Molecular phylogeny of the operculated land snail family Pupinidae (Caenogastropoda, Cyclophoroidea) in mainland Southeast Asia
Abstract
The operculated land snail family Pupinidae from mainland Southeast Asia has been systematically revised based on shell morphology. Despite previous morphological studies, the evolutionary relationships within this family remained unclear. This study represents the first comprehensive molecular phylogeny of this snail group, utilising two mitochondrial (COI and 16S rRNA) and two nuclear (5.8S rRNA + ITS2 and 28S rRNA) genetic markers. Additionally, we conducted phylogenetic analyses of Pupina species from 1106 loci generated through double-digest restriction site-associated DNA sequencing (ddRADseq). It turned out that Southeast Asian Pollicaria emerged as a sister clade to Central American Aperostoma of the Megalomastomatidae, leading to the resurrection of the Pollicariidae. Among the remaining pupinid genera, Tortulosa was nested within the Coptocheilus clade, while Pupina and Pupinella were not monophyletic. The previously recognised Pupina arula species group was found to be monophyletic and was reclassified into Tylotoechus (formerly a Pupina subgenus), based on distinctive conchological characters such as an extending parietal tooth from a parietal callus and a wide, outward-curving posterior canal. However, some Pupina and Tylotoechus species were not retrieved as monophyletic, suggesting the presence of multiple ‘cryptic species’. Divergence time estimation indicated that the Pupinidae split could date back to the Late Triassic to Early Cretaceous, with the first diversification of pupinid genera occurring during the Middle Jurassic and Early Cretaceous. This successful reconstruction of a robust phylogeny using ddRADseq loci demonstrates the significant potential of RADseq techniques in elucidating the evolutionary relationships of deeply divergent taxa. Further studies incorporating the type species Tylotoechus destructus and Pupina keraudrenii are necessary to justify the usage of these genera.
1 INTRODUCTION
Mainland Southeast Asia, or Indochina, is well-known for its high flora and fauna diversity, as well as their endemicity to the area, due to highly diverse types of habitats. This region includes the countries of Cambodia, Laos, Myanmar, Thailand, Vietnam and Peninsular Malaysia. Most of the region falls within the Indo-Burma biodiversity hotspot, while Peninsular Malaysia is included in the Sundaland counterpart (Myers et al., 2000; Sodhi et al., 2004; Tordoff et al., 2012). Recently, land snail diversity in mainland Southeast Asia has been systematically documented in several countries, for example, Laos (Inkhavilay et al., 2019), Cambodia (Sutcharit et al., 2020) and peninsular Malaysia (Foon et al., 2017; Hausdorf, 2019). More comprehensive taxonomic and systematic studies have also been conducted for both land operculated snails (e.g., Jirapatrasilp, Páll-Gergely, et al., 2021; Páll-Gergely, 2023; Páll-Gergely, Gargominy, et al., 2017; Páll-Gergely, Hunyadi, et al., 2017; Tongkerd et al., 2023) and eupulmonates (e.g., Man et al., 2024; Pholyotha et al., 2020; Sutcharit & Panha, 2021; Tongkerd et al., 2024).
The family Pupinidae Pfeiffer, 1853 (superfamily Cyclophoroidea, subclass Caenogastropoda; Bouchet et al., 2017) is an operculated land snail group characterised by its pupoid to conical shell shape and a tubular bursa copulatrix (Tielecke, 1940). Currently, the Pupinidae comprises approximately 32 extant and 11 extinct genera (MolluscaBase, 2024c; Yu et al., 2023), with their current distribution spanning from South and East Asia down to some parts of Australia and western Pacific Islands, typically found in various natural habitats and particularly abundant in limestone regions (see literature cited in Kongim et al., 2013).
In mainland Southeast Asia, 11 pupinid genera have been documented and classified into two subfamilies: the Pupininae and the Pupinellinae Kobelt, 1902 (Do & Nguyen, 2023; Jirapatrasilp, Sutcharit, & Panha, 2022; Páll-Gergely et al., 2015; Thach, 2017). These two subfamilies were distinguished only by the shell surface: a glossy and completely smooth surface covered by glaze in the Pupininae, and either a striated, matt or silky-shiny surface without glaze in the Pupinellinae (Egorov, 2013; Kobelt, 1902). Yet, this diagnostic character is not exclusive to all members in each respective subfamily (Jirapatrasilp, Sutcharit, & Panha, 2022). Another controversial family-level taxon related to the Pupinidae containing only one genus, Pollicaria Gould, 1856, was either adopted as its own family, Pollicariidae, by Egorov (2005), as a subfamily of the Pupinidae by Wenz (1938a), Egorov (2013) and MolluscaBase (2024a), or treated as a synonym of the Pupinellinae by Bouchet et al. (2017). Therefore, the validity of this (sub)familial classification needs further reinvestigation by means of molecular phylogenetics.
Taxonomic studies in the past decade have focused on several genera, including Pollicaria (Kongim et al., 2013; Minton et al., 2017), Rhaphaulus Pfeiffer, 1856 and Streptaulus Benson, 1857 (Páll-Gergely et al., 2014; Páll-Gergely, Gargominy, et al., 2017; Páll-Gergely, Hunyadi, et al., 2017), Pseudopomatias Möllendorff, 1885 and Vargapupa Páll-Gergely, 2015 (Páll-Gergely et al., 2015; Páll-Gergely & Grego, 2019), Coptocheilus Gould, 1862 (Bui & Páll-Gergely, 2020; Páll-Gergely et al., 2019) and Pupina Vignard, 1829, Pupinella Gray, 1850 and Tortulosa Gray, 1847 (Jirapatrasilp, Sutcharit, & Panha, 2022). These studies focused mainly on taxonomic revisions and the introduction of new taxa, based solely on shell and opercular characteristics. Most pupinid genera are well-defined, except for Pupina, whose subgeneric classification is still inconsistent due to limited diagnostic traits (Jirapatrasilp, Sutcharit, & Panha, 2022). As former studies were based on morphological data alone, the issues of species delimitation, cryptic diversity and overlap in morphological characteristics still remain a challenge.
Molecular data, particularly DNA sequences obtained through Sanger sequencing of mitochondrial and nuclear marker genes, have now been extensively used to illustrate the evolutionary relationship within land snail taxa, often leading to an updated classification scheme (Jirapatrasilp, Huang, et al., 2022; Páll-Gergely et al., 2024; Saadi & Wade, 2019; Zhang et al., 2024). Molecular phylogenetic frameworks at the family level have been recently adopted in Southeast Asian land operculated snails, for example, Cyclophoridae (Páll-Gergely et al., 2024) and Diplommatinidae (Webster et al., 2012) and more widely in the taxonomic scope of some eupulmonates, for example, Streptaxidae (Siriboon et al., 2020), Dyakiidae (Jirapatrasilp, Tongkerd, et al., 2021) and Euconulidae (Pholyotha et al., 2023). Although there is one molecular phylogenetic study focusing on the taxonomic position of the pupinid genus Pollicaria (Minton et al., 2017), and some pupinid species have been incorporated in the molecular phylogenies of other terrestrial cyclophoroid families (Páll-Gergely et al., 2024; Webster et al., 2012), there is currently none that clearly outline the evolutionary relationships among the various taxa within the Pupinidae.
