Comprehensive and Rapid Real-Time PCR Analysis of 21 Foodborne Outbreaks
Abstract
A set of four duplex SYBR Green I PCR (SG-PCR) assay combined with DNA extraction using QIAamp DNA Stool Mini kit was evaluated for the detection of foodborne bacteria from 21 foodborne outbreaks. The causative pathogens were detected in almost all cases in 2 hours or less. The first run was for the detection of 8 main foodborne pathogens in 5 stool specimens within 2 hours and the second run was for the detection of other unusual suspect pathogens within a further 45 minutes. After 2 to 4 days, the causative agents were isolated and identified. The results proved that for comprehensive and rapid molecular diagnosis in foodborne outbreaks, Duplex SG-PCR assay is not only very useful, but is also economically viable for one-step differentiation of causative pathogens in fecal specimens obtained from symptomatic patients. This then allows for effective diagnosis and management of foodborne outbreaks.
1. Introduction
The introduction of real-time PCR in foodborne outbreak investigations provides an opportunity for rapid detection of pathogens in food and clinical settings [1]. The benefits to public health administration from rapid real-time PCR assays are most notable after comprehensive and rapid detection of bacteria. The results can quickly inform a public health administrator about the causative pathogens of foodborne outbreak, allowing a more accurate, effective, and timely response. Abubakar et al. [2] implied in the Health Technology Assessment program (now part of the National Institute for Health Research, UK) that the feasibility of conversion to rapid methods such as multiplex PCR and DNA microarrays is dependent on localized considerations, including the community prevalence rates for specific pathogens, the skill base, and subsequent training costs for laboratory staff and spare capacity available to ensure adequate laboratory space for new equipment. Although these tests look promising, further studies are necessary to assess their usefulness [2].
Apart from saving time, real-time PCR is sensitive, highly specific and offers the potential for quantification [3]. The risk of cross-contamination is significantly reduced, and high-throughput performance and automation are possible since no post-PCR manipulations are required [4]. In principle, two different chemistries are available for real-time detection of PCR products: fluorescent probes that bind specifically to certain DNA sequences and fluorescent dyes that intercalate into any double-stranded DNA. Fluorescent-probe based real-time PCR (TaqMan PCR) studies to detect causative pathogens from foodborne outbreaks in feces using TaqMan probes have been carried out [3–6]. TaqMan PCR assays require the availability of primers and probes that must be selected according to very rigid criteria. Use of simple, cheaper double-stranded DNA-binding dye SYBR green I for detection of PCR amplicons (SG-PCR) overcomes this limitation. Therefore, real-time PCR could be applied without the need for fluorescent probes [7]. In the absence of probes, the specificityof the reaction is determined on the basis of the melting temperature (Tm). The advantages of SG-PCR over TaqMan PCR include the relative simplicity and reduced cost of SYBR Green I compared to TaqMan probes [8]. Recently, the application of SG-PCR for the detection of foodborne bacteria in different samples has been increased [1, 9–12]. Duplex SG-PCR assays have been carried out to detect causative bacteria in feces from foodborne outbreaks [4, 10, 13].
We previously reported a set of four duplex SG-PCR assays for one-step differentiation of 8 genes of foodborne pathogens in DNA extracted from 5 feces using 32 capillary tubes of LightCycler (Roche). The first run was for the detection of 8 main foodborne pathogens and the second run was for the other pathogens. We reported here that improved diagnostic duplex SG-PCR assays were upgraded with new highly sensitive primer pairs for 11 foodborne pathogens. These assays successfully identified the causative pathogens of foodborne outbreaks caused by enteropathogenic Escherichia coli, enterohemorrhagic E. coli, astA-positive E. coli, Plesiomonas shigelloides, Vibrio parahaemolyticus, Campylobacter jejuni, Clostridium perfringens, Bacillus cereus, or Staphylococcus aureus in 21 cases from 2002 to 2007. This assay is simple, rapid, inexpensive, reliable as well as suitable for comprehensive, rapid detection of causative pathogens in foodborne outbreaks.
2. Material and Methods
2.1. Bacterial Strains
The 27 foodborne bacteria used in this study are E. coli (enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), and enteroaggregative E. coli (EAEC)), Shigellasonnei, Salmonella Enteritidis, Yersinia enterocolitica, Yersinia pseudotuberculosis, Providencia alcalifaciens, Plesiomonas shigelloides, Campylobacter jejuni, C. coli, Vibrio cholerae, TDH-positive V. parahaemolyticus, TRH-positive V. parahaemolyticus, Aeromonas hydrophila, Staphylococcus aureus, emetic Bacillus cereus, enterotoxigenic B. cereus, and Clostridium perfringens (Table 1). Bacterial cultures and viable-cell counting were described in a previous report [10]. For template DNA of each foodborne pathogen as a PCR control, 200 μL of each bacterial culture (108 CFU/mL) was treated with a QIAamp DNA Stool Mini kit (Qiagen) in the same procedure as the following stool treatments.
