is especially well established in PDCs [15, 16]. Interestingly, in addition to being produced as a result of TLR7 ligation, IFNα enhances TLR7 signaling in PDCs [17, 18], forming a positive feedback loop.
Despite these data and the strong association between SLE-associated autoantibodies and serum IFNα, SLE-associated autoantibodies are not sufficient for high serum IFNα in humans in vivo [19]. Healthy subjects with anti-Ro antibodies do not have high serum IFN-α, while a significant proportion of anti-Ro positive patients with SLE or Sjogren’s syndrome do have high serum IFNα, suggesting that these autoantibodies require other disease-associated factors to result in high serum IFNα in humans.
2.2. IFNα and Adaptive Immune Regulation
Excess serum IFNα and IFN-response gene expression are characteristics of lupus and are most likely the result of excessive PDC activation. Such high levels of interferon could contribute to lupus by promoting immune activation rather than tolerance. Dendritic cells are the primary activators of T cells, and affect both T-cell tolerance and activation, depending on the state of the dendritic cell. When treated with interferon alpha, dendritic cells mature and become more prone to activate T cells [20, 21]. Myeloid dendritic cells from lupus patients are able to phagocytose and present self-antigens to T cells in a stimulatory, rather than regulatory manner, a process which is interferon-dependent [22]. Such a process likely contributes to loss of T-cell tolerance to self-antigens and subsequent autoimmunity.
Exposure of the dendritic cell to IFNα contributes to T cell polarity. When CD4+ T cells are activated in the presence of IFNα-producing dendritic cells, their polarity is shifted towards IFN-γ producing cells rather than IL-4 producing cells [23, 24], which may promote autoimmunity or immune dysregulation. The T-cells activated by IFNα-treated dendritic cells also are enriched for T-follicular helper cells, a recently described cell type which are adept at activating B cells and driving antibody production [25].
Regulatory T cells (T-reg) are attracting increased attention as a mechanism of immune regulation and suppression of autoimmunity. In lupus, T-regs are often, though not always, found in lower numbers than in controls [26–31]. Those T-regs that are present in lupus are inefficient at suppressing inflammation and T-cell proliferation [27, 29, 30, 32]. T-reg development is suppressed by treatment of dendritic cells with IFNα [33]. In lupus patients, T-reg activity is diminished, due at least in part to the action of IFNα [34] indicating that increased IFNα levels in lupus patients is likely contributing to the development or maintenance of autoimmunity through suppression of T-reg cells.
B cells are important in lupus, since humoral autoimmunity is a hallmark of the disease. IFNα can prevent apoptosis and enhance proliferation of primary B cells, even in the absence of mitogenic stimuli [35]. Interestingly, isolated B cells are inhibited from developing into antibody-producing plasma cells by IFNα treatment [36]. However, this inhibition is reversed if the B cells are allowed to come into contact with monocytes, in which case IFNα actually stimulates B-cell development and antibody production [37].
The ability of IFNα to influence the activation and function of many major immune cell subsets is a testament to the wide and far-reaching effects of this cytokine. It is clear that interferon is dysregulated in lupus and that overexpression of IFNα can result from the autoantibodies present in lupus. Many components of the molecular pathways through which IFNα and TLRs drive immune activation include genetic risk factors for lupus, further implicating IFNα in lupus etiology and pathogenesis.
3. IFN and IFN-Related Genes Associated with SLE Risk
Lupus involves a combination of both environmental and genetic factors. Support for a genetic component includes a high sibling risk ratio [8–29], high heritability (greater than 66%), and higher concordance rates between monozygotic twins (20 to 40%) as compared to other full siblings and dizygotic twins (2 to 5%) [38, 39]. A large number of genetic risk factors are associated with increased susceptibility to the SLE. This genetically determined increased risk status has been referred to as a “threshold liability” [40], which is expected to be highly polygenic in nature and widely variable between individuals. Environmental factors also affect lupus susceptibility and likely interact with this “threshold liability”, but as in the case of genetic factors, there is no single environmental cause. A person may have only a few of the genetic risk variations and never get SLE despite exposure to environmental triggers. In contrast, another person may have many of these variations and then develop SLE on first exposure to an environmental trigger.
3.1. Lupus-Associated Risk Loci
Research into the etiopathogenesis of SLE has recently been advanced by several large scale case-control genetic studies, including genome-wide association scans. There is now a pool of approximately 30 genes that have been associated with SLE susceptibility with a high degree of statistical certainty and many others with probable evidence for association (reviewed in [41–45]). With this large number of SLE-associated genes, we can begin to group the list of identified SLE associated genes which should provide insight into initial disease pathogenesis into functional categories. These categories include TLR and IFN signaling, apoptosis and clearance of immune complexes, and B- and T-cell signaling. Several genes affecting the interferon pathway have been associated with risk for lupus. The Interferon pathway normally serves an important function in defense against viral infection. Yet in people with genetic predisposition, environmental triggers such as viral infections may tip the scales in favor of autoimmunity.