The present study is the first to elucidate the evolutionary relationships within the Pupinidae from mainland Southeast Asia using molecular phylogenetic analyses of two mitochondrial (cytochrome c oxidase subunit I (COI) and 16S ribosomal (r)RNA) and two nuclear (5.8S rRNA + ITS2 and 28S rRNA) markers. The molecular phylogeny of some closely related Pupina species in the region was also constructed from a novel dataset of hundreds of genome-wide markers retrieved from double-digest restriction site-associated DNA sequencing (ddRADseq: Franchini et al., 2017; Peterson et al., 2012), where the relevant techniques in previous studies yielded a robust species-level land snail phylogeny (Becher et al., 2025; Haponski et al., 2019; Raheem et al., 2023). We also conduct a time-calibrated phylogenetic inference in order to postulate the evolutionary history underlying the diversification of the family Pupinidae in mainland Southeast Asia. Finally, the phylogenetic relationship retrieved in this study would be a foundation to update the systematics of this land operculated snail family.
2 MATERIALS AND METHODS
2.1 Specimen preparation and species identification
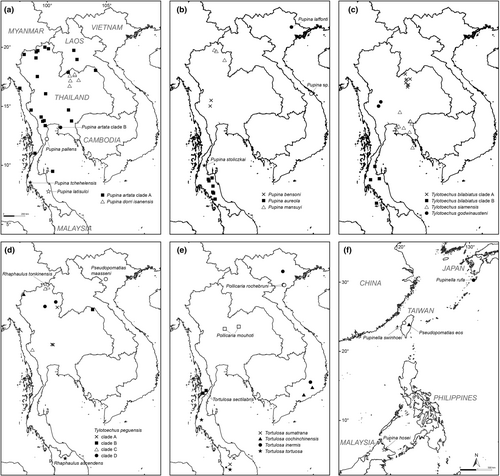
The specimens of the family Pupinidae in this study were based on voucher specimens deposited in Chulalongkorn University Museum of Zoology (CUMZ), Bangkok, Thailand and National Museum of Natural Science of Taiwan (NMNS), Taichung, Taiwan (see Figure 1, Table S1). The specimens were photographed and killed by the two-step method for euthanasia (AVMA, 2020) before being preserved in 70% ethanol for anatomical studies or preserved in 95% (v/v) ethanol for molecular analyses. The handling of animals in this study was approved by Chulalongkorn University Animal Care and Use Committee (CU-ACUC) under the approval number 1723018 and 2523009. Species identification and generic assignment followed Minton et al. (2017), Páll-Gergely et al. (2019) and Jirapatrasilp, Sutcharit, and Panha (2022) and specimens were compared with the relevant type specimens if possible.
2.2 DNA extraction and sanger sequencing
Total genomic DNA was extracted from a small piece of foot muscle tissue following the protocol of Sokolov (2000), with slight modifications from Scheel and Hausdorf (2012), from a total of 127 specimens. Among these, 98 specimens were subjected to Sanger sequencing (Table S1). DNA concentrations were measured using the Qubit 4 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) with the QuantiFluor dsDNA assay kit (Promega Corp., Madison, WI, USA). Three partial gene fragments mostly used in land snail systematics, mitochondrial cytochrome c oxidase subunit I (COI) and 16S ribosomal RNA (16S) and nuclear 5.8S + internal transcribed spacer 2 (ITS2) + 28S rRNA, were sequenced in this study. The primer pairs and the annealing temperature (Ta) for DNA amplification of each gene fragment are as follows: the newly designed LCO_Cyc (5′-ATTTTCTACAAATCATAARGATATTGG-3′) + HCO2198 (Folmer et al., 1994) or the newly designed HCO_Dio (5′-AATGTTGATATAAAATAGGATCYCC-3′) and Ta = 50°C for COI, 16Sar (Palumbi et al., 1991) + the newly designed 16Sbr_Cyc (5′-ACGCCGGTCTGAACTCAGATCATGT-3′) and Ta = 55°C for 16S and LSU1 + LSU3 (Wade & Mordan, 2000) and Ta = 58°C for 5.8S + ITS2 + 28S rRNA. PCR amplifications were performed in 11 μL volumes containing 7.74 μL ddH2O, 2 μL 10× PCR buffer (biolabproducts, Bebensee, Germany), 0.3 μL MgCl2 (50 mM, biolabproducts), 0.4 μL dNTP mix (5 mM each, biolabproducts), 0.2 μL of each primer (10 μM), 0.16 μL Platinum Taq DNA polymerase (1 unit/μL; Invitrogen) and 1 μL of the template DNA. The PCR thermal cycling conditions were as follows: an initial denaturation step at 94°C for 2 min, and 35 PCR cycles (94°C for 30 s, the respective Ta for 1 min, 68°C for 1 min). Both strands of the amplified products were sequenced at Macrogen Europe Laboratory (Amsterdam, The Netherlands).
2.3 ddRAD library preparation and sequencing, and ddRAD locus assembly
DNA extracts of some Pupina species and Pupinella mansuyi (Dautzenberg & Fischer, 1908) were selected for the ddRAD library preparation, following the protocol called quaddRAD in Franchini et al. (2017) with slight modifications as described by Xu and Hausdorf (2021). A total of 77 specimens were subjected to ddRAD analysis, and among these, 48 specimens were shared with Sanger sequencing (Table S1). The details of the protocol used are given in Becher et al. (2025), with the use of restriction enzymes FastDigest PstI and MspI (Thermo Fisher Scientific). In summary, the genomic DNA was digested and ligated to adaptors with inner barcodes and four random bases for the recognition of PCR duplicates in the same reaction. We modified the barcode sequence of adapter quaddRAD-i5_#01 to TATCGT (quaddRAD-i5-top_#01: 5′-CGCTCTTCCGATCTNNNNTATCGTTGCA-PHOS-3′; quaddRAD-i5-bottom_#01: 5′-PHOS-ACGATANNNNAGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGT-3′). Then an equal amount by weight of different samples, each with different inner barcode combinations, was pooled. Next, each pool of the digested/ligated DNA was amplified by PCR (15 cycles) with Phusion Polymerase (Thermo Fisher Scientific) and primers with outer dual Illumina indices. Aliquots of each pool with equimolar amounts of fragments in the targeted size range of 400–500 bp were pooled into one final library. Paired-end next-generation sequencing (2 × 150 bp) of the library was performed in one Illumina NovaSeq X lane at GENEWIZ (Leipzig, Germany). Demultiplexed paired-end quaddRAD-seq data for each sample have been deposited in the NCBI Sequence Read Archive database (BioProject PRJNA1242557).
