Molecular Phylogeny of Mouse-Tailed Bats (Chiroptera: Rhinopomatidae): Evolutionary Insights Into a Desert-Dwelling Family
Funding: This work was supported by Ministry of Culture of the Czech Republic, DKRVO 2024-2028/6.I.b, National Museum, 00023272.
ABSTRACT
The family Rhinopomatidae comprises six species of mouse-tailed bats within the genus Rhinopoma, inhabiting arid regions of the Old World from western Africa to Myanmar. While their phylogenetic relationships have been explored through morphology and molecular analyses, many populations still remain insufficiently studied using molecular approaches. This study aims to analyse samples representing all species and populations within the family, employing one mitochondrial and four nuclear genetic markers. These sequences were used to construct phylogenetic trees, estimate divergence times and evaluate biogeographic patterns. Our results revealed the presence of five major genetic lineages within Rhinopoma. Notably, the morphologically distinct East African R. macinnesi was genetically embedded within R. cystops, suggesting synonymisation of the former name and expanding the distribution range of R. cystops southward into eastern Africa. The study also identified greater mitochondrial diversity compared to nuclear markers, uncovering nine mitochondrial lineages across the family, including three polytypic species: R. cystops, R. hardwickii and R. muscatellum. Divergence time estimation placed the family's origin at approximately 45 million years ago. Initial diversification into current species occurred 9.5 million years ago, likely originating in the Arabian Peninsula or Iran, regions that still host the highest diversity of Rhinopoma. This study provides the most comprehensive molecular phylogeny of Rhinopomatidae to date, reducing the recognised species count to five and offering new insights into the evolutionary history and biogeography of this desert-adapted bat family.
1 Introduction
The genetic era has significantly advanced our understanding of biodiversity, unveiling morphologically cryptic lineages and driving numerous intra- and interspecific taxonomic revisions. These revisions have either split existing species into distinct entities or, less commonly, lumped previously separated taxa into a single entity. Despite these advances, many mammal groups, including bats, remain taxonomically complex and understudied, particularly those that have received limited systematic attention (Burgin et al. 2018). The family of mouse-tailed bats, Rhinopomatidae Bonaparte, 1838, exemplifies such a group. To date, no comprehensive study has encompassed all extant species in this family using a molecular genetic approach.
The family of mouse-tailed bats, Rhinopomatidae, derives its vernacular name from their long, slender tails, which are almost entirely free from the uropatagium. This family comprises a single genus, Rhinopoma Geoffroy, 1818, currently including six recognised species (Hulva et al. 2007; Horáček 2019; Mammal Diversity database 2025). These desert-dwelling bats are further distinguished by their small nose-leaf, relatively long forearms, short wing fingers and jointed ears. Their distribution primarily spans a belt of arid and semi-arid habitats stretching across northern Africa, the Middle East and southern Asia, from Morocco, Senegal and Kenya to the Levant, Arabia, Iran, India and Myanmar. Rare occurrences have also been reported in Thailand and Indonesia (Hill 1977; Koopman 1994; Van Cakenberghe and De Vree 1994).
Previous genetic studies have redefined the taxonomic placement of Rhinopomatidae (Teeling et al. 2002, 2005; Hulva and Horáček 2002; Eick et al. 2005; Hulva et al. 2007). Traditionally grouped with Craseonycteridae Hill, 1974, in the superfamily Emballonuroidea Gervais, 1856, these studies placed Rhinopomatidae and Craseonycteridae within the Rhinolophoidea Grauy, 1825 superfamily, alongside Megadermatidae Allen, 1864. Compared to other clades of Rhinolophoidea, such as Rhinolophidae Gray, 1825 (one genus, 114 species), Hipposideridae Lydekker, 1891 (7 genera, 92 species) and Rhinonycteridae (4 genera, 9 species). Rhinopomatidae is much less diverse with only six currently recognised species, akin to its sister families Megadermatidae (6 genera, 7 species) and Craseonycteridae (one genus, one species) (Wilson and Mittermeier 2019; Mammal Diversity Database 2025; Feng et al. 2025).
Over the last century, the taxonomy of the genus Rhinopoma has undergone significant revisions. Initially, only two species were distinguished based on body size: the large-sized Rhinopoma microphyllum (Brünnich, 1782) and the small-sized R. hardwickii Gray, 1831, both considered to span the entire distribution range of the family. In the early 20th century, two additional species were added. Rhinopoma cystops Thomas, 1903, was proposed for a smaller form found in Egypt, and R. muscatellum Thomas, 1903, distinguished by its distinctive narial swellings and found in Arabia (Thomas 1903). Later taxonomic treatments synthesised these four species back into two, based primarily on size, with multiple subspecies recognised within each (Ellerman and Morrison-Scott 1951; Harrison 1964; Kock 1969). However, the distinct morphology of R. muscatellum, along with the sympatric occurrence with similarly sized R. hardwickii, prompted its reconsideration as a separate species (DeBlase et al. 1973; Hill 1977).
Subsequent morphological revision identified an additional species, R. macinnesi Hayman, 1937. This species, characterised by its smaller body size and the moderately developed narial swellings (Van Cakenberghe and De Vree 1994), was initially classified as a subspecies of R. cystops (Hayman 1937), R. hardwickii (Ellerman and Morrison-Scott 1951; Kock 1969; Hill 1977) or even R. muscatellum in Eritrean populations (Kock 1969; Largen et al. 1974). The elevation of R. macinnesi to species rank was accepted by subsequent authors (Schlitter and Qumsiyeh 1996; Kock et al. 2001; Simmons 2005; Horáček 2019; Mammal Diversity Database 2025).
Finally, molecular data further expanded the family to six species (Hulva et al. 2007; Benda et al. 2009). The deep divergence between two mitochondrial lineages within R. hardwickii led to its division into two separate species: the Afro-Arabian R. cystops and Irano-Indian R. hardwickii s.str. (Hulva et al. 2007). Similarly, a genetically and morphologically distinct Yemeni population originally assigned to R. muscatellum (Kock et al. 2001) resulted in the description of R. hadramauticum Benda, 2009 (Hulva et al. 2007; Benda et al. 2009). Altogether, six species are currently recognised as valid, with several subspecies identified within most of them (Van Cakenberghe and De Vree 1994; Simmons 2005; Hulva et al. 2007; Benda et al. 2012; Horáček 2019).