Once a genetic variation is identified, functional inference then characterization is necessary to move from identification to an understanding of how the variation affects the etiology or pathogenesis of SLE. Since most of the genes involved in genetic susceptibility to SLE have been identified only recently, there remains much work to identify the functional differences in the genetic associations. However, work done thus far in human cohorts is promising, and the categories of genes and loci associated with risk of lupus already suggest pathways that are of high importance.
3.2. Interferon Regulatory Factors
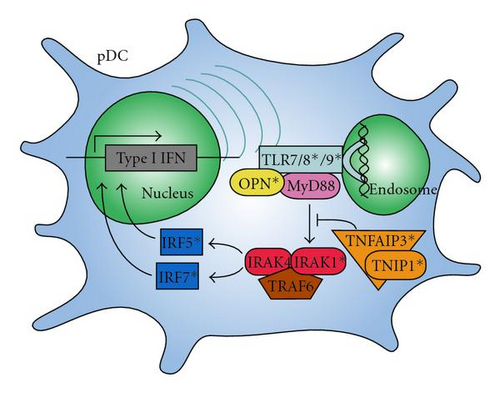
Certain lupus-associated genetic variations have been shown to directly increase IFNα levels or response to IFNα signaling. Interferon regulatory factor 5 (IRF5) has been confirmed as a risk locus in several different ethnic groups [46–50]. Three main functional variants in IRF5 have been described, which combine to form a risk haplotype in individuals of European descent [51]. One of these loci, at rs2004640, creates an alternate splice site (exon 1B) in the untranslated first exon. Another is a copy number variation of a 30-bp insertion/deletion sequence in exon 6, and the final is rs10954213, which creates an alternate polyadenylation site, resulting in shorter, stabler mRNA [52].
Since IRF5 activates IFNα production, these more stable variants may pose a risk due to their ability to produce excess IFNα. In fact, studies of this gene in human SLE cohorts have shown that the risk variant predisposes to greater serum IFN-α, supporting the idea that the risk haplotype is a gain-of-function variant [53]. IRF5 itself is activated by IFNα signaling, producing a potential positive feedback loop. Another IRF, IRF7, has been highlighted by the association of the IRF7/KIAA1542 locus with lupus in recent studies [54, 55]. Several SNPs in this area were shown to correlate with IFNα levels and alter autoantibody profiles in certain ethnicities [56].
IRF5 and IRF7 are activated by signaling through the endosomal toll-like receptors (TLRs) 7, 8, and 9. Interestingly, both of the IRF variants which are implicated in SLE predispose to higher serum IFN-α, but only in the presence of SLE-associated autoantibodies [53, 56] suggesting that these autoantibodies may provide chronic stimulation of the endosomal TLR pathway of IFN-α generation that when combined with gain-of-function polymorphisms in the IRFs results in dysregulation of the pathway in vivo. Additionally, TLRs 8 and 9 were identified in recent studies as containing susceptibility loci to SLE [57, 58]. The role of TLRs in the interferon production was discussed above.
3.3. Interferon-Associated Genes
Another confirmed locus of susceptibility is in the gene encoding IL1 receptor-associated kinase 1 (IRAK1). This kinase is part of the signal transduction which follows TLR ligation. In a mouse model of lupus, IRAK deficiency eliminated most lupus symptoms, showing the importance of this key intermediate [59]. Since this gene is on the X chromosome, gene dosage may contribute to the risk and the prevalence of the disease in women [59].
Two interacting proteins involved in inflammation, TNFα-induced protein 3 (TNFAIP3) and TNFAIP3-interacting protein 1 (TNIP1), have been identified as risk loci [60–64]. TNFAIP3 encodes the protein A20, which helps turn off signaling through NFκB after an inflammatory response [65, 66]. TNIP1 interacts with TNFAIP3 and is involved in several signal transduction pathways.
Signal transducer and activator of transcription 4 (STAT4) is another risk locus with direct links to the interferon pathway. It is involved in proliferation, differentiation, and apoptosis. STAT4 has 2 risk loci, one at rs7574865 which has been shown to increase sensitivity to IFNα [67], and another at rs3821236 which increases STAT4 transcription and interacts with IRF5 susceptibility loci [68]. The presence of both of these risk alleles gives an additive effect, increasing risk to SLE [69]. Osteopontin (OPN) is a key molecule for IFNα production in pDCs [70]. Presence of a lupus risk-associated form of this gene was recently tied to high IFN levels in males and young-onset female lupus patients [71].
Possible interactions of the IFN-associated genes that have been linked to lupus are shown in Figure 2. The risk variants of these genes influence the production of and response to IFNα, likely driving the increased levels seen in lupus patients and affecting the clinical manifestations of the disease.
4. Clinical Aspects of IFNα in Lupus
Lupus primarily affects women in the reproductive years; however people of all ages, genders, and ancestral backgrounds are susceptible. Disease features range from mild manifestations such as rash or arthritis to life-threatening end-organ manifestations such as glomerulonephritis or thrombosis, and it is difficult to predict which manifestations will affect a given patient.