| Bacterial strains | Sourcese | PCR results with each primer set (see Table 2) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eae | JMS1 | JMS2 | LT | STa | EAST-1 | aggR-Z | virA | Styinva | yadA-X | PAG | PSG | AB | CC ceuE | hlyA | tdh | trh | AHH1 | FemB | ces-TM | SG | GAP | ||
| Escherichia coli -EPECO55(eaeA) | EC-2736b | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -EPEC O153 (eaeA and astA) | EC-2649b | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -EHEC O26:H11(Stx1) | SE-02005 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -EHEC O157:H7 (Stx2) | SE020025 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -EHEC O157:H7 (Stx1 and Stx2) | SE-02027 | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -ETEC O148 (LT, ST and astA) | EC-3515b | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -ETEC O169 (ST and astA) | EC-4725b | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli- EAEC O111 (aggR and astA) | EC-4131b | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| E. coli -EIEC O124:HNM (virA) | EA32a | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Shigella sonnei | I00031 | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Salmonella Enteritidis | Sal-2339 | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Yersinia enterocolitica O3/B4 | Pa241 | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Y. pseudotuberculosis O4b | SP988 | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Providencia alcalifaciens | NIID124C | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Plesiomonas shigelloides | NIID123C | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Campylobacter jejuni | SC 009 | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| Campylobacter coli | SC 011 | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Vibrio cholerae O1 | ATCC14035 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| V. cholerae O139 | NIID63-93C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| V. cholerae non- O1 | SVP84 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| V. parahaemoliticus O3:K6 (tdh) | SVP02 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − |
| V. parahaemoliticus O3:K6 (trh) | NIIDK4C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| Aeromonas hydrophila O1 | ATCC7966 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| Staphylococcus aureus | SS 05e | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − |
| Emetic Bacillus cereus | No.127e | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − |
| Enterotoxigenic B. cereus | No.1e | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| Clostridium perfringens | H2d | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
- Strain kindly donated by K. Sugiyamaa, Shizuoka Prefectural Institute of Public Health, Shizuoka; J. Yatsuyanagib, Akita Prefectural Institute of Public Health, Akita; M. Tamura and E. Arakawac, fOther strains except for ATCC numbers are our own collections.
2.2. Primer Design
The 22 primer pairs used in this study for the detection of E. coli (EIEC, EPEC, EHEC, ETEC, and EAEC), Salmonellaenterica, Shigella spp., Y. enterocolitica, Y. pseudotuberculosis, P. alcalifaciens, C. jejuni, C. coli, V. cholerae, V. parahaemolyticus, A. hydrophila, P. shigelloides, S. aureus, C. perfringens, and B. cereus were described in our previous reports [10, 13] for cases 1 to 19. The newly designed 22 primer pairs listed in Table 2 were used for cases 19 to 21. In this study, 10 primer pairs (marked with * in Table 2) were newly designed or selected from earlier publications (see Table 2 references). The 4 primer pairs (ces, yadA-X, CCceuE, and aggR-Z) were newly designed. The ces primer was constructed from cereulide synthetase gene of emetic B. cereus [4], the yadA-X primer from yadA gene on the plasmid present in virulent Yersinia spp. [24], the CCceuE primer from ceuE gene encoding of a lipoprotein component of a binding-protein-dependent transport system for the siderophore enterochelin of C. coli [25], and the aggR-Z primer from aggR gene encoding of a transcriptional activator for EAEC aggregative adherence fimbria I expression [26]. To determine the specific primers ces, yadA-X, CCceuE, and aggR-Z, the genes of ces, yadA, ceuE, and aggR that were expected to be unique were selected with the Basic Local Alignment Search Tool (BLAST) program within GenBank and were designed by Biosearch Technologies Inc. (USA). Other primer pairs were those used in earlier publications (see Table 2 references). All oligonucleotide primers were synthesized by Invitrogen (Yokohama, Japan) or Biosearch Technologies Inc. (USA).