The raw fastq files of each pool obtained from the Illumina run were first demultiplexed by the sequencing provider using the outer dual Illumina indices. quaddRAD-seq data were then processed using the de novo assembly option of ipyrad v. 0.9.90 (Eaton & Overcast, 2020), in which the sequences were clustered by the tool vsearch and the homology was inferred based on sequence similarity. The demultiplexed fastq files of each pool were demultiplexed again by sorting reads by the inner barcodes specific to each sample, using the datatype ‘pair3rad’ and the restriction-site overhangs for PstI and MspI as ‘TGCAG, CGG’, and allowing two barcode mismatches. The reads were filtered by applying a maximum of five low-quality bases, and the restriction sites, barcodes and Illumina adapters were trimmed from raw sequence reads using the strict filter option. Bases with low quality scores (Phred-score < 20) were replaced with N. Reads were matched together and aligned within samples at a clustering threshold of 75%. Clusters of aligned loci with a minimum depth of coverage <6 were discarded. The maximum numbers of uncalled bases and heterozygous bases allowed in consensus sequences were both set at 5%. The concatenation of all loci was applied to maximise phylogenetic signal (Gadagkar et al., 2005). The final concatenated alignment was created with the minimum number of 12 samples per locus, and the maximum number of eight indels were allowed in a final locus. This final step also allowed a maximum of 50% of heterozygous sites and 20% SNPs per locus. An output file for the final concatenated dataset was generated for further phylogenetic reconstruction. Patterns of locus-sharing among individuals were assessed by calculating the pairwise Jaccard's distances (Jaccard, 1908) from a locus presence–absence matrix (Hipp et al., 2014) using the function ‘locus.dist’ in the R package RADami (Hipp, 2017). A graphical representation of locus-sharing similarity matrix was plotted with the phylogeny retrieved from IQ-TREE (see below) using the function ‘plot.locus.dist’.
2.4 Phylogenetic reconstruction
Chromatograms of DNA Sanger sequence data were checked manually for misreads, and sequences were then trimmed in MEGA X (Kumar et al., 2018). The accession numbers of newly generated sequences deposited in the GenBank database, as well as other relevant pupinid sequences retrieved from GenBank which were used in subsequent analyses are given in Table S1. Two sets of outgroups were also incorporated into the dataset: (1) outgroup taxa within the Cyclophoroidea, largely following Webster et al. (2012), which cover the families Megalomastomatidae, Hainesiidae, Cochlostomatidae and Cyclophoridae, and (2) outgroup taxa outside the Cyclophoroidea, but still within Caenogastropoda, including Koreoleptoxis tegulata (Martens, 1894) (Semisulcospiridae), Elimia catenaria (Say, 1822) (Pleuroceridae) and Holandriana holandrii (Pfeiffer, 1828) (Amphimelaniidae) of the superfamily Cerithioidea (see Table S1).
The sequence alignments of each gene fragment were performed separately using MAFFT v.7, which is available online (https://mafft.cbrc.jp/alignment/server/index.html), with default options (Katoh et al., 2017). The nuclear 5.8S + ITS2 + 28S rRNA gene marker was divided into two fragments for finding the different best-fitting model: 5.8S + ITS2 and 28S rRNA, as the genes encoding rRNA and transcribed spacers have a different degree of substitution rate (Choudhary et al., 2015). The recognition of each gene fragment was done by annotating the ITS2, 5.8S and 28S flanking regions via the ITS2-Annotation module (Keller et al., 2009) at http://its2.bioapps.biozentrum.uni-wuerzburg.de/ (Koetschan et al., 2010). The concatenated dataset was prepared in Kakusan4 (Tanabe, 2011) with the best-fitting model adjustment for Bayesian inference (BI) analyses. The BI analysis was performed with the best-fitting models of each gene fragment and each codon position of COI (generalised time reversible (GTR) + gamma (G) for all codon positions of COI, Hasegawa–Kishono–Yano (HKY85) + G for 16S rRNA and 5.8S + ITS2 and Kimura 2-parameter (K80) + G for 28S rRNA) using MrBayes on XSEDE v.3.2.6 (Ronquist et al., 2012) in the CIPRES Science Gateway (Miller et al., 2010). Two independent analyses were run simultaneously and consisted of four chains of five million generations, sampling every 500 generations and discarding the first 50% of samples as burn-in. Convergence of the two runs was achieved if the average standard deviation of split frequencies was ≤0.01, and adequate Markov chain Monte Carlo (MCMC) samples from the posterior probability distributions were assessed if potential scale reduction factor (PSRF) values of each parameter approached 1.0 (Ronquist et al., 2012).
The maximum likelihood (ML) analysis was also performed using the IQ-TREE webserver (see http://iqtree.cibiv.univie.ac.at), with the integrated ModelFinder function (Kalyaanamoorthy et al., 2017; Nguyen et al., 2015; Trifinopoulos et al., 2016). Concatenated sequences of ddRAD loci were also subjected to ML analysis performed with IQ-TREE on ACCESS v.2.3.2 in the CIPRES Science Gateway. Branch support was estimated using 10,000 ultra-fast bootstrap replicates (Hoang et al., 2018), the Shimodaira and Hasegawa-approximate likelihood-ratio (SH-aLRT) test and the approximate Bayes (aBayes) test (Anisimova et al., 2011). A clade was considered to be well-supported if the SH-aLRT support values were ≥80%, aBayes support values were ≥0.95, ultra-fast bootstrap support (BS) values were ≥95% and Bayesian posterior probability values (PP) were ≥.95 (Anisimova et al., 2011; Hoang et al., 2018; San Mauro & Agorreta, 2010). All trees were rooted with the superfamily Cerithioidea, except for the ML tree of ddRAD loci, which adopted the midpoint rooting. The alignment files and phylogenetic trees are available from TreeBase (study accession number 32047).
2.5 Divergence time estimation
The approximate divergence time among the species and genera of the Pupinidae was estimated using BEAST2 on ACCESS v.2.6.7-2.7.7 (Bouckaert et al., 2014) in the CIPRES Science Gateway. The dataset was based on the same nuclear and mitochondrial sequence data with the partitioning and nucleotides substitution models as in the BI analysis with MrBayes. The divergence time estimation employed the linked optimised lognormal relaxed clock model, which is more efficient and operates well whether the rates and branch lengths are correlated or not (Douglas et al., 2021), and a Yule process model for a tree prior with a random starting tree. All taxa within the superfamily Cyclophoroidea were constrained as monophyletic. The tree calibration points were applied at three nodes. The first two nodes belong to the ingroup: (1) the crown node of Coptocheilus (including Tortulosa, see Section 3) and (2) the crown node of Pseudopomatias, applying the age of fossil records of these genera in Burmese amber (98.79 ± 0.62 Ma) (Hirano et al., 2019; Shi et al., 2012; Yu et al., 2023). The third node, belonging to the outgroup, is the crown node of Cochlostoma of the Cochlostomatidae, based on the oldest fossil record in the Thanetian or the Late Palaeocene (<56 Ma) (Manganelli & Giusti, 1997; Wenz, 1923a). All fossil calibrations were given an exponential prior distribution (mean = 1, offset = 98.17 for the crown nodes of Coptocheilus + Tortulosa and Pseudopomatias and mean = 1, offset = 56 for the crown node of Cochlostoma). Three runs of 100 million generations each were executed, where the parameters were sampled every 1000 generations. LogCombiner v. 2.7 (Drummond & Rambaut, 2007) was used to combine the three runs and resample the tree files every 5000 generations, while assessing the effective sample size (ESS) values for all parameters to be above 200 and the convergence in Tracer v. 1.7.2 (Rambaut et al., 2018). TreeAnnotator v. 2.7.7 (Drummond & Rambaut, 2007) was used to construct the maximum clade credibility tree using median heights as node heights, after discarding the first 10% of the trees as burn-in. The final ultrametric tree with the node bar indicating 95% highest posterior density intervals of divergence times (95% HPD interval) on each branch was displayed via FigTree v. 1.4.4 (Rambaut, 2018), and the phylogeny was plotted with the geological time scale by the geoscalePhylo function from the R package strap v. 1.6.1 (Bell & Lloyd, 2015).