4.1. IFN-α as a Causal Factor in Human Lupus
A number of patients treated with IFNα have developed lupus or lupus-like syndrome [72–74]. In these reports, many specific manifestations of idiopathic lupus such as malar or discoid rash, oral ulcers, photosensitivity, renal involvement, and anti-Sm and anti-dsDNA antibodies were represented, suggesting that these cases were not “drug-induced” SLE but instead resembled idiopathic SLE [73]. Discontinuation of IFNα typically resulted in remission of SLE symptoms [73], supporting a causal relationship with IFN-α. While only a minority of patients treated with IFNα develop SLE (<1% of patients) [75], these data support the idea that IFNα can be sufficient to induce SLE in some individuals. Many more IFNα-treated individuals develop a “lupus-like” syndrome [74], with some SLE symptoms which are insufficient to meet formal diagnostic criteria for SLE [76]. IFN-induced SLE can be severe, and there are reports of life-threatening multiorgan SLE involvement including glomerulonephritis, serositis, discoid rash, myopericarditis, and vasculitis [77, 78].
Another finding which supports the hypothesis of IFNα as a primary causal factor in human SLE is the clustering of high serum IFNα in lupus families [79]. Patients with lupus and their healthy relatives have higher serum IFNα activity as compared to healthy unrelated individuals [79]. Strong familial correlations in serum IFNα were observed regardless of disease status (affecteds versus unaffecteds), and SLE probands in the same family tended to have similar IFNα levels [79]. Spouses of SLE patients did not have high serum IFNα activity, and taken together these data suggest that high serum IFNα is a heritable risk factor for SLE. Interestingly, age-related patterns of serum IFNα were also observed in SLE families in which the ages of highest IFNα mirrored the ages of peak SLE incidence [80, 81]. The discovery of several lupus risk loci in IFN-related genes provides further support for the above observation that serum IFN-α is heritable, and the SLE risk variants of each of these genes result in a gain of function increase in IFNα signaling as detailed above.
4.2. Clinical Correlations with IFN Alpha
A very strong correlation is consistently observed between the presence of SLE-associated autoantibodies, such as anti-Ro, anti-La, anti-Sm, anti-RNP, and anti-dsDNA [79, 82]. Lupus patients with high serum IFNα had a significantly higher prevalence of cutaneous and renal disease in most studies [82–84]. It is interesting that both of these clinical manifestations share an association with a particular serology (rash with anti-Ro and nephritis with anti-dsDNA), and whether these clinical manifestations are associated independent of serology has not been shown to our knowledge.
A number of studies have shown that IFNα correlates with disease activity when assessed cross sectionally [82–85]. Results are conflicting regarding the potential fluctuation of IFNα with disease activity in SLE, and there are a number of studies which did not find a longitudinal correlation [86, 87]. In these studies, a cross-sectional relationship between IFNα and disease activity is still observed, suggesting that IFNα may indicate those patients who generally have higher disease activity as compared to other patients. A recent prospective study evaluated the utility of serum interferon-regulated chemokine levels as potential biomarkers of SLE disease activity [88]. In this study, IFNα-induced chemokines correlated with disease activity cross sectionally, rose at the time of a flare, and decreased as the disease remitted [88]. In this same study, high chemokine levels were predictive of SLE flare over the next year in a subset of patients.
4.3. Anti-IFNα Therapies in Lupus
Given all of the studies presented above, there has been considerable interest in therapies which block IFNα. To date there is one published study describing a phase I trial of a fully human monoclonal antibody that binds to the majority of the subtypes of human interferon alpha [89]. Treatment with this anti-IFN antibody resulted in a dose-dependent inhibition of interferon-induced gene expression in peripheral blood cells as well as skin lesions in patients with mild to moderate SLE [89]. No obvious safety signals were reported during the phase I trial of anti-IFN therapy, and the proof-of-principle analyses supported a biological effect blocking the IFN pathway in humans. Phase two trials to assess efficacy of these agents in treating SLE are currently underway. There are many known predictors of high serum IFNα in SLE patients, including both serologic and genetic markers outlined in this paper. We anticipate that incorporation of these variables into clinical trial design would enhance efficacy and potentially minimize side effects by targeting the most relevant patient group. Long-term safety data will be important, since IFNα is such a highly conserved and important immunological mediator of viral defense.
5. Conclusions
IFN-α is associated with SLE through multiple lines of evidence. These include genetic, immunological/serological, and clinical associations, as described in this review. It is likely that IFN-α plays a key role in SLE etiology, pathogenesis, and/or disease persistence. Despite this large body of evidence associating IFN-alpha with lupus, the association between interferon alpha and SLE is largely inferential. The exact cellular and immunological mechanisms through which IFN affects lupus also remain undiscovered for the most part. These mechanisms and pathways are potentially fertile areas for future investigation. Such studies will likely lead to new therapeutic targets as well as a greater understanding of lupus as a disease.