| Primer set for duplex PCR | Species and subgroups | Target gene | PCR primers | GenBank accession no. | location | Product size (bp) | Tm valuesa | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Forward or revers | primers′ sequences (5′ - 3′) | |||||||||
| First run | 1 Escherichia coli | eaeA | *b | eae | F2 | CATTGATCAGGATTTTTCTGGTGATA | Z11541 | 899-924 | 106 | 83.2 ± 0.2 | [14] |
| EPEC and EHEC | R | CTCATGCGGAAATAGCCGTTA | 1000-979 | ||||||||
| Staphylococcus aureus | femB | * | FemB | fw | AATTAACGAAATGGGCAGAAACA | AF106850 | 277-299 | 93 | 80.8 ± 0.3 | [15] | |
| rv | TGCGCAACACCCTGAACTT | 370-351 | |||||||||
| 2 Campylobacter jejuni | C. jejuni- specific DNA | * | AB | F | CTGAATTTGATACCTTAAGTGCAGC | AL111168 | 381135 | 86 | 79.1 ± 0.4 | [8] | |
| DNA | R | AGGCACGCCTAAACCTATAGCT | 381185 | ||||||||
| EAEC | astA | EAST-1 | S | GCCATCAACACAGTATATCC | L11241 | 63-82 | 106 | 84.9 ± 0.6 | [16] | ||
| AS | GAGTGACGGCTTTGTAGTCC | 168-148 | |||||||||
| 3 Vibrio parahaemoliticus | tdh | Tdh199 | F | GGTACTAAATGGCTGACATC | X54341 | 601-582 | 251 | 81.6 ± 0.3 | [17] | ||
| R | CCACTACCACTCTCATATGC | 351-370 | |||||||||
| Emetic Bacillus cereus | ces | * | ces-TM | F | GATGTTTGCGACGATGCAA | DQ360825 | 8689-8707 | 65 | 80.4 ± 0.1 | This study | |
| R | CTTTCGGCGTGATACCCATT | 8793-8734 | |||||||||
| 4 Salmonella spp. | invA | * | Styinva | JHO-2-right | TCGTCATTCCATTACCTACC | M90846 | 167-186 | 119 | 81.3 ± 0.4 | [5] | |
| JHO-3-left | AAACGTTGAAAAACTGAGGA | 285-234 | |||||||||
| Clostridium perfringens | cpe | GAP | 11 | GGTTCATTAATTGAAACTGGTG | X81849 | 583-604 | 154 | 78.3 ± 0.4 | [18] | ||
| 12 | AACGCCAATCATATAAATTACAGC | 712-736 | |||||||||
| Second and third runs | 5 ETEC (ST) | ST | STa | F | GCTAATGTTGGCAATTTTTATTTCTGTA | M25607 | 294-321 | 190 | 78.5 ± 0.2 | [19] | |
| R | AGGATTACAACAAAGTTCACAGCAGTAA | 483-456 | |||||||||
| Plesiomonas shigelloides | gyrB | PSG | 237-F | TTCCAGTACGAGATCCTGGCTAA | AJ300545 | 237-259 | 68 | 83.1 ± 0.2 | [13] | ||
| 304-R | TGAATCGACACGCCAGAGTTC | 304-284 | |||||||||
| 6 EAggEC | aggR | * | aggR-Z | F | CAGAATCGTCAGCATCAGCTACA | Z18751 | 432-454 | 97 | 79.5 ± 0.3 | This study | |
| R | GATGCCCTGATGATAATATACGGAA | 358-382 | |||||||||
| EIEC & Shigella spp. | virA | virA | F | CTGCATTCTGGCAATCTCTTCACA | D26468 | 1589-1622 | 215 | 82.4 ± 0.3 | [20] | ||
| R | TGATGAGCTAACTTCGTAAGCCCTCC | 1813-1788 | |||||||||
| 7 Aeromonas hydrophila | ahh1 | * | AHH1 | F | GCCGAGCGCCCAGAAGGTGAGTT | CP000462 | 1653360-82 | 133 | 89.8 ± 0.4 | [21] | |
| R | GAGCGGCTGGATGCGGTTGT | 1653492-73 | |||||||||
| ETEC (LT) | LT | LT | 1 | AGCAGGTTTCCCACCGGATCACCA | S60731 | 613-636 | 132 | 82.0 ± 0.3 | [22] | ||
| 2 | GTGCTCAGATTCTGGGTCTC | 744-725 | |||||||||
| 8 Providencia alcalifaciens | gyrB | PAG | 38F | TCTGCACGGTGTGGGTGTT | AJ300547 | 38-56 | 73 | 81.0 ± 0.2 | [13] | ||
| 110R | ACCGTCACGGCGGATTACT | 110-92 | |||||||||
| Enterotoxigenic B. cereus | nheB | * | SG | F3 | GCACTTATGGCAGTATTTGCAGC | DQ153257 | 2101-2123 | 152 | 82.7 ± 0.4 | [23] | |
| R3 | GCATCTTTTAAGCCTTCTGGTC | 2252-2231 | |||||||||
| 9 Yersinia enterocolitica | yadA | * | yadA-X | F | CCAGAACCAATTGCAATGCCT | X13882 | 1564-1543 | 100 | 81.6 ± 0.2 | This study | |
| Y. pseudotuberculosis | R | CTTTAAACAGCTTGTTCCAGCCA | 1465-1487 | 81.1 ± 0.3 | |||||||
| Campylobacter coli | ceuE | * | CCceuE | 825F | ACGCGCACAAGGCATACTT | X88849 | 3513-3531 | 91 | 77.6 ± 0.3 | This study | |
| 915R | CCAGTATTCAGGATCAAGATAAATGATTT | 3603-3575 | |||||||||