3 RESULTS
3.1 Phylogenetic analyses of DNA sanger sequences and genus-level relationships
The final concatenated sequence alignment of 125 taxa had a length of 2319 bp including 1364 (58.8%) variable sites and 1140 (49.2%) parsimony-informative sites. The alignment includes 660 bp of COI (366 variable and 318 parsimony-informative sites), 555 bp of 16S rRNA (366 variable and 334 parsimony-informative sites), 774 bp of 5.8S + ITS2 (581 variable and 454 parsimony-informative sites) and 330 bp of 28S rRNA (51 variable and 34 parsimony-informative sites). GenBank accession numbers for COI are PV225414–PV225508, for 16S are PV221581–PV221669 and for ITS2 + 28S are PV221670–PV221746 (Table S1).
The phylogenetic analyses gave largely consistent topologies for both strongly supported and less supported nodes (Figure 2, showing ML topology with unresolved polytomies collapsed). These analyses were based on the concatenated datasets and rooted with the superfamily Cerithioidea, analysed by ML in IQ-TREE (Figure S1), and BI from both MrBayes (Figure S2) and BEAST2 (Figure 3). All taxa belonging to the Cyclophoroidea in this study were retrieved as monophyletic with well-supported values, except that this was not supported in BEAST2. The monophyly of each of the families Cyclophoridae, Hainesiidae, Cochlostomatidae, as well as the clade Hainesiidae + Cochlostomatidae, was well-supported in all analyses.

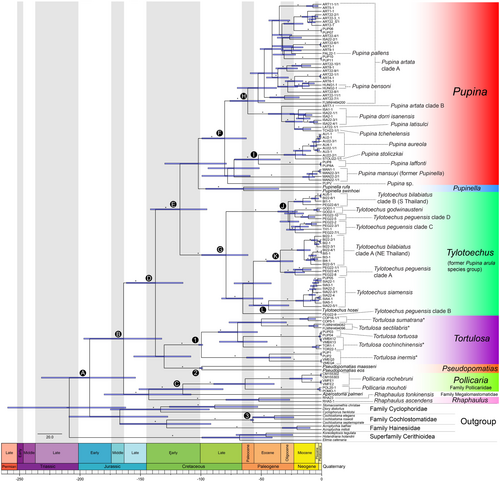
The results of this study reveal significant taxonomic changes within the family Pupinidae, based on well-supported phylogenetic analyses. The study also clarifies broader phylogenetic relationships of the Pupinidae at the family level. For example, Aperostoma palmeri (Bartsch & Morrison, 1942) of the Megalomastomatidae was nested within the Pupinidae, in that this taxon is a sister clade of the pupinid genus Pollicaria, being well supported in all analyses. This leads to the monophyly of the Pupinidae + A. palmeri, which is also well supported in all analyses except in BEAST2 (Figure 3).
The family Pupinidae in its current sense could be separated into three well-supported clades: (1) Rhaphaulus, (2) A. palmeri + Pollicaria and (3) the main clade comprising the remaining taxa of this family, where the relationship among these three clades was not resolved. The main clade of the remaining taxa could be further separated into three clades: (1) Pseudopomatias, (2) Coptocheilus + Tortulosa and (3) Pupina + Pupinella. The clade Pseudopomatias was well-supported by all analyses and the clade Coptocheilus + Tortulosa was well-supported by SH-aLRT and aBayes support values and BEAST2 analysis.
This study also identifies cases of non-monophyly and proposes taxonomic reassignments at the genus level. One case is that, within the clade Coptocheilus + Tortulosa, T. tortuosa (Férussac, 1821), the type species of Tortulosa, was nested within the other Coptocheilus species, therefore rendering Coptocheilus polyphyletic. As the priority of the genus name Tortulosa preceded Coptocheilus, we propose to move all Coptocheilus species from mainland Southeast Asia into Tortulosa (Table 1). Another case is Pupinella which was not monophyletic, as East Asian Pupinella rufa (A. Adams & Sowerby II, 1864) and Pupinella swinhoei H. Adams, 1866 constitute a clade distinct from the mainland Southeast Asian clade, consisting of Pupinella mansuyi, the Pupina artata and Pupina aureola species groups. Therefore, Pupinella mansuyi is here reclassified as Pupina mansuyi, also based on glossy shell surface as in other Pupina species. We also propose to reclassify all remaining Pupinella species from mainland Southeast Asia in Pupina (Table 1).
| Former generic classification | Generic combination proposed in this study |
|---|---|
| Pupina as recognised in Jirapatrasilp, Sutcharit, and Panha (2022) | Tylotoechus arula (Benson, 1856) comb. nov. |
| Tylotoechus bilabiatus (Jirapatrasilp, 2022) comb. nov. | |
| Tylotoechus crosseanus (Morlet, 1883) comb. nov. | |
| Tylotoechus excisus (Möllendorff, 1902) comb. nov. | |
| Tylotoechus exclamationis (Mabille, 1887) comb. nov. | |
| Tylotoechus godwinausteni (Jirapatrasilp, 2022) comb. nov. | |
| Tylotoechus hosei (Godwin-Austen, 1889) comb. nov. | |
| Tylotoechus mouhoti (Pfeiffer, 1861) comb. nov. | |
| Tylotoechus peguensis (Benson, 1860) comb. nov. | |
| Tylotoechus perakensis (Möllendorff, 1891) comb. nov. | |
| Tylotoechus siamensis (Möllendorff, 1902) comb. nov. | |
| Tylotoechus vescoi (Morelet, 1862) comb. nov. | |
| Pupinella as recognised in Jirapatrasilp, Sutcharit, and Panha (2022) | Pupina illustris Mabille, 1887 |
| Pupina mansuyi (Dautzenberg & Fischer, 1908) | |
| Pupina sonlaensis Do, 2017 | |
| Pupina thaitranbaii Do, 2017 | |
| Coptocheilus as recognised in Bui and Páll-Gergely (2020) | Tortulosa cochinchinensis (Rochebrune, 1882) comb. nov. |
| Tortulosa inermis (Bavay & Dautzenberg, 1909) comb. nov. | |
| Tortulosa maunautim (Bui & Páll-Gergely, 2020) comb. nov. | |
| Tortulosa maydelineae (Páll-Gergely, Nguyen & Chen, 2019) comb. nov. | |
| Tortulosa messageri (Bavay & Dautzenberg, 1909) comb. nov. | |
| Tortulosa sectilabris (Gould, 1844) comb. nov. | |
| Tortulosa sumatrana (Dohrn, 1881) comb. nov. |
3.2 Phylogenetic relationships of Pupinella and Pupina species groups
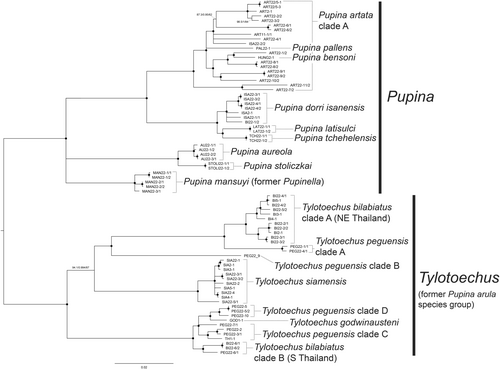
The study provides further insights into the phylogenetic structure of the clade containing Pupinella and the three Pupina species groups sensu Jirapatrasilp, Sutcharit, and Panha (2022). All species in the Pupina arula species group in this study (P. bilabiata Jirapatrasilp, 2022, P. godwinausteni Jirapatrasilp, 2022, P. peguensis Benson, 1860 and P. siamensis Möllendorff, 1902), including Pupina hosei Godwin-Austen, 1889 from Sabah, Borneo, were retrieved together as monophyletic with all well-supported values. This clade of the P. arula species group is a sister clade to another clade of the remaining Pupina and Pupinella taxa. Pupina peguensis was separated into four distinct clades, three of which are mostly distributed in northern Thailand (Figure 1d), whereas P. bilabiata was separated into two clades belonging to two different geographical regions: northeastern and southern Thailand (Figure 1c).