The widespread R. microphyllum is distinctive for its large body size, a tail shorter than its forearm, a high sagittal crest on its skull and a pentagonal rostrum shape (Van Cakenberghe and De Vree 1994). Its current distribution extends from Senegal, along the edges of the Sahara, through the Levant and Arabian Peninsula, and further east to India (Horáček 2019). A small number of specimens have also been documented from Sumatra (Van Cakenberghe and De Vree 1994). Historically, up to six subspecies of R. microphyllum were recognised based on geographic distribution and minute variations in body size (Kock 1969; Schlitter and DeBlase 1974; Hill 1977; Nader and Kock 1982; Schlitter and Qumsiyeh 1996). These include microphyllum, spanning from western Africa to the Levant; asirensis Nader and Kock, 1982, from south-western Arabia; harrisoni Schlitter and DeBlase, 1974, from south-western Iran; kinneari Wroughton, 1912, from Iran to India; sumatrae Thomas, 1903, from Sumatra; and tropicalis Kock, 1969, from the African Sahel. However, a detailed morphological revision reduced the number of subspecies to four by synonymising harrisoni and tropicalis with microphyllum (Van Cakenberghe and De Vree 1994).
More recently, phylogeographic studies based on mitochondrial DNA from the Middle East have suggested differences between lineages, supporting the validity of at least three subspecies: microphyllum, harrisoni and kinneari (Akmali et al. 2011; Bagherfard et al. 2021). Despite these findings, the validity of the subspecies remains debated. The primary justification for recognising these subspecies continues to be their distinct geographic ranges (Hill 1977; Van Cakenberghe and De Vree 1994), though some authors have proposed treating R. microphyllum as monotypic (Hulva et al. 2007; Horáček 2019).
Rhinopoma hardwickii is distinguishable from R. microphyllum by its smaller size and cranial features, from R. muscatellum by the lesser development of narial swellings, from R. macinnesi by the position and shape of its narial swellings (Van Cakenberghe and De Vree 1994), and from R. cystops by a slightly larger body size and genetic traits (Hulva et al. 2007; Benda et al. 2009). Its distribution range stretches from Iraq to Bangladesh and possibly includes the Sunda Islands (Van Cakenberghe and De Vree 1994; Horáček 2019). The species currently comprises two subspecies (Horáček 2019): hardwickii, found from Iraq to India, and sondaicum Van Cakenberghe et De Vree, 1994, restricted to the Sunda Islands. Morphologically, these two subspecies closely resemble each other. Their distinction is based primarily on the geographic gap between the two populations, analogous to the situation observed in R. microphyllum (Van Cakenberghe and De Vree 1994).
The first genetic study of the genus Rhinopoma revealed significant divergence within R. hardwickii s.l. (Hulva et al. 2007), resulting in the elevation of its Afro-Arabian lineage to a species rank as R. cystops Thomas, 1903, with two subspecies cystops and arabium Thomas, 1913. Originally, the small-sized subspecies R. h. cystops was known from central belt of the Sahara, including Upper Egypt and southern Algeria, and the second, large-sized subspecies, R. h. arabium, was reported to occur in northern Africa from Morocco and Senegal to Egypt and Somalia (excluding the Saharan interior), Levant, Arabia and Iran (Kock 1969; Hill 1977; Van Cakenberghe and De Vree 1994; Horáček et al. 2000). However, the genetic study combined with morphology showed that cystops is distributed in whole northern Africa, while arabium is found in the Levant, Arabia and Socotra (Hulva et al. 2007). Later, the latter lineage/subspecies was also found in the Horn of Africa (Benda et al. 2019).
Rhinopoma muscatellum Thomas, 1903, is primarily found around the Persian Gulf, including Oman, UAE, Iran, Afghanistan, Pakistan and possibly south-western India. It is distinguishable by its well-developed narial swellings (Van Cakenberghe and De Vree 1994; Simmons 2005; Horáček 2019). Based on both body measurements and genetic data, this species has been divided into two subspecies (DeBlase et al. 1973; Hill 1977; Van Cakenberghe and De Vree 1994; Benda et al. 2012; Horáček 2019): the smaller muscatellum and the larger seianum Thomas, 1913. Originally, muscatellum was thought to inhabit Oman and Iran, while seianum was considered to range from easternmost Iran to Afghanistan and Pakistan (DeBlase et al. 1973; Hill 1977; Van Cakenberghe and De Vree 1994). However, genetic studies have revised this understanding, suggesting that muscatellum is restricted to the Arabian Peninsula (Oman and UAE), whereas seianum occupies mainland Asia (Iran, Pakistan and possibly India) (Benda et al. 2012; Horáček 2019; Kafaei et al. 2020).
The sole African-only Rhinopoma species, R. macinnesi, was originally considered as a subspecies, either of R. cystops, R. hardwickii or R. muscatellum (Hayman 1937; Kock 1969; Largen et al. 1974). Being smaller in body size than other species and having narial swelling moderately developed, it was regarded as a separate species (Van Cakenberghe and De Vree 1994). This monotypic species is reported to occur in eastern Africa from Kenya and South Sudan to Somalia and Eritrea (Kock 1969; Van Cakenberghe and De Vree 1994; Simmons 2005; Horáček 2019).
The most recently described species, R. hadramauticum, inhabits the Hadramaut Province of eastern Yemen (Benda et al. 2009). Its distinctiveness in body size was initially proposed by Kock et al. (2001), yet it was still assigned as R. muscatellum, and in the genetic differences proposed by Hulva et al. (2007), showing a deep divergence between the Iranian and Yemeni populations. The following thorough morphologic comparison conformed with previous conclusions and led to the description of a new species (Benda et al. 2009).
Intriguingly, the family Rhinopomatidae is only scarcely present in the fossil record. For a long time, only two records were reported from the Late Pleistocene of Israel (Tschernov 1984). However, more fossils unquestionably belonging to Rhinopoma sp. from the Late Miocene (8–10 million years ago, Mya) were found in Greece (Hulva et al. 2007). In addition to the Rhinopoma fossils, two other genera and species have been described as members of this family: Qarunycteris moerisae Gunnell, Simons et Seiffert, 2008 from the Late Eocene (~36 Mya) of Egypt (Gunnell et al. 2008) and Cobarhina handae, Sigé, Mein, Jousse et Aguilar, 2014 from the Early Miocene (~16 Mya) of southern France (Sigé et al. 2014). Molecular analyses of fossil-calibrated phylogenies suggested the radiation of the family/genus Rhinopoma began in the Oligocene (~29 Mya), with origins tracing back to the Early Eocene (~50–55 Mya) (Teeling et al. 2002; Eick et al. 2005). The area of origin was estimated in the archipelago south of the Western Tethyan seaway or in India (Hulva et al. 2007). Considering these fossil records, it seems clear that the evolutionary history of the whole family is not identical to that of its only extant genus.