| 10 Vibrio cholerae | hlyA | hlyA | 2272-F | AGCAGCGTGTGGGACAAGA | X51746 | 2272-2291 | 71 | 82.4 ± 0.1 | [13] | ||
| 2272-F | GCGGACCCTAATGCATCAAT | 2342-2323 | |||||||||
| Vibrio parahaemoliticus | trh | Trh | 250-F | GGCTCAAAATGGTTAAGCG | DQ359748 | 256-274 | 250 | 81.1 ± 0.1 | [17] | ||
| 251-R | CATTTCCGCTCTCATATGC | 505-487 | |||||||||
| Singl PCR | EHEC (Stx 1) | Stx1 | JMS1 | F | GTCACAGTAACAAACCGTAACA | EF441598 | 509-488 | 95 | 81.1 ± 1.0 | [12] | |
| R | TCGTTGACTACTTCTTATCTGGA | 415-437 | |||||||||
| Singl PCR | EHEC (Stx 2) | Stx2 | JMS2 | F | CGACCCCTCTTGAACATA | EF441616 | 140-157 | 108 | 81.7 ± 0.3 | [12] | |
| R | GATAGACATCAAGCCCTCGT | 247-228 | |||||||||
- a: Average ± standard deviation of Tm values of 10 tests: b: *:new selected or designed primer
2.3. Duplex SG-PCR with Feces
Feces (1 g) from 5 patients were weighed aseptically from the mass sample collected for virological inspection, placed into sterile tubes, and homogenized with 9 mL of distilled water. Then, 200 μL of stool suspension was treated with a QIAamp DNA Stool Mini kit. For real-time PCR, we used SYBR Premix EX Taq (Takara, Japan), 32 glass capillary tubes, and a LightCycler instrument (Roche Diagnostics, Mannheim, Germany) as described by the manufacturer. Duplex SG-PCR was performed using 32 glass capillary tubes with 4 groups of 2 primer sets on the LC instrument for each run. Analysis of each group of primer pairs was made in 8 glass capillary tubes; each of which included 1 negative DNA control consisting of PCR-grade water, 2 positive controls, and template DNA from 5 feces. The first run of duplex SG-PCR was analyzed using 4 primer sets selected from 11 primer sets described in our previous reports [10, 13]. The newly first run primer set including eae plus FemB, AB plus EAST1, Tdh plus Ces-TM, and Styinva plus GAP (see Table 2) was used for analysis of cases 19 to 21. The second run was analyzed using 4 primer sets selected from the following primer sets: LT plus AHH1, STa plus PSG, aggR-Z plus virA, SG plus PAG and the third run using yadA-X plus CCceuE, and hlyA plus Trh. The eaeA-positive samples were analyzed by simple PCR using primers JMS1 and JMS2. Each reaction tube contained 10 μL of SYBR Premix EX Taq, 6.8 μL of PCR-grade H2O, 0.4 μL of both forward and reverse primers (10 μM) for the target gene of two foodborne pathogens, and 2 μL of template DNA in a 20 μL PCR mixture. The assay cycling profile was 95 °C for 10 minutes, followed by 30 cycles of denaturation at 95 °C for 5 seconds and then annealing at 60 °C for 20 seconds. Fluorescence signals were measured once per cycle at the end of the extension step. After PCR amplification, a melting temperature curve analysis was done. Next, the LightCycler PCR products were cooled to 65 °C and then heated to 95 °C at a rate of 0.1 °C per second. The fluorescence signals obtained were continuously monitored to confirm amplification specificity during 1 hour of analysis. The products’ melting temperature peaks were calculated by performing 10 or more assays per sample and were based on the initial fluorescence curve found by plotting the negative derivative of fluorescence over temperature versus temperature. To quantify target bacteria in feces, DNA samples extracted with the QIAamp DNA Stool Mini kit from target bacteria were used to form a standard curve. Two microliters of a serial 10-fold dilution of DNA (Easy Dilution from Takara, Japan) were prepared and analyzed under the conditions specified above.
2.4. Duplex SG-PCR Analysis in 21 Foodborne Outbreaks
21 foodborne outbreak cases examined by duplex SG-PCR in Shimane Prefecture, Japan from 2002 to 2007 are shown in Table 3.
| Case No. | Date ocurred (day/mo/yr) | Days for examination after occurrence | Infected group | Source of infection (suspected source) | No. of patients/total | No. of examined patients | Causative pathogens | Stool samples (No. of positive/ examined samples) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG-PCR | Isolation | ||||||||||||