The clade of the remaining Pupinella taxa, and Pupina artata and Pupina aureola species groups was retrieved as monophyletic in MrBayes analysis (Figure S2). Excluding Pupinella rufa and Pupinella swinhoei from East Asia, the clade with remaining mainland Southeast Asian taxa could be separated into four subclades, where the relationships among these subclades yielded an unresolved polytomy. Three out of four subclades are (1) Pupina mansuyi, (2) Pupina sp. from Da Nang, Vietnam and (3) Pupina aureola Stoliczka, 1872 with two other species from the same Pupina aureola species group (P. stoliczkai Jirapatrasilp, 2022 and P. laffonti Ancey, 1899).
The fourth subclade could be further separated into two well-supported subclades. The first subclade is the P. artata clade A representing P. artata species group, consisting of the majority of P. artata Benson, 1856, with two species, P. pallens Möllendorff, 1894 and P. bensoni Jirapatrasilp, 2022, nested within the P. artata clade A. The second subclade contains some taxa of P. aureola species group, including P. tchehelensis Morgan, 1885, P. dorri isanensis Jirapatrasilp, 2022 and P. latisulci Jirapatrasilp, 2022, plus P. artata clade B from eastern Thailand. Therefore, the species P. artata, as well as P. artata species group and P. aureola species group were not monophyletic.
As Pupina species in this study are polyphyletic with the nesting of Pupinella, several species within Pupina were reassessed, leading to taxonomic reclassification. The Pupina arula species group constitutes its own clade; therefore, this clade should bear its own genus. This species group is characterised by an extending parietal tooth from a parietal callus, continuing horizontally when observed from the lateral view and a wide and outward-curving posterior canal, which is always visible at the apertural view. These characters also share morphological features with Pupina destructa Heude, 1885, the type species of the subgenus Tylotoechus Kobelt & Möllendorff, 1897 (Jirapatrasilp, Sutcharit, & Panha, 2022: fig. 17). Although ‘Pupina’ destructa was not included in this molecular phylogeny, we refrain from proposing a new genus for the Pupina arula species group and opt to elevate the subgenus Tylotoechus to the generic rank instead. This clade of Pupina arula species group was then assigned to Tylotoechus, and the remaining species within this species group from mainland Southeast Asia were reclassified in this genus (Table 1). Members of Pupina artata and Pupina aureola species groups remain in the genus Pupina.
3.3 SNP calling and phylogenetic analysis of ddRADseq data
A total of 124.96 million raw reads with a length of 151 bp each were retrieved across 79 samples. Among these, 43 samples belong to Pupina and 36 samples to Tylotoechus (see Table S1). We then removed two samples (one Pupina and one Tylotoechus) owing to low read numbers (<100,000 raw reads). After the first quality and adapter filtering step, a total of 124.79 million filtered reads (99.9%) across 77 samples were retained. The number of filtered reads per sample ranged from 124,495 to 9,280,173 reads, with an average ± standard deviation of 1.62 ± 1.48 million reads (Table S2).
After processing with ipyrad, the number of consensus reads per sample ranged from 1730 to 31,284 reads, with an average ± standard deviation of 17,177 ± 5480 reads. These consensus reads were clustered into 267,389 loci, which were then filtered by the removal of PCR duplicates, maximum numbers of indels, SNPs and heterozygous sites per locus, retaining 260,155 loci. After filtering by the minimum number of 12 samples per locus, the final concatenated sequence matrix size was 274,923 bp from a total of 1106 loci, where the matrix contains 18.01% of the total matrix data. The number of loci coverage for each sample ranged from 16 to 928 loci, with an average ± standard deviation of 202 ± 310 loci (Table S2).
The phylogenetic analyses of these ddRADseq loci yielded a tree topology (Figure 4) generally similar to those of phylogenies retrieved from Sanger sequence analyses, even though the number of loci coverage was as low as 16 loci retrieved from one P. mansuyi sample (MAN22/3-1; Table S2), and this sample still belonged to the same clade as other P. mansuyi samples with well-supported values. One difference from the phylogeny of ddRADseq loci is that Tylotoechus peguensis clade B was retrieved as a sister clade to the clade of Tylotoechus bilabiatus clade A + T. peguensis clade A with well-supported values, whereas in phylogenies retrieved from Sanger sequence analyses, T. peguensis clade B was retrieved as a sister clade to the clade of (T. bilabiatus clade A + T. peguensis clade A) + (T. siamensis + T. hosei) (Figures 2 and 3). The plot of the pairwise shared-locus matrix showed that more loci were shared among individuals of the closely related species within the same major clade (Figure 5a), especially the clade C of T. peguensis clade A + T. bilabiatus clade A (Figure 5b), showing a percentage of shared loci to total loci between 41.8% and 81.6% (mean = 72.5%, SD = 11.4%). The remaining major clades have much lower percentages (Table 2).