Despite the recent advances in genetics, the phylogeny and biogeographic history of extant Rhinopomatidae have not yet been studied in sufficient depth or satisfactorily resolved. In the genetic studies, this family was never studied completely, and the rather low sampling has not covered most of the populations within the family. Moreover, the recent studies have relied solely on uniparental mitochondrial markers, which are prone to biases such as mitochondrial introgression, sex-biased dispersal (Flanders et al. 2009; Vallo et al. 2013; Platt et al. 2018) and/or are misleading due to pseudogenes or nuclear mitochondrial DNA segments (NUMTs) (Shaw 2002; Song et al. 2008). Therefore, to address these gaps, this study aims to: (1) reconstruct the evolutionary history of extant Rhinopomatidae, (2) estimate divergence times within this family using the updated calibration points and compare these estimates across Rhinolophidea and (3) identify the geographic origins of the family. To achieve these objectives, we compiled a novel multi-locus dataset for 144 specimens spanning almost the entire geographic range of the Rhinopoma genus, supplemented with available data (Hulva et al. 2007; Benda et al. 2012, 2019).
2 Material and Methods
2.1 Sampling
A total of 163 specimens of the Rhinopoma genus from the collection of the National Museum, Prague, Czech Republic (NMP) were used to extract DNA for genetic analysis (Table S1). This dataset was supplemented with publicly available sequences from GenBank, obtained from previous studies (Figure 1; Benda et al. 2012, 2019; Foley et al. 2015). As an outgroup, GenBank sequences of species from other families within the Rhinolophoidea superfamily were added, including the sister families of Megadermatidae and Craseonycteridae (Puechmaille et al. 2011; Foley et al. 2015; Simoes et al. 2019), as well as the families Rhinolophidae, Rhinonycteridae and Hipposideridae (Dool et al. 2016; Demos et al. 2019; Patterson et al. 2020; Rossoni et al. 2021; Yuzefovich et al. 2021, 2022; Benda et al. 2019; Uvizl et al. 2024). Further details on the origin of samples and GenBank accession numbers are provided in Table S1.

2.2 Amplification and Sequencing
Genomic DNA was extracted from alcohol-preserved tissue samples using the Geneaid Genomic DNA Mini Kit. We targeted one mitochondrial marker (mtDNA), consisting of 1140 bp of cytochrome b (Cyt-b), and four nuclear markers (nDNA): 508 bp of acyl-coenzyme A oxidase 2 intron (ACOX), 644 bp of COP9 signalsom subunit 7A intron (COPS), 449 bp of the rogdi atypical leucine zipper (ROGDI), 551 bp of the signal transducer and activator of transcription 5A intron (STAT). The primers used for both PCR amplification and sequencing were specifically designed for the order Chiroptera, and their effectiveness has been validated in previous studies (see e.g., Puechmaille et al. 2011; Dool et al. 2016; Salicini et al. 2011; Thong et al. 2012). Primer names, sequences and annealing temperatures are provided in Table S2. The purified PCR products were sequenced bidirectionally using the Sanger method by Macrogen Inc. (Amsterdam, The Netherlands).
2.3 Phylogenetic Reconstruction
Sequences were edited and aligned using the MAFFT plugin (Katoh and Standley 2013) with a default setting in Geneious 11.0.5 (https://www.geneious.com) and subsequently manually edited and trimmed using Gblocks (Castresana 2000). Heterozygous positions in the nDNA markers were coded with the IUPAC codes, and ambiguous positions or missing data were coded as ‘N’. Indels were treated as gaps. Sequences of protein-coding markers were translated to amino acids to check for the presence of stop codons, which would indicate the amplification of pseudogenes. The two final datasets, mitochondrial and nuclear, were generated. The mitochondrial dataset contained Cyt-b sequences with a total length of 1140 bp, and the nuclear dataset consisted of ACOX, COPS, ROGDI and STAT sequences with a combined length of 2103 bp. The nuclear dataset was partitioned by gene.
Phylogenetic trees for both the mitochondrial and nuclear datasets were constructed using maximum likelihood (ML). The appropriate nucleotide substitution model for each partition was selected based on the Bayesian information criterion using ModelFinder (Kalyaanamoorthy et al. 2017) (see Table S3). The ML analysis was performed using IQ-TREE (Chernomor et al. 2016; Nguyen et al. 2015). The best-scoring ML tree was identified through ultrafast bootstrap (UFBoot; Hoang et al. 2018) with 1000 bootstrap replicates and 1000 topology replicates. To assess the robustness of the ML tree, branch supports were evaluated using the SH-like approximate likelihood ratio test (SH-aLRT; Guindon et al. 2010) and a Bayesian-like transformation of aLRT (aBayes; Anisimova et al. 2011). SH-aLRT was performed with 1000 replications. aBayes branch support was used instead of Bayesian posterior probabilities, as it is more conservative, more robust to model violation, and exhibits greater statistical power (Anisimova et al. 2011). The ML, SH-aLRT and aBayes analyses were run on the IQ-TREE web server (Trifinopoulos et al. 2016).
We further inferred a phylogenetic network from the Rinopoma mitochondrial sequences using the neighbour-net algorithm (Bryant and Moulton 2004) implemented in SplitsTree v.4 (Huson and Bryant 2006). Uncorrected p-distances between haplotypes were calculated for the Cyt-b in MEGA11 (Tamura et al. 2021). The bootstrap was performed with 1000 replications.
2.4 Divergence Time Estimation
For the molecular dating analyses, the nuclear dataset was pruned to include one sample per species. The final alignment was 2059 bp long, consisting of 51 concatenated sequences, each containing at least two out of the four nuclear markers. Divergence time estimation was set up in BEAUti and run in BEAST v2.5.0 (Bouckaert et al. 2014). We followed the settings outlined in Chornelia and Hughes (2022) and Álvarez-Carretero et al. (2022) applying strict molecular clocks and the Yule speciation process (Yule 1925; Gernhard 2008) for all genes. The substitution model was adopted from the phylogenetic reconstructions (see above). To calibrate the analysis, we employed two primary calibration points: the split between the Rhinolophidae and Hipposideridae families, estimated at 47 million years ago (Mya), and the split between the Megadermatidae and Craseonycteridae families, estimated at 41 Mya, based on previous studies (Álvarez-Carretero et al. 2022). A lognormal prior distribution was used for these calibration points. BEAST was run three times, each for 20 million generations, with trees saved every 2000 generations. Adequate mixing of the MCMC chains and effective sample sizes (ESS > 200) were confirmed using Tracer v1.6 (Rambaut et al. 2015). LogCombiner was used to discard the first 25% of the sampled trees (burn-in) and to merge the resulting tree files. TreeAnnotator was then used to identify the maximum clade credibility tree. All analyses were performed on the MetaCentrum computational platform (metacentrum.cz).