| 1st test | 2nd test | 3rd test | Final test | Total | |||||||||
| 1 | 4-Oct-02 | 6 | School excursion in a mountain area | Stream watera | 23/33 | 22 | *EPEC O:125, O:166, O:UT *astA-positive E. coli O:1, O:UT | 1/7 | — | — | 4/22 | 7/22 | 5/22 |
| 2 | 03-Sep-03 | 3 | Protective care school | Catering box lunch | 22/46 | 10 | astA-positive E. coli O:18, | 1/5 | — | — | 6/10 | 6/10 | 3/10 |
| O:20, O:114, O:159, O:UT | |||||||||||||
| [Norovirus | 6/7] | ||||||||||||
| 3 | 01-Oct-03 | 2 | Celebration in a company | Catering box lunch | 437/1354 | 12 | * C. perfringens O:13, O:16 | 5/5 | — | — | 7/12 | 7/12 | 10/12 |
| 4 | 11-Jun-04 | 6 | Camping group of high school | Grilled meat (beef, bovine intestinal meat) | 4/8 | 4 | C. jejuni | 1/4 | — | — | 1/8 | 1/8 | 5/8 |
| 5 | 12,13-Jun-04 | 6 ~ 7 | 9 citizen groups in Chophouse | Grilled meat (beef, bovine intestinal meat) | 30/UN | 12 | C. jejuni | 4/5 | — | — | 8/12 | 8/12 | 10/12 |
| 6 | 17-Jun-04 | 5 | Cooking practise in a high school | Shelf-cooked lunch (salada mixed chicken) | 31/41 | 20 | * C. jejuni | 4/5 | — | — | 12/14 | 12/14 | 17/20 |
| 7 | 07-Jul-04 | 1 | Citizen in Chinese restaurant | Fried riceb | 6/6 | 6 | * B. cereus | 1/1 | — | — | 2/6 | 2/6 | 2/6 |
| 8 | 11-Oct-04 | 3 | Sport club in a high school | Shelf-cooked lunch | 26/47 | 6 | * C. perfringens O:16, OUT | 1/5 | — | — | 3/6 | 3/6 | 4/6 |
| 9 | 5~7-Nov-04 | 5 ~ 7 | Restaurant | Unknown | 5 | 5 | C. jejuni | 2/5 | — | — | 2/5 | 2/5 | |
| 10 | Unknown | Several days (19-Jur-05) | Nursery | Unknown | 24/73 | 22 | *EHEC O26 | 8/22 | 8/22 | 8/22 | |||
| [Norovirus | 20/22] | ||||||||||||
| 11 | 28~30-Sep-05 | 1 ~ 3 | Prisoners in a prison | Shelf-cooked mealc | 113/600 | 61 | astA -positive E. coli | — | — | 14/14 | 14/14 | 41/46 | |
| C. perfringens (sporadic case) | 1/5 | 1/46 | |||||||||||
| 12 | 2~6-Oct-05 | 1 ~ 5 | Elementary and high school children | Unknown (School lunch) | 39/94 | 39 | astA -positve E. coli | 5/5 | — | — | 5/5 | IMf | |
| EPEC | 2/5 | IM | |||||||||||
| A. hidrophila | 1/5 | 1/16 | |||||||||||
| C. jejuni (sporadic case) | 1/5 | IM | |||||||||||
| 13 | 28~30-May-06 | 0 ~ 2 | Citizens at restaurant | Lunch (pilaf and scrambled aggd) | 27/34 | 27 | * S. aureus | 2/5 | — | — | 2/5 | 4/8 | |
| astA-positve E. coli | 1/5 | ||||||||||||
| 14 | 4-Jul-06 | 0 | Boarder of high school | Catering box lunch | 34/51 | 34 | * C. perfringens | 5/5 | — | — | 8/8 | 8/8 | 19/50 |
| 15 | 16-Aug-06 | 1 | Citizens at restaurant | Fried rice | 15/34 | 15 | * B. cereus | 1/4 | — | — | 1/4 | 2/4 | |
| 16 | 23~29-Aug-06 | 2 ~ 8 | Boarder of training high school | Supper (contaminated sliced cabbagee) | 19/43 | 18 | * C. jejuni | 3/5 | — | — | 6/9 | 8/9 | 9/14 |
| astA -positve E. coli | 4/5 | 5/9 | IM | ||||||||||
| 17 | 2-Sep-06 | 3 | Citizens in Buddhist service | Catering box lunch | 14/49 | 4 | V. parahaemolyticus | 4/5 | — | — | 4/6 | 4/6 | 3/6 |
| 18 | 22-Dec-06 | 5 | Citizens at restaurant | Supper (chiken) | 12/12 | 8 | * C. jejuni | 3/5 | — | — | 4/9 | 4/9 | 4/10 |
| 19 | 4-Jul-07 | 6 | Citizens at restaurant | Supper (chiken) | 7/11 | 7 | * C. jejuni | 1/2 | — | — | 1/2 | 2/3 | |
| 20 | 21-Oct-07 | 1 | Citizens at restaurant | Supper | 7/13 | 7 | *EPEC | 2/5 | — | 4/5 | IM | ||
| P. shigelloides | 2/5 | 2/5 | |||||||||||
| 21 | 29-Nov-07 | 1 | Citizens at restaurant | Supper (raw chiken liver) | 8/8 | 7 | * C. jejuni | 3/5 | — | — | 4/7 | 5/7 | 4/7 |
| astA -positve E. coli | 1/5 | 1/7 | |||||||||||
| Total | 54/93 | 111/191 | 160/276 | ||||||||||
| 58.1% | 58.1% | 58.0% | |||||||||||
- a: EPEC O : 166, O : UT and astA -positive E. coli O : 27, O : UT strains were isolated from stream water drunk by patients in case 1., b: B cereus was isolated from cooked pork in case 7. c: astA genes were detected from 5 food samples in case 11., d: S. aureus was isolated from pilaf and scrambled egg in case 13., e: C. jejuni specific gene was detected from 5 food samples in case 17. f: Impossible isolation. *: 14 cases examined by SG-qPCR and viable cell count.