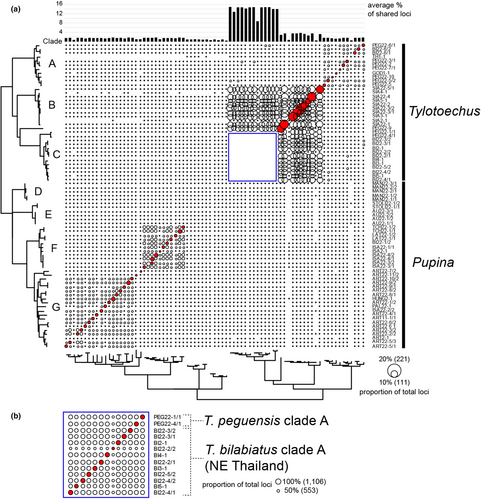
| Clade | Species | % range | % average ± SD |
|---|---|---|---|
| A | Tylotoechus bilabiatus clade B + T. peguensis clade C + T. peguensis clade D + T. godwinausteni | 1.3–4.9 | 3.2 ± 1.0 |
| B | T. siamensis | 0.6–13.1 | 7.5 ± 4.6 |
| C | T. peguensis clade A + T. bilabiatus clade A | 41.8–81.6 | 72.5 ± 11.4 |
| D | Pupina mansuyi | 1.2–2.3 | 1.5 ± 0.3 |
| E | Pupina aureola + P. stoliczkai | 1.4–2.5 | 2.0 ± 0.3 |
| F | Pupina dorri isanensis + P. latisulci + P. tchehelensis | 2.9–6.1 | 4.7 ± 0.7 |
| G | Pupina artata clade, including P. pallens and P. bensoni | 1.0–5.4 | 3.3 ± 0.9 |
- Note: The lettering of the clades follows those in Figure 5.
3.4 Divergence time estimation
The chronogram (Figure 3) illustrated the split between the family Pupinidae and the other families in the Cyclophoroidea within the timespan between the Late Triassic and Late Jurassic (node A: 95% HPD interval = 154–232 Ma; mean = 192 Ma, Early Jurassic). The earliest event that led to the split between the clade Pollicaria + Aperostoma + Rhaphaulus and the clade of the remaining taxa occurred between the Early Jurassic and Early Cretaceous (node B: 95% HPD interval = 132–197 Ma; mean = 164 Ma, Middle Jurassic). The split between Pollicaria from Southeast Asia and Aperostoma from Central America occurred between the Late Jurassic and Late Cretaceous (node C: 95% HPD interval = 81–151 Ma; mean = 115 Ma, Early Cretaceous).
The later split among the majority of the pupinid genera in this study occurred between the Late Jurassic and Early Cretaceous (node D: 95% HPD interval = 115–161 Ma; mean = 137 Ma, Early Cretaceous). More speciose genera (i.e., Pupina and Tylotoechus) were split apart between the Early to Late Cretaceous (node E: 95% HPD interval = 95–142 Ma; mean = 117 Ma, Early Cretaceous) and started to diversify within the timespan between the Late Cretaceous and Palaeocene (node F: 95% HPD interval = 62–99 Ma; mean = 80 Ma, Late Cretaceous; node G: 61–99 Ma; mean = 79 Ma, Late Cretaceous). Some later species diversification of Pupina occurred between Late Cretaceous and Eocene (node H: HPD interval = 47–76 Ma; mean = 61 Ma, Palaeocene; node I: 34–72 Ma; mean = 52 Ma, Eocene), while diversification into more Tylotoechus species happened between Palaeocene and Miocene (node J: HPD interval = 20–36 Ma; mean = 28 Ma, Oligocene; node K: 23–45 Ma; mean = 34 Ma, Eocene; node L: 27–60 Ma; mean = 44 Ma, Eocene).
4 DISCUSSION
4.1 Monophyly of Pupinidae and resurrection of Pollicariidae Thiele, 1929
The monophyly of the Pupinidae or, in other words, which taxa should be classified in Pupinidae, is still questionable because it is not clear which of the morphological states that characterise the Pupinidae according to Thiele (1929) and Tielecke (1940) could be autapomorphies of the taxon. In addition, the relationships between some genera, for example, Rhaphaulus, and between them and the outgroups also remain partly unresolved (Figure 2).
Based on the findings of this study, the Pupinidae in the current sense are not monophyletic as the molecular phylogenetic analyses showed that Pollicaria proved to be a sister clade of the representative of the Neotropical Megalomastomatidae, Aperostoma palmeri. Although A. palmeri was included in a clade containing all the pupinid genera in the current sense, we do not consider including Megalomastomatidae in the Pupinidae appropriate as these two groups are distinct from each other in terms of genitalia and geographical distribution (Kongim et al., 2013; Thompson, 1969; Tielecke, 1940). Therefore, as all major genera in mainland Southeast Asia have been included in the phylogenetic analysis of this study, we resurrect the family Pollicariidae for Pollicaria in order to keep most of the remaining pupinid genera together as monophyletic. The diagnostic of this family follows that of the genus as proposed by Thiele (1929), who firstly introduced this family-level taxon, the tribe Pollicarieae [sic] to accommodate this sole genus. Pollicaria includes by far the largest ‘pupinids’ endemic to mainland Southeast Asia, and could be differentiated from the other pupinid genera by a larger (1.5–2×) shell size with a shallow posterior angled groove as a breathing device, sometimes with a parietal declining shoulder inside the peristome, a calcareous operculum and a bright yellow-orange to pale orange body (Kongim et al., 2013). As not all taxa of the Pupinidae and Megalomastomatidae were included in this study, future studies including a broader representation of taxa from each family are needed to further update this classification scheme.
The taxonomic position of Rhaphaulus is uncertain, as the phylogenetic relationships among the three clades of Rhaphaulus, the remaining Pupinidae and Pollicariidae + Megalomastomatidae are still unresolved in ML and MrBayes analyses, whereas Rhaphaulus was retrieved as a sister clade to the clade Pollicariidae + Megalomastomatidae in the BEAST2 analysis. The systematics of Rhaphaulus is only based on shell and operculum characters, whereas the genitalia information is still lacking. The shell of Rhaphaulus is similar to Pollicaria in having a pupoid shell, with a large body whorl and a shouldered penultimate whorl but is at least twice smaller than Pollicaria and possesses a tube as a breathing device instead of a shallow posterior angled groove as in Pollicaria. The operculum of Rhaphaulus is also thin and corneous, while being calcareous in Pollicaria (Jirapatrasilp, Sutcharit, & Panha, 2022; Páll-Gergely et al., 2014; Sajan et al., 2019). Another large pupinid genus, Braziera Smith, 1887 from New Guinea, with its sole member Braziera brazierae (Smith, 1887) and its subspecies B. brazierae aignanensis (Hedley, 1891), also resembles Pollicaria in shell shape with a round aperture without any tube, but with a smaller shell and corneous operculum more like Rhaphaulus (Egorov, 2013; Hedley, 1891; Iredale, 1941; Smith, 1887). Therefore, the examination of genitalia as well as the inclusion of more mitochondrial and nuclear sequences are needed to pinpoint the exact taxonomic position of both Rhaphaulus and Braziera.
4.2 Phylogenetic relationships within Pupinidae
The Pupinidae from mainland Southeast Asia have been classified into two subfamilies: Pupininae and Pupinellinae. The two subfamilies only differ in the shell surface, being glossy and completely smooth with glaze in the Pupininae, and being either striated, matt or silky-shiny without glaze in the Pupinellinae (Egorov, 2013; Kobelt, 1902). However, this study revealed that the Pupinellinae in its current sense sensu Kobelt (1902) (Pseudopomatias, Pollicaria, Pupinella, Rhaphaulus, Tortulosa) are paraphyletic. The studied species of Pupinella (the type genus of the Pupinellinae) form two lineages, which are nested within the representatives of the Pupininae, Pupina (the type genus of the Pupininae) and Tylotoechus. Therefore, the subfamilial classification of the Pupinidae has to be revised.