2.5 Biogeographic Analysis
The ancestral geographic range analyses were conducted using probabilistic modelling within the R package “BioGeoBEARS version 1.1.3” (Matzke 2013). We applied three models of geographic range evolution: the Dispersal–Extinction–Cladogenesis (DEC) model of LAGRANGE (Ree and Smith 2008), the DIVALIKE model (a likelihood implementation of the DIVA parsimony process; Ronquist 1997) and the BAYAREALIKE model (Landis et al. 2013), each with different assumptions about anagenetic and cladogenetic processes.
To account for founder-event speciation (jump dispersal), we also tested extended versions of each model by adding a free parameter “j” (DEC+J, DIVALIKE+J and BAYAREALIKE+J), with DEC+J nested within DEC, DIVALIKE+J nested within DIVALIKE and BAYAREALIKE+J nested within BAYAREALIKE (Matzke 2013). The statistical comparison of these models was performed to select the best-fitting biogeographic scenario for the Rhinopomatidae family.
To infer the ancestral biogeographic ranges of Rhinopomatidae, we used a calibrated tree generated in this study (see above). We conducted two separate analyses: one for the Rhinolophoidea superfamily and another for the Rhinopomatidae family specifically. In addition to the calibrated tree, the analyses required a geography file containing data on the current distribution of extant taxa, which was sourced from data from Burgin et al. (2020), available in Maps of Life (mol.org). Zoogeographic zones were defined based on the geologic history of the regions and areas of endemism, following previous studies (Holt et al. 2013; Foley et al. 2015; Chornelia and Hughes 2022).
3 Results
In this study, we generated 144 new Cyt-b sequences for Rhinopoma species, including 92 for R. cystops, 6 for R. hadramauticum, 4 for R. hardwickii, 2 for R. macinnesi, 8 for R. microphyllum and 32 for R. muscatellum. These sequences represented 85 unique sequences overall: 51 for R. cystops, 5 for R. hadramauticum, 3 for R. hardwickii, 2 for R. macinnesi, 4 for R. microphyllum and 19 for R. muscatellum. After including GenBank data, the final Cyt-b dataset consisted of 140 unique sequences, distributed across species as follows: 51 for R. cystops, 5 for R. hadramauticum, 8 for R. hardwickii, 2 for R. macinnesi, 8 for R. microphyllum, 27 for R. muscatellum and 34 from outgroup species.
For the nuclear dataset, we generated sequences for four introns: 89 for ACOX, 49 for COPS, 90 for ROGDI and 84 for STAT, supplemented by 159 GenBank sequences. Only specimens with at least two sequenced introns were included in the analysis. This criterion was met by 92 specimens: 48 for R. cystops, 3 for R. hadramauticum, 7 for R. hardwickii, 2 for R. macinnesi, 5 for R. microphyllum and 27 for R. muscatellum. This was reduced to a final nuclear dataset of 97 unique haplotypes/sequences, including 23 for R. cystops, 3 for R. hadramauticum, 6 for R. hardwickii, 2 for R. macinnesi, 6 for R. microphyllum, 13 for R. muscatellum and 44 from outgroups.
The final Cyt-b dataset was 1140 bp long, with 675 parsimony informative positions (59.21% of the total length). The concatenated nuclear dataset was 2152 bp long (508 bp for ACOX, 644 bp for COPS, 449 bp for ROGDI and 551 bp for STAT), with 585 parsimony-informative positions (27.18%). Missing data accounted for 19.23% of the nuclear dataset. The sequences for R. macinnesi were obtained for the first time, and we were able to recover data for all used markers in this study. The total sequence coverage for this species was 97.89% in the Cyt-b dataset and 82.16% and 81.88% in the concatenated nuclear dataset. This level of completeness supports the robustness of the phylogenetic placement of R. macinnesi within the dataset.
3.1 Phylogenetic Reconstruction
The phylogenetic trees generated through ML analysis of the mtDNA dataset (Figure 2) identified 10 lineages within the extant rhinopomatids, divided into two major groups. The monophyly of the family was strongly supported (UFBoot = 100, aBayes = 1), and Rhinopomatidae was placed as a sister family to the families Craseonycteridae and Megadermatidae (not shown in the figure).
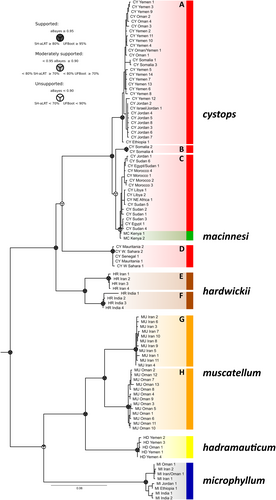
The first group (UFBoot = 99, aBayes = 1) contained six lineages corresponding to three recognised species: Rhinopoma cystops (lineages A–D), R. macinnesi (lineage C) and R. hardwickii (lineages E, F). These formed three subgroups: A–C, D and E–F, although the close relationships between subgroups within the group were not supported. The second group comprised four lineages of three recognised species: R. muscatellum (lineages G, H), R. hadramauticum (lineage I) and R. microphyllum (lineage J). The placement of R. microphyllum in this group was supported only by aBayes (UFBoot = 85, aBayes = 0.99).
In more detail, lineage A consisted of the haplotypes of R. cystops from the Arabian Peninsula and the Horn of Africa, while lineage B contained exclusively the samples of R. cystops from Somalia. Lineage C included R. cystops from northern and north-eastern Africa and the Levant, with R. macinnesi from Kenya embedded within this lineage. The remaining haplotypes of R. cystops from western Africa formed a separate subgroup, lineage D, whose relationship to either R. cystops or R. hardwickii was unresolved. Lineages E and F represented two populations of R. hardwickii, one from Iran (E) and the other from India (F). Similarly, lineages G and H divided R. muscatellum populations, one from Iran (G) and the other from Oman (H). The two remaining species, R. microphyllum and R. hadramauticum, formed lineages with low internal variation, despite the wide distribution of R. microphyllum, or very restricted range of R. hadramauticum.