3. Results and Discussion
3.1. Duplex SG-PCR Procedures
We previously reported duplex SG-PCR assays for detection of 19 species of foodborne pathogens using 22 primer pairs [10, 13]. After that, more accurate duplex SG-PCR assays were designed by 10 more sensitive and specific primers including 6 primers (FemB, AB, ces-TM, Styinva, SG, and AHH1) selected from earlier publications (see references in Table 2) and 5 new primers (eae, aggR-Z, yadA-X, and CCceuE) constructed in this study. The new primer set was used for cases 19 to 21. Real-time SG-PCR procedures using 22 primer pairs for the detection of 15 bacterial species, including 5 E coli subgroups, were developed for the duplex assay. The primer sequence, target, SG-PCR product size, Tm values (mean plus standard deviation from a range of 10 assays), specificities, and references are summarized and listed in Tables 1 and 2. The primer virA detects virA gene of Shigella spp. and EIEC; the primer eae detects eaeA gene of EPEC and EHEC, and the primer EAST-1 detects astA gene of EAEC and ETEC. Primer hlyA detected hlyA gene of V. cholerae strains O1 and O139 as well as non-O1 strains. The primer SG for the detection of nheB (nonhemolytic enterotoxin B) gene of B. cereus cross-reacts with enterotoxigenic and emetic strains and the primer ces-TM detects cereulide synthetase gene of emetic strain of B. cereus. The nheB and ces gene positive strains were identified with emetic strains and the nheB gene positive and ces gene negative strains with enterotoxigenic strains. A new primer yadA-X for Yersinia adhesion reacts with virulent Y. enterocolitica and Y. pseudotuberculosis, but not with nonpathogenic strains of Yersinia spp. (data not shown). Other primers, including new primers aggR-Z and CCceuE, specifically detect each gene of EAEC and C. coli. Food-borne Outbreak Investigation Report (http://www.mhlw.go.jp/topics/syokuchu/), Ministry of Health, Labor and Welfare, Japan during 2005 to 2007 shows that 97% of foodborne outbreaks were caused by the following 7 species of foodborne pathogens: S. enterica (58.3%), C. jejuni (15.2%), TDH-producing V. parahaemolyticus (8.3%), S. aureus (7.2%), C. perfringens (3.6%), emetic B. cereus (1.6%), EHEC (2.9%), and other virulent E. coli (2.1%) which include astA-positive E. coli which is a strain of E. coli that does not possess any diarrheagenic characteristics except the EAEC heat-stable toxin 1 (EAST1) gene and is frequently isolated in diarrhea outbreaks [27]. Using of 4 primer sets of 2 primer pairs, including newly selected or designed 6 primer pairs, for the detection of 7 main foodborne pathogens and astA-positive E. coli in the first run of duplex SG-PCR brought out the comprehensive, rapid, and sensitive detection of causative pathogens in foodborne pathogens to cases 19 to 21 (Table 2 and Figures 1 and 2). The second run of duplex SG-PCR used 4 primer sets and the final run utilized 2 primer sets selected from the remaining 4 primer pairs. The primers JMS1 and JMS2 were used for the single PCR detection of stx1 and/or stx2 genes from the eaeA gene-positive samples for the confirmation of EHEC. Figures 1 and 2 show the Tm curves of the duplex SG-PCR products of the template DNA samples in each run. In duplex SG-PCR assay with two primer pairs, each PCR product was generated with a different Tm curve. These could be resolved in a LightCycler by using Tm curve analysis when a target bacterium was present in the reaction tube.