Among all the pupinid genera in this study, Coptocheilus and Pupinella were not retrieved as monophyletic. Coptocheilus is paraphyletic with regard to Tortulosa. Currently, Tortulosa exhibits a disjunct distribution range, with species distributed in the Western Ghats, India and Sri Lanka classified under the subgenus Eucataulus Kobelt, 1902, and a single species from the Malay Peninsula, Tortulosa tortuosa (Férussac, 1821) which is a type species of the genus (Jirapatrasilp, Sutcharit, & Panha, 2022; Raheem et al., 2014). Coptocheilus has a wider distribution from the Himalaya, East Asia down to Southeast Asia (Páll-Gergely et al., 2019). Given that the position of Tortulosa within the mainland Southeast Asian Coptocheilus species is strongly supported by the data, we suggest including all Coptocheilus species in this study as well as the remaining Vietnamese Coptocheilus species in Tortulosa (Table 1). The relationship between Eucataulus and Tortulosa has to be confirmed by a molecular phylogenetic analysis. The type species of Coptocheilus, C. altus (Sowerby I, 1842) from the Philippines, and additional species from other regions should also be sequenced to check whether they belong to the Tortulosa clade and Coptocheilus is synonymous with the older name Tortulosa or whether it represents a distinct group.
The genus Pupinella has a wide distribution from East Asia, Southeast Asia down to New Guinea (Kobelt, 1902; MolluscaBase, 2024b). Although only two representative species from East Asia (P. rufa and P. swinhoei) and one from mainland Southeast Asia (P. mansuyi) are included in the phylogenetic analysis, Pupinella was not covered as monophyletic as P. mansuyi is more closely related to other Pupina species from mainland Southeast Asia. Pupinella could only be diagnosed by the presence of an umbilical passage or a funnel-like anterior canal forming a tube opening at both ends, while it shares other conchological characters with Pupina (Jirapatrasilp, Sutcharit, & Panha, 2022; Varga & Páll-Gergely, 2017). Therefore, it could be assumed that the anterior canal character is homoplasious, and the comprehensive inclusion of Pupinella species from a wider distribution range into a phylogenetic framework is crucial for the revision of this genus. The position of Pupinella mansuyi has already been questioned by Jirapatrasilp, Sutcharit, and Panha (2022) because the species has a glossy surface like the Pupina species. Because the phylogenetic tree shows that Pupinella mansuyi is more closely related to the Pupina species than to the other Pupinella species, Pupinella mansuyi is thus transferred to Pupina (Table 1). On the other hand, two east Asian species, Pupinella swinhoei, which is also the type species of the subgenus Pupinopsis H. Adams, 1866, and Pupinella rufa were in the meanwhile kept in Pupinella. The inclusion of Pupinella pupiniformis (Sowerby I, 1842) from the Philippines, the type species of Pupinella, is necessary to identify the group for which the name Pupinella is appropriate, and whether Pupinopsis should be raised to a full genus.
On the other hand, different states of the parietal tooth and the posterior canal are found to be specific to Pupina and Tylotoechus. Tylotoechus was formerly classified as a subgenus of Pupina. This name is here applied to the Pupina arula species group sensu Jirapatrasilp, Sutcharit, and Panha (2022) (Table 1), diagnosed by an extending parietal tooth from a parietal callus and a wide and outward-curving posterior canal. The name Pupina is now applied to the species of the Pupina artata and Pupina aureola species groups, and other Pupinella species from mainland Southeast Asia, exhibiting a triangular or fin-shaped parietal tooth either covering the posterior canal or located next to the slit-like or wide but not outward-curving posterior canal. The inclusion of T. destructus from China and Pupina keraudrenii Vignard, 1829 from New Guinea, the type species of Tylotoechus and Pupina, respectively, in the molecular phylogenetic analysis is necessary to decide whether the usage of the name Tylotoechus and Pupina for each respective group is justified.
The investigation of phylogenetic relationships among pupinid genera, although using a limited set of taxa that is not fully representative in terms of taxon sampling, still provides initial insights into Asian pupinid taxa. As many of these taxa have distributions extending beyond mainland Southeast Asia, the findings of this study pave the way for future research that includes a broader taxonomic and geographical scope.
4.3 Implication of ‘cryptic species’ in Pupina and Tylotoechus
Cryptic diversity in land snails has been frequently uncovered through extensive molecular and morphological studies (Modica et al., 2016; Razkin et al., 2017), with the term ‘cryptic species’ often reflecting taxonomists' initial inability to identify meaningful diagnostic characters rather than a true biological phenomenon (Horsáková et al., 2019, 2022). Particularly in cyclophoroid land snails, species diagnoses have historically relied primarily on shell characters (Gude, 1921; Kobelt, 1902), whereas those traditional macroscopic conchological differences may initially seem absent. Anatomical characters are also reported in very few species because of difficulties in dissection or else they are highly similar to be rendered less useful in species differentiation (Kasinathan, 1975; Páll-Gergely et al., 2020; Tielecke, 1940; Tongkerd et al., 2023). However, taxonomists now increasingly incorporate microscopic data and geometric morphometric analyses to support molecular phylogenetic species delimitations of many cyclophoroid taxa (Nantarat et al., 2014, 2019; Prasankok et al., 2023; von Oheimb et al., 2019).
The extent of ‘cryptic species’ could be implied in Pupina and Tylotoechus, as some nominal species are not monophyletic in this study. One prominent example, T. bilabiatus, characterised by an ovate-fusiform to fusiform shell with a distinct furrow between inner and outer peristomes making the lip appear as doubled (Jirapatrasilp, Sutcharit, & Panha, 2022), is separated into two different clades that are not closely related to each other and belong to two different geographic regions. Another Tylotoechus morphospecies, T. peguensis, was separated into four distinct clades, whereas in Pupina, P. artata was separated into two clades, where one clade illustrates a species complex that also incorporates other Pupina species (P. pallens and P. bensoni). As these nominal species are solely identified based on macroscopic shell characters, such as shell and parietal tooth shape (Jirapatrasilp, Sutcharit, & Panha, 2022), the reexamination of microscopic traits as well as quantitative analyses of morphological data would promisingly yield diagnostic characters of purportedly Pupina and Tylotoechus ‘cryptic’ species.
Nevertheless, it is highly possible that later analyses or reexaminations would not reveal any morphological diagnoses. Another alternative is to employ unique nucleotide substitutions of gene fragments as molecular diagnostic characters (Brower & DeSalle, 2024; Renner, 2016; Rheindt et al., 2023), in order to prevent the ‘taxonomic crypsis’ the situation of not describing any morphologically cryptic species (Schlick-Steiner et al., 2007). This sole practice as ‘DNA taxonomy’ or ‘molecular taxonomy’ (Ahrens, 2024; Jörger & Schrödl, 2013; Miralles et al., 2024) has been adopted in diverse groups of gastropods (e.g., Churchill et al., 2014; Gittenberger & Gittenberger, 2011; Johnson et al., 2015; Puillandre et al., 2017), although still to a lesser extent in land snails (von Oheimb et al., 2019). However, it should be an ultimate aim for any taxonomists, attempting their best to uncover any elusive morphological diagnoses and combine them together with molecular diagnoses in an integrative taxonomic framework (Zamani et al., 2022).