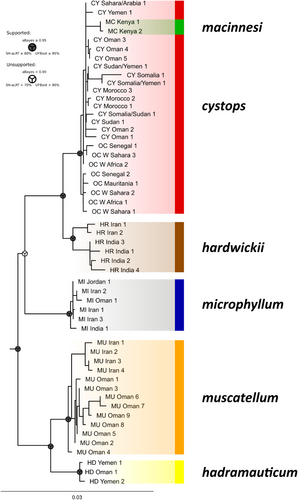
The results of ML analysis on the nDNA dataset (Figure 3) also indicated the monophyly of Rhinopoma (UFBoot = 100, aBayes = 1), but identified only five basic lineages, one for each species except R. macinnesi. All four mitochondrial lineages of R. cystops (A–D) formed a single nuclear lineage, which also included samples of R. macinnesi, mirroring the mitochondrial results. The nuclear data showed the lineages of R. hardwickii (E, F) and R. muscatellum (G, H) each formed cohesive groups, consistent with the mitochondrial data. Overall, the nDNA lineages exhibited low internal variation. The relationships between lineages within the family were only partially resolved: the subgroup comprising lineages of R. cystops was in sister position to that of R. hardwickii (UFBoot = 100, aBayes = 1), and the lineage representing R. muscatellum was a sister group to that of R. hadramauticum (UFBoot = 100, aBayes = 1), in concert with mtDNA analysis results. However, the placement of R. microphyllum relative to these species' pairs remained unresolved.
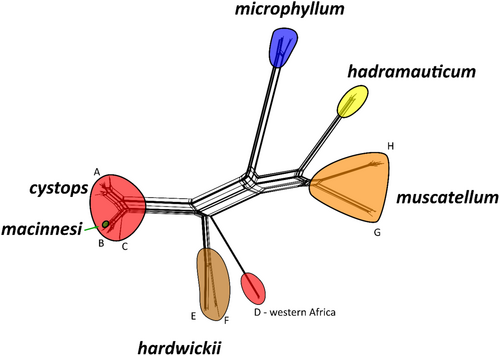
The phylogenetic network constructed using SplitsTree (Figure 4) based on the mtDNA dataset, identified the same 10 lineages as the mtDNA tree, with largely consistent topology. Rhinopoma macinnesi was nested within lineage C of R. cystops, while lineage D of R. cystops diverged from lineages A–C at the same point as lineages E and F of R. hardwickii. In this network, R. microphyllum was positioned between the cystops-hardwickii group and the hadramauticum-muscatellum group. The uncorrected genetic distances between species within the family Rhinopomatidae based on mtDNA dataset ranged from 3.97% to 7.10%, while the distances within species ranged from 0% to 4.88% (Table S4).
3.2 Divergence Time Estimation
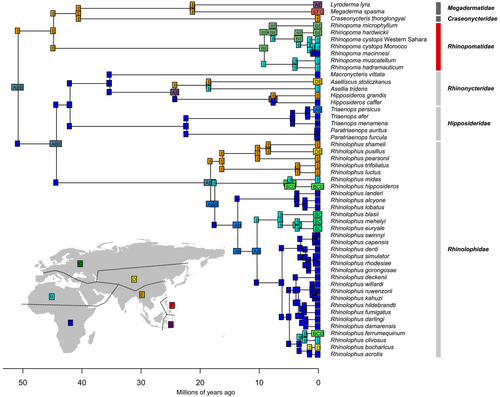
The time-calibrated tree for the superfamily Rhinolophoidea (Figure 5) showed the same topology for the extant family Rhinopomatidae as the nDNA tree. Within Rhinopomatidae, all nodes were strongly supported (PP > 0.99) except for R. microphyllum (PP = 0.90), consistent with the results from the nDNA tree. The only other node of interest with weak support was the split between the family Rhinopomatidae and its two sister families, Craseonycteridae and Megadermatidae (PP = 0.80), which was estimated to have occurred 44.94 million years ago (Mya; 95% highest posterior density [HPD]: 35.16–54.65 Mya).

The divergence of Rhinopoma was estimated to have occurred at 9.46 Mya (95% HPD: 5.63–14.20 Mya), when the R. hadramauticum-muscatellum group split from the remaining species. The two species within this group diverged from each other by approximately 4.06 Mya (95% HPD: 1.57–6.99 Mya). Subsequently, R. microphyllum diverged from other species about 7.84 Mya (95% HPD: 4.33–11.85 Mya). The split between R. hardwickii and R. cystops occurred more recently, around 3.41 Mya (95% HPD: 1.53–5.67 Mya).
For this analysis, R. cystops was divided into three lineages based on previous results to trace the onset of intraspecific diversification. The first lineage corresponded to mitochondrial subgroup D from western Africa, represented by a specimen from Western Sahara. The second lineage included remaining subgroups (A–C), excluding the Kenyan samples, and was represented by a specimen from Morocco. The third lineage comprised a specimen from Kenya, referred to as “macinnesi” (based on morphological traits; Benda et al. (2024). Our results indicated that the divergence of R. cystops began in western Africa approximately 1.44 Mya (95% HPD: 0.47–2.84 Mya). The diversification expanded eastward, with a subsequent split between the Moroccan and Kenyan samples (within a single mitochondrial subgroup) occurring around 0.90 Mya (95% HPD: 0.21–1.76 Mya).
In general, our results showed that the divergence times of extant species within Rhinopomatidae were comparable to those within the family Rhinolophidae. However, the diversification of Rhinopoma began much later, around 9.46 Mya, than in other families within Rhinolophoidea. For instance, diversification in Hipposideridae started at 35.32 Mya, in Rhinonycteridae at 22.55 Mya, in Megadermatidae at 21.33 Mya and in Rhinolophidae at 18.43 Mya (Figure 5). This notably later diversification of Rhinopomatidae underscores its distinct evolutionary trajectory within the superfamily and supports the existence of extinct clades that emerged earlier in the family's evolutionary history.
3.3 Biogeographic Analysis
The nuclear time-calibrated tree for the superfamily Rhinolophoidea (Figure 5) was used to conduct the biogeographic analysis (Figure 6). Our results, based on the likelihood ratio test (LRT) with a chi-squared one-tailed test, showed higher log likelihood (natural log) values for models that included founder events (+j models) (Table S5). The most supported model for the entire Rhinolophoidea was DEC + J (Lnl = −102.97), whereas the remaining models had lower log-likelihood values (Lnl < −104.43). However, although the DEC + J model had some Akaike Information Criterion (AIC) support, its estimate of j is nearly 0, indicating little support for the founder playing a significant role in this family's evolution.
Focusing on the genus Rhinopoma (Figure 7), both DEC + J and DIVALIKE + J had similar log likelihoods (Lnl = −15.85 vs. −15.94). However, when compared with their nested versions, DIVALIKE was preferred based on AIC (36.23), while other models had higher AIC values (AIC > 37.70).