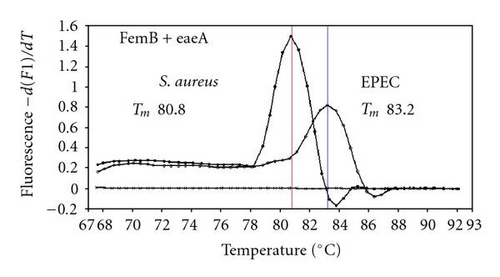
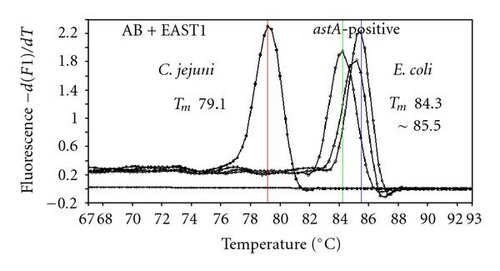
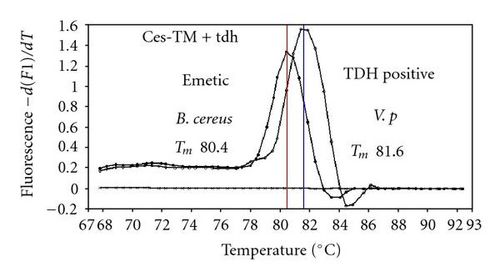
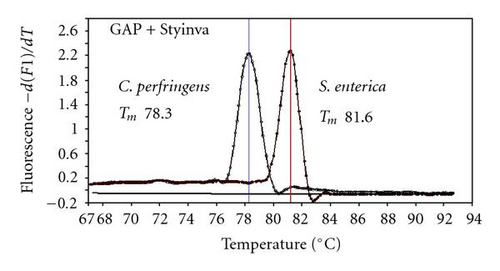
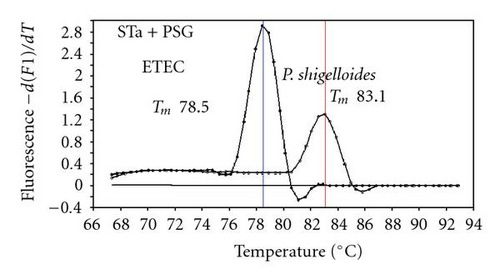
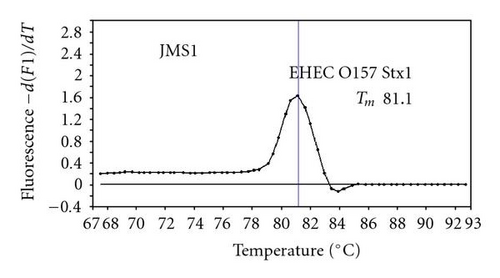
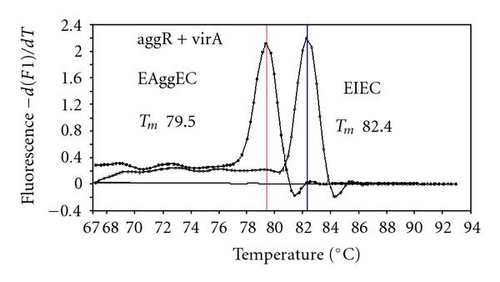
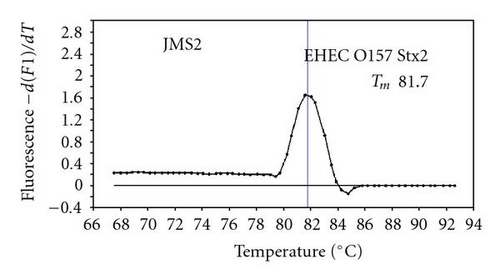
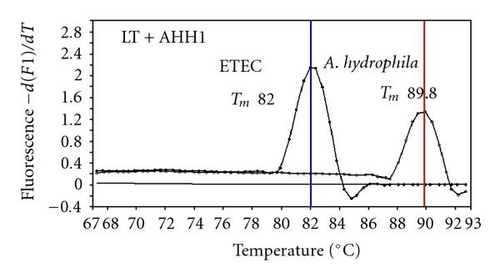

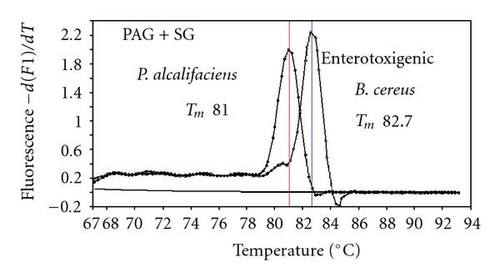
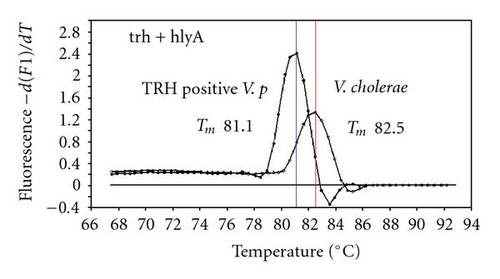

3.2. Using Duplex SG-PCR for Identification of the Causative Agent in 21 Foodborne Outbreaks
Table 3 shows epidemiological and clinical investigations in 21 foodborne outbreaks examined by duplex SG-PCR analysis in Shimane Prefecture, Japan from 2002 to 2007. From samples of feces, we used a combination of duplex SG-PCR assay with DNA extraction using a QIAamp DNA Stool Mini kit. The SG-PCR assay is rapid, specific, and sensitive as a detection technique. The DNA extraction of 5 stool specimens with the QIAamp DNA Stool Mini kit was carried out within 1 hour and it effectively removed inhibitors present in feces. The duplex SG-PCR assay was also carried out within 1 hour. The 7 species (listed previously) of foodborne bacteria, which included 3 groups of E. coli, were detected from 111 (58.1%) of 191 feces in 21 cases by duplex SG-PCR. Then these causative agents were isolated and identified after 2 to 4 days. With the exception of two cases (cases 10 and 11), the first run of duplex SG-PCR confirmed the presence of a pathogen in 54 (58.1%) of 93 feces in 19 (90.5%) cases within 2 hours. The exceptions were case 10 where a confirmation test was necessary to detect the eaeA gene of EHEC O26 and case 11 where astA-positive E. coli was detected on the third run. In the first run, DNA samples extracted from 5 feces (1, 3, 4, or 7 feces in 6 cases) of symptomatic patients were used and the causative pathogens were detected from 1 to 5 samples: 1 (in 8 cases: 1, 2, 4, 7, 8, 15, 19, and 21), 2 (in 3 cases: 9, 13, and 20), 3 (in 3 cases: 16, 18, and 21), 4 (in 3 cases: 5, 6, and 17), and 5 samples (in 3 cases: 3, 12, and 14). Then the causative pathogens were later isolated in a routine laboratory. In cases 11 and 12, C. perfringens or C. jejuni was detected by duplex SG-qPCR with more than 105 CFU/g feces from only 1 sample and C. perfringens was then also isolated from only 1 of 46 samples and C. jejuni from only 1 of 16 samples by culture method. Therefore, the infections with both these pathogens were determined to be sporadic cases and they were immediately eliminated as causative pathogens in cases 11 and 12. It was confirmed that duplex SG-PCR analysis of 5 feces collected from symptomatic patients was ultimately the most effective screening method for foodborne pathogens in foodborne outbreaks [10, 13].