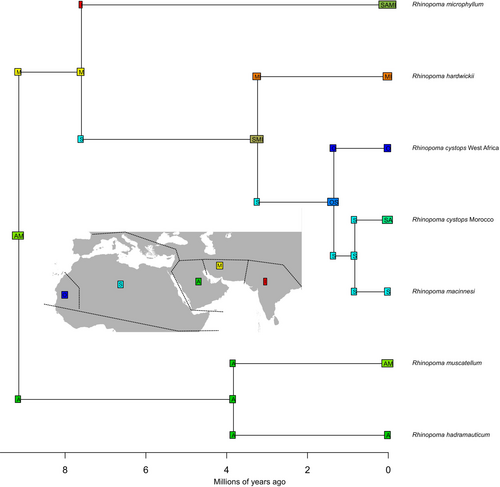
Our biogeographical analysis suggests that the common ancestor of the extant family Rhinopomatidae likely originated in the Oriental region, where species such as R. microphyllum and R. hardwickii are distributed. The diversification of the family into the current species likely occurred either in the Oriental region or the southern Palaearctic region (Figure 6). However, this result encompasses the entire distribution range of the Rhinopoma genus. A more detailed regional analysis indicates that the earliest divergences within Rhinopoma occurred in the area of the Arabian Peninsula and Iran (Figure 7), i.e., the Afrotropic–Oriental realms transition, which remains the primary region where the largest species diversity is still found today.
4 Discussion
This study provides new insights into the evolutionary history of the Rhinopomatidae family through multi-locus genetic data. By incorporating samples representing all six currently recognised species, we expanded on previous studies (e.g., Hulva et al. 2007; Benda et al. 2009, 2012) and identified significant revisions of the family's taxonomic arrangement.
4.1 Phylogeny of Extant Rhinopomatidae
The extant Rhinopomatidae family forms a monophyletic and monogeneric clade according to both morphology (Miller Jr. 1907; Ellerman and Morrison-Scott 1951; Kock 1969; DeBlase et al. 1973; Hill 1977; Van Cakenberghe and De Vree 1994) and genetics (Teeling et al. 2002, 2005; Hulva and Horáček 2002; Eick et al. 2005; Hulva et al. 2007). It is a part of the superfamily Rhinolophoidea and currently contains six species (Horáček 2019; Mammal Diversity Database 2025). Our phylogenetic analyses results, particularly the one based on the nDNA dataset, mostly conform with the previous views, although they showed one substantial difference, because it revealed only five distinct lineages instead of six.
Nuclear DNA is often considered a robust marker for species delimitation (e.g., Eick et al. 2005; Dool et al. 2016; Uvizl et al. 2024; Győrössy et al. 2024), and our nDNA tree of Rhinopoma supported the sister relationships of R. cystops with R. hardwickii, and R. muscatellum with R. hadramauticum, proposed by Hulva et al. (2007) in the study based on a shorter fragment of the mitochondrial Cyt-b gene. Intriguingly, the sequences of R. macinnesi were nested within those of the R. cystops lineage, particularly aligning with samples from north-eastern Africa and Arabia. This finding undermines a separate position of the R. macinnesi populations and instead indicates R. cystops as a widespread lineage spanning northern Africa southward to Kenya and the Arabian Peninsula. The low internal diversity of nDNA across Rhinopoma species, even in widely distributed taxa such as R. microphyllum, underscores their rather remarkable morphologic uniformity, possibly linked to stabilising selection due to specific ecological niches in their evolutionary trajectory (Hulva et al. 2007; Horáček 2019).
In contrast, the mtDNA tree revealed greater intra-specific diversity, likely due to its faster coalescence time (Palumbi et al. 2001). Our mtDNA analysis results doubled the number of recognised lineages compared to the nDNA analysis, a pattern commonly observed in bats (e.g., Dool et al. 2016; Zhang et al. 2018; Patterson et al. 2025). Notably, R. cystops exhibited four mtDNA lineages, geographically structured into populations from Arabia and north-eastern Africa (lineage A), Somalia (B), northern Africa and the Levant (C) and western Africa (D). Similarly, R. hardwickii and R. muscatellum each formed two mtDNA lineages from Iran (E) and India (F), and from Iran (G) and Oman (H), respectively. Such a diversity was suggested by Benda et al. (2012); nonetheless, it was described to a much lesser extent due to the limited sampling.
Meanwhile, R. microphyllum and R. hadramauticum maintained a single lineage each, although the former species showed an insignificant geographic pattern (two shallow sublineages, one from the Persian Gulf region and the other from the remaining part of its distribution, including the Levant and Arabian Peninsula, see Benda et al. 2019). Notably, our knowledge of R. hadramauticum's occurrence area has expanded with the inclusion of a sample from Oman, supplementing specimens that were previously known only from the type locality in Yemen. The geographic structuring of these mitochondrial lineages suggests female philopatry as a significant driver of genetic divergence due to the reduced mitochondrial gene flow, a phenomenon increasingly recognised in bats (e.g., Funk and Omland 2003; Rivers et al. 2005; Rossiter et al. 2007; Toews and Brelsford 2012; Moussy et al. 2013).
Overall, some of these mitochondrial lineages could be aligned with the morphotypes assigned to recognised subspecies (Van Cakenberghe and De Vree 1994; Simmons 2005; Benda et al. 2012; Horáček 2019), and thus, our results can suggest taxonomic implications with significant impact for the entire Rhinopomatidae family.
4.2 Current Systematics of Rhinopoma
Our findings indicate a discrepancy between the number of currently recognised species, primarily based on morphological comparisons (see the review by Horáček 2019), and the number of genetic lineages identified through our analyses. Using samples from all six morphologically defined species/lineages, our genetic analyses revealed the presence of only five distinct genetic species/lineages. These results led us to propose a revision of the taxonomic understanding of the Rhinopoma genus, suggesting a reduction in the number of recognised species and an increase in the number of subspecies.
The most impactful result concerns R. macinnesi, a small-sized form of eastern African desert. It forms a distinct morphotype, prompting earlier classifications as either a subspecies within the R. cystops/hardwickii complex (Hayman 1937; Ellerman and Morrison-Scott 1951; Kock 1969; Largen et al. 1974; Hill 1977) or a separate species (Van Cakenberghe and De Vree 1994; Simmons 2005; Horáček 2019; Mammal Diversity Database 2025). However, R. macinnesi has not been included in genetic studies until now, limiting its phylogenetic placement within Rhinopoma.
Our genetic analyses, using two samples from central Kenya, the region where the type material of R. macinnesi was collected (Bat Island, Lake Turkana; Hayman 1937), revealed that it nests consistently within the R. cystops clade in both mitochondrial and nuclear DNA trees. In the mtDNA tree, R. macinnesi clustered with lineage C, alongside geographically close populations from northern and northeastern Africa and the Levant. In the nDNA tree, it aligns within the sole R. cystops lineage. As a comparison, Rhinopoma samples from Somalia also grouped with R. cystops, clustering either within mtDNA lineage C, forming a distinct lineage B or within the shared nuclear lineage. Would there be any discordance between mtDNA and nucDNA datasets, we could have considered an introgression of a genome part of one species into the genome of another species (Mao and Rossiter 2020; Toews and Brelsford 2012) or incomplete lineage sorting (e.g., Funk and Omland 2003). In this case, the congruence between mitochondrial and nuclear datasets supports the conclusion that the populations assigned to R. macinnesi should be lumped with R. cystops, making the name macinnesi Hayman a junior subjective synonym of cystops Thomas, without the justification for subspecific position as originally suggested by Hayman (1937). Consequently, the distribution range of R. cystops now extends significantly southward, encompassing Kenya and, presumably, the Horn of Africa (Eritrea and Somalia), where the macinnesi morphotype has been reported to occur (Van Cakenberghe and De Vree 1994; Lanza et al. 2015; Horáček 2019).
Further, the branching of the mtDNA tree suggests subspecific diversity in three species, making R. cystops, R. hardwickii and R. muscatellum polytypic. Previously, R. cystops was divided into two subspecies based on the mtDNA data (Hulva et al. 2007). However, more extensive sampling in our study revealed four geographically distinct lineages within R. cystops. Lineage A spans the Levant, Arabia and Socotra (Hulva et al. 2007), as well as, interestingly, Ethiopia and Somalia (Benda et al. 2019; our results). This area overlaps with the proposed range of R. c. arabium (Hulva et al. 2007) and covers geographically the type locality of this subspecies (Wasil, Yemen; Thomas 1913). Lineage C, comprising populations from northern and eastern Africa from Morocco through Egypt and the Sudan to Somalia and Kenya, and, surprisingly, the Levant, corresponds to the nominotypical subspecies (type locality: Luxor, Upper Egypt; Thomas 1903) and incorporates the Kenyan populations formerly assigned to R. macinnesi. However, the occurrence of these three lineages (A–C) geographically overlaps in the marginal parts of their ranges, in the Levant (A and C) and Horn of Africa (A and B).
Lineage D, restricted to western Africa (Senegal, Mauritania and Western Sahara), is distinct based on mtDNA (8.5%–9.5% uncorrected p-distances). While this deep divergence suggests potential species status, its morphological similarity to other African populations (Kock 1969; Koch-Weser 1984; Van Cakenberghe and De Vree 1994) and the shared nDNA lineage indicates that this mtDNA lineage represents a population within the species rank of R. cystops rather than a species of its own, whose taxonomic status warrants further study. Similarly, lineage B, comprising two distinct samples from Somalia, represents a fourth divergent population within R. cystops based on mtDNA. The mitochondrial divergence in specimens from a single colony could be explained by mtDNA introgression from a closely distributed population (of R. c. arabium, in this case). Nevertheless, the intraspecific taxonomy of R. cystops requires a further revision.
In R. muscatellum, genetic results corroborate two subspecies R. m. muscatellum and R. m. seianum (cf. DeBlase et al. 1973; Hill 1977; Van Cakenberghe and De Vree 1994). Our results are also in concert with previous genetic studies, which limited the small-sized R. m. muscatellum to Oman and extended the variable-sized R. m. seianum across southern Iran, Afghanistan and Pakistan (Benda et al. 2012; Kafaei et al. 2020). Similarly, within R. hardwickii, the mtDNA analysis revealed two distinct populations, but the absence of samples from the Sunda Islands (distribution range of R. h. sondaicum) limits our full understanding of the intraspecific arrangement within this taxon. Nevertheless, our findings suggest the presence of an additional taxon in Iran in accordance with the previous suggestions (Hulva et al. 2007; Benda et al. 2012). The nominotypical populations of R. h. hardwickii are thus restricted to India and adjacent areas.
In contrast, our results differ from previous studies regarding the intraspecific diversity in R. microphyllum. Morphological studies recognised up to six subspecies (Kock 1969; Schlitter and DeBlase 1974; Hill 1977; Nader and Kock 1982; Schlitter and Qumsiyeh 1996), while phylogeographic studies suggested minute differences between populations, identifying three subspecies at maximum (Levin et al. 2008; Akmali et al. 2011; Bagherfard et al. 2021). In our study, this species was divided into two shallow mitochondrial sublineages: one from the Persian Gulf region (Oman and Iran) and another from the remaining parts of its distribution. Nonetheless, the differences between the two sublineages were insufficient to warrant the recognition of subspecies. Consequently, we propose to consider R. microphyllum a monotypic species, consistent with the suggestion of Horáček (2019). For R. hadramauticum, previously regarded as monotypic (Benda et al. 2009; Horáček 2019), our results support this taxonomic arrangement.
4.3 Origin and Biogeography of Rhinopomatidae
Our study provides a detailed insights into the evolutionary origin and biogeography of the Rhinopomatidae family by integrating comprehensive phylogenetic, molecular dating and biogeographic analyses. The position of Rhinopomatidae as a sister clade to the families Megadermatidae and Craseonycteridae is consistently supported across various studies (e.g., Teeling et al. 2005; Hulva et al. 2007; Meredith et al. 2011; Foley et al. 2015; Amador et al. 2018; Álvarez-Carretero et al. 2022). Results of our analysis conform with these suggestions, regardless of the number of species included. However, some studies suggested alternate placements, such as a sister relationship with Craseonycteridae alone (Bininda-Emonds et al. 2007; Agnarsson et al. 2011) or with the broader Rhinolophoidea clade (Agnarsson et al. 2011).
Our results estimate the split between Rhinopomatidae and its sister families at approximately 45 million years ago (Mya), during the Middle Eocene, which is generally earlier but in the confidential intervals suggested by other studies (Teeling et al. 2005; Meredith et al. 2011; Foley et al. 2015; Álvarez-Carretero et al. 2022) or of a similar age (Hulva et al. 2007; Agnarsson et al. 2011; Amador et al. 2018).
The timing of diversification within Rhinopoma shows greater variation across studies. Our findings placed the start of divergence at around 10 Mya, during the Upper Miocene. This is congruent with the results of Foley et al. (2015), who estimated the split between R. microphyllum and R. hardwickii at 9 Mya, closely aligning with our estimate of 8 Mya. This split was of a similar age also with that revealed by Álvarez-Carretero et al. (2022), however, they dated the diversification of Rhinopomatidae to 24 Mya. Other studies put the beginning of the diversification to the age of 14–28 Mya (Hulva et al. 2007; Agnarsson et al. 2011; Amador et al. 2018; Bagherfard et al. 2021; Kafaei et al. 2020). Nevertheless, the fossil evidence corroborates our timing, with the oldest known Rhinopoma fossils from Greece, dated to 8–10 Mya (Hulva et al. 2007). The morphotype of this fossil is like recent R. hardwickii, which, according to our results, diverged from R. microphyllum at this age.