Duplex SG-PCR rapidly and accurately demonstrated that 12 (57.1%) of 21 cases were caused with a single foodborne pathogen such as C. jejuni (6 cases), C. perfringens (3 cases), B. cereus (2 cases), and TDH-producing V. parahaemolyticus (one case). There were also 7 (33.3%) cases with plural foodborne bacterial pathogens (such as astA-positive E. coli, EPEC, C. jejuni, C. perfringens, A. hydrophila, and P. shigelloides) and 2 (9.5%) cases with foodborne bacterial pathogens (astA-positive E. coli or EHEC O:26) and norovirus. In cases 2 and 10, although detection of norovirus is out of the scope of our work, norovirus and foodborne bacterial pathogens were concomitantly detected by conventional PCR analysis in our virological laboratory. In case 2 in which norovirus was detected in 6 of 7 feces, the astA gene of EAEC was detected from 7 of 10 feces and then astA-positive E. coli strains were isolated from 6 samples. In case 10 in which norovirus was detected from 20 of 22 feces, the eae gene of EPEC or EHEC was detected from 8 of 22 feces and EHEC O26 strains were isolated from 8 of 22 feces. In 7 cases (cases 1, 11, 12, 13, 16, 20, and 21), the pathogenic E. coli strains belonging to astA-positive E. coli and/or EPEC were concomitantly detected with other foodborne bacterial pathogens. In case 1, the eae gene of EPEC or EHEC was detected from 4 of 22 feces and the astA gene of EAEC was detected in 3 other feces. However, duplex SG-PCR could not detect other virulent genes, including the stx1 and stx2 genes of EHEC. Then EPEC strains were later isolated from 5 feces and astA-positive E. coli from 4 other feces. In case 12, the astA gene of EAEC was detected in all 5 feces and the eae gene of EPEC or EHEC in 2 feces, but duplex SG-PCR could not detect other E. coli virulent genes. The subsequent bacteriological examination could not isolate pathogenic E. coli among nonpathogenic E. coli flora. In case 16, the C. jejuni specific gene was detected in 6 of 9 feces and the astA gene of EAEC was detected in 5 feces (both genes from 3 feces). C. jejuni strains were then isolated from 9 of 14 feces, but we were not able to isolate the pathogenic E. coli strain among nonpathogenic E. coli flora. In cases 19 to 21 analyzed improved real-time PCR using 8 primers for the detection of 7 main foodborne bacteria and astA-positive E. coli, C. jejuni, EPEC, or astA-positive E. coli were detected from 1 to 3 fecal samples on the first run and the absence of the other main foodborne bacteria in the analyzed samples was readily confirmed. In case 20, the eae gene of EPEC or EHEC was detected from 2 of 5 fecal samples on the first run and the gyrB gene of P. shigelloides was detected separately from other 2 fecal samples on the second run. Then P. shigelloides strains were isolated from 2 feces, but isolation of the EPEC strain was very difficult due to the presence of large nonpathogenic E. coli flora in the feces.
In almost all cases, the duplex SG-PCR assay first run detected these causative agents from more than one of the five feces. Then, in almost all cases, the presence of a causative agent (presumed from duplex SG-PCR assay) was confirmed by the results of the final SG-PCR assay run and the bacteriological cultivation of additional feces. These findings confirmed that for foodborne outbreaks duplex SG-PCR is a useful tool for the rapid detection of both single and multiple pathogens.
3.3. Quantification of the Causative Agent in 14 Foodborne Outbreak Cases
Figure 2 shows the relationship between CFU and DNA copy of foodborne pathogens using SG-quantitative PCR (qPCR) assay in 71 feces from 14 cases examined by viable cell counting. There was no correlation (r2 = 0.1183) between CFU and DNA copy of foodborne pathogens in feces, although almost all pathogens were detected by SG-PCR from feces registering more than 103 CFU/g by viable cell counting. By using SG- qPCR assay combined with DNA extraction using the QIAamp DNA Stool Mini kit, Bibbal et al. [28] reported a significant correlation between CFU and DNA copy of ampicillin-resistant Enterobacteriaceae in swine feces. Fu et al. [29] reported a significant correlation between CFU and DNA copy of Lactobacillus and total anaerobic bacteria in dog feces but found no correlation between CFU and DNA copy of C. perfringens. Although accurate quantifications of foodborne pathogens, including C. jejuni and C. perfringens, in feces were not completely performed by SG-qPCR in this study, the presence of any foodborne pathogens at more than 103 CFU/g feces was certainly confirmed by melting curve analysis. There are two major problems for these differences. One cause is different sample preparation that was used for CFU from the feces stored in the transport medium and for qPCR using the mass sample collected for virological inspection. Another cause is the approach used to construct the standard curves that were prepared from pure bacterial cultures. These curves do not relate with the “real” situation of a bacterial quantification in a faecal sample and can in part explain the absence of correlation between CFU and DNA copy of foodborne pathogens in faeces.
In our routine bacteriological diagnostic laboratory, we used duplex SYBR Green I PCR assay combined with DNA extraction via QIAamp DNA Stool Mini kit for the detection of foodborne bacteria from 21 foodborne outbreak cases. The causative bacteria were detected in almost all cases in 2 hours or less. The first run was for the detection of 8 main foodborne bacteria and the second run was for the detection of other unusual suspect bacteria. The results proved that for comprehensive and rapid molecular diagnosis in foodborne outbreaks, duplex SG-PCR assay is not only very useful, but is also economically viable for one-step differentiation of causative bacteria in fecal specimens obtained from symptomatic patients. This then allows for effective diagnosis and management of foodborne outbreak.
Acknowledgment
This work was supported in part by a grant-in-aid of the Japanese Ministry of Health, Labor and Welfare (H19-Kenki-011).