The geographic origin of the Rhinopoma genus has been less studied than its divergence timing. Based on morphological evidence, ancestral trait diversity and geological history, the Indian subcontinent was previously suggested as the source area of the genus radiation (Hulva et al. 2007). Our biogeographic analysis supports this view, identifying southern and south-eastern Asia as the likely region of origin. In a broader biogeographic study of Rhinolophoidea (Foley et al. 2015), the authors treated the Indian subcontinent and south-eastern Asia as separate areas and recognised the distribution of extant Rhinopomatidae in south-eastern Asia, which resulted in south-eastern Asia being the source area in their study.
Additionally, we investigated the origins of divergence within the genus Rhinopoma. A broader analysis of Rhinolophoidea indicated that diversification occurred within a wide geographic belt extending from north-western Africa to south-eastern Asia. However, our genus-level analysis focused on Rhinopoma revealed that its diversification likely began in western Asia, particularly in regions corresponding to modern-day Iran and Arabia. Earlier studies dismissed Iran as a potential origin area (Hulva et al. 2007), citing its submersion as part of the Tethys Sea during the Oligocene–Miocene (Rögl 1999; Barrier and Vrielynck 2008; Zhang et al. 2014). However, our findings suggest that diversification began in the Miocene, after the closure of the Tethys (Rögl 1999; Zhang et al. 2014), when the Iranian Highlands had risen above sea level, presenting a fertile environment conducive to Rhinopoma dispersal and further colonisation, prior to the onset of the Messinian salinity crisis.
This scenario aligns with tectonic and climatic events during the Late Miocene (7–11 Mya), when continuity of the Tethys Sea was disrupted by uplift of the Arabian Peninsula (Barrier and Vrielynck 2008) as well as with the timing of Rhinopoma diversification (approximately 10 Mya). This period also coincides with increasing expected aridisation in the Sahara, which had arid conditions as early as 8 Mya (Ruddiman et al. 1989; de Menocal 1995; Feakins et al. 2013) and fully desert-like conditions by 7 Mya (Schuster et al. 2006). These environmental shifts, linked to the shrinking Tethys Sea (Zhang et al. 2014), may have facilitated the dispersal of desert-adapted Rhinopoma species across expanding arid habitats. Subsequent diversification within Rhinopoma may have been driven by oscillations between arid and humid climates, which began in the Late Miocene and persist to the present day (Schuster et al. 2009; Larrasoaña et al. 2013). These climatic fluctuations likely shaped the formation of distinct lineages by creating temporary barriers and opportunities for dispersal or isolation.
Importantly, the fossil records suggest that the evolutionary history of Rhinopomatidae as a family predates and extends beyond the single extant genus Rhinopoma. The earliest known fossil attributed to this family is Qarunycteris moerisae from the Late Eocene (~36 Mya) of Egypt (Gunnell et al. 2008). Another extinct taxon, Cobarhina handae, is dated roughly to the Early/Middle Miocene (~16–13 Mya) of southern France (Sigé et al. 2014). These fossils indicate a broader historical range for the family, including an early western expansion into the Mediterranean region during warmer climatic periods, either during the Eocene or the early Miocene. However, subsequent climate cooling and increasing aridisation after ~10 Mya (Steinthorsdottir et al. 2021) likely led to the extinction of the western, tropic-adapted clades of Rhinopomatidae. In contrast, our findings suggest that the extant genus Rhinopoma represents a southeastern clade that began radiating during or after these climatic shifts.
Thus, the evolutionary history of the family is not identical to that of its sole extant genus, and interpreting current diversity without reference to these extinct lineages would underestimate the historical complexity of Rhinopomatidae. Further research incorporating additional fossil and molecular data is needed to fully elucidate these evolutionary processes at finer spatial and temporal scales.
5 Conclusions
This study presents the most comprehensive molecular phylogeny of the Rhinopoma genus to date, incorporating a significantly expanded sample set spanning almost the entire distribution range. Our findings reveal ten mitochondrial and five nuclear lineages, offering a refined understanding of the systematic arrangement and relationships among the taxa within the family.
We propose a revision of the number of recognised species and subspecies in the Rhinopoma genus reduced to five, lumping R. macinnesi with R. cystops. The nuclear diversity aligns with the species boundaries, while the mitochondrial lineages reflect subspecific divisions. Specifically, we suggest the existence of four possible subspecies within R. cystops and two in R. muscatellum and R. hardwickii. The geographical variations detected in R. microphyllum and R. hadramauticum do not support their intraspecific taxonomic divisions.
Concerning the origin of Rhinopomatidae, our study demonstrated somewhat earlier time estimates for the origin as well as for species diversification of this family than previous studies. We estimated that Rhinopomatidae formed a separate lineage in the period around 45 Mya in India. However, the diversification of the Rhinopoma genus likely began in western Asia approximately 10 Mya. This region, characterised by the highest diversity of the extant Rhinopoma species, likely served as the source for subsequent speciation events and/or for spreading of populations that later created a unique lineage in separated areas.
This study presents a most complete molecular phylogeny of the extant family Rhinopomatidae to date, significantly increasing the number of used samples from a wide area covering almost the whole distribution range of the family. However, some parts of this distribution remain unsampled and some species, for example R. microphyllum, are underrepresented in the margins of its distribution. Future increased sampling and use of phylogenomic markers may improve the current phylogeny elucidate inter-population dynamics and provide deeper insights into the evolution of the Rhinopoma genus.
Author Contributions
Marek Uvizl: methodology, formal analysis, investigation, data curation, writing – original draft, visualisation, project administration, funding acquisition. Peter Vallo: methodology, formal analysis, investigation, writing – review and editing. Petr Benda: conceptualisation, resources, writing – review and editing, supervision, project administration, funding acquisition.
Acknowledgements
We thank C. Srinivasulu for sharing sequences of Indian samples. We also thank the editor and two anonymous reviewers for manysuggestions that greatly improved successive versions of the study. The preparation of this contribution was supported by the Ministry of Culture of the Czech Republic (# DKRVO 2024–2028/6.I.b, National Museum, 00023272). Computational resources were provided by the e-INFRA CZ project (# 90254), supported by the Ministry of Education, Youth and Sports of the Czech Republic. Open access publishing facilitated by Charles University, as part of the Wiley - CzechELib agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The DNA sequences analysed in this study are available in NCBI GenBank, as detailed in the text and tables above. Specimens are deposited in the National Museum (Prague, Czechia).