Although the phylogeny does not cover taxa across their entire geographical range, it includes the greatest number of representatives with different shell forms, making it a meaningful step toward understanding their evolutionary relationships. A more detailed phylogenetic analysis of these two genera will certainly reveal the exact species diversity within the region.
4.4 Divergence time and evolutionary history of the family Pupinidae
Our study estimated the split of the Central American Aperostoma from the Southeast Asian Pollicariidae between the Late Jurassic and Late Cretaceous, which is concordant with the estimation of the split between Aperostoma and the Pupinidae in Gervascio (2017). This is not in accordance with the hypothesis that the divergence between American and Asian cyclophoroid taxa was triggered by the breakup of Pangea, which began well in the Triassic, and the subsequent opening of the central Atlantic Ocean during the Early Jurassic (Frizon de Lamotte et al., 2015; Zhu et al., 2021). The inclusion of more Neotropical cyclophoroids in the phylogenetic framework and the age calibration with the Neotropical fossil taxa are required to clarify the evolutionary history of deep divergence between Neotropical and Indomalayan cyclophoroid groups.
The fossils of the Pupinidae could be dated back to the Late Cretaceous, the epoch of which several extant and related extinct genera were already evolved (Balashov et al., 2021; Bullis et al., 2020; Hirano et al., 2019; Hrubesch, 1965a, 1965b; Jochum et al., 2021; Neubauer et al., 2019; Tausch, 1886; Yu et al., 2018, 2019, 2021, 2023). However, some pupinid genera in the Late Cretaceous of Europe, for example, Cyclomastoma Hrubesch, 1965, Praebadchisestis Hrubesch, 1965, Rognacia Oppenheim, 1895, and even the younger ones from Palaeocene to Miocene, for example, Ischurostoma Bourguignat, 1874, Protocallia Wenz, 1925, Ventriculus Wenz, 1914, lack an umbilical keel, apertural tubes or canals (Bourguignat, 1874; Hrubesch, 1965a, 1965b; Oppenheim, 1895; Wenz, 1914, 1923b, 1925, 1938b). These extinct genera might belong to the family Pollicariidae instead.
The discovery of these diverse fossils in the Late Cretaceous suggests that the diversification of the Pupinidae and its split from the related cyclophoroid families occurred much earlier than the timeframe proposed by Hirano et al. (2019), alleging that the first divergence within the Cyclophoroidea occurred during the Jurassic and Cretaceous, and the diversification of cyclophoroid families and genera occurred during the Late Cretaceous and Palaeocene. In this study, the time-calibrated phylogeny inferred the split of the Pupinidae during the Late Triassic and Early Cretaceous with the mean age in the Middle Jurassic, and the first diversification of pupinid genera started during the Middle Jurassic and Early Cretaceous with the mean age in the Early Cretaceous (Figure 3), which corresponds more to the estimation by Gervascio (2017). During these periods, Southeast Asia was deemed the center of diversification for angiosperms (Buerki et al., 2014; Malik, 2019). It has been proposed that complex palaeogeographical histories along with the features of island biogeography and a wide range of ecological niches could have fueled the diversification of taxa in this region during the Late Jurassic and Early Cretaceous (Buerki et al., 2014), and it could be implied that these factors might also play a role in the diversification of the Pupinidae. Despite different age estimations, the finding also supports the notion of extreme conchological conservatism in the Pupinidae for at least 100 million years (Bichain et al., 2022; Hirano et al., 2019).
4.5 The implementation of RADseq related techniques for deeply divergent taxa
This study illustrates the success of using ddRADseq data to construct a phylogeny of two related, deeply divergent pupinid genera, Pupina and Tylotoechus, with the divergence age of c. 120 Ma or in the Early Cretaceous (Figure 3). Comparing with other land snail research, RADseq-related techniques have successfully revealed the genetic differentiation of populations (Hausdorf et al., 2024; Oswald et al., 2022) and the hybridisation of closely related species (Korábek & Hausdorf, 2024; Xu & Hausdorf, 2021), as well as resolved the phylogeny (Raheem et al., 2023; Whelan et al., 2023) and delimited species in radiations within a species complex (Bamberger et al., 2022; Becher et al., 2025; Haponski et al., 2019; Razkin et al., 2016). However, RADseq-related techniques have never been applied to resolve the phylogeny of deeply divergent land snail taxa, while this has been done in other animal groups up to 80-million-year divergence or the Late Cretaceous (Herrera et al., 2015; Herrera & Shank, 2016).
Despite incomplete ddRADseq data, it still provides useful and reliable information for estimating phylogenetic relationships within or among deeply divergent genera, as demonstrated in this study. Although it is well known that downstream analyses would be challenged by missing data, caused by more allele dropouts among distantly related taxa (DaCosta & Sorenson, 2016; Zhou et al., 2022), the remaining data are still informative enough to construct a robust phylogeny with most nodes well supported (Figure 4), and the topology is congruent with the phylogeny retrieved from Sanger sequences (Figure 2). This is consistent with previous analyses in that the inclusion of missing sites within their datasets is more beneficial in providing more phylogenetic signal (Eaton et al., 2017; Hodel et al., 2017; Huang & Knowles, 2016; Tripp et al., 2017), although still not without repercussions of a missing data artefact (Dell'Ampio et al., 2014; Smith et al., 2020).
At present, the cost of RADseq related techniques continues to decline (Herry et al., 2023; Lowry et al., 2017) and the techniques do not require the taxon-specific probe sets as in the acquisition of ultraconserved elements (UCEs) (McCormack et al., 2012) or loci from the anchored hybrid enrichment method (Lemmon et al., 2012). Moreover, compared to Sanger sequencing, data retrieved from next-generation sequencing is significantly cheaper per data generated ($500/Mb vs. $0.50/Mb; Canadian Agency for Drugs and Technologies in Health, 2014), and the costs of ddRADseq in particular are at least five times less than other related RADseq techniques (Peterson et al., 2012; Toonen et al., 2013). Therefore, the RADseq-related techniques, especially ddRADseq, may become a more promising alternative to Sanger sequencing for constructing phylogenies of more deeply divergent taxa.
ACKNOWLEDGEMENTS
The authors are grateful to all members of the Animal Systematics Research Unit Chulalongkorn University, for their kind help during field trips. Special thanks go to Chaowalit Songsangchote and Minh Hoang for assistance in specimen collection, and Jennifer Lauschke for help with laboratory work. We also thank Thor-Seng Liew and Nicole B. Webster for their valuable comments that greatly improved the manuscript. This research project is supported by the Second Century Fund (C2F), Chulalongkorn University to PJ and CS. This work and the stay of PJ at the Leibniz Institute for the Analysis of Biodiversity Change, Hamburg, Germany, were funded by the Georg Forster Research Fellowship, Alexander von Humboldt Foundation.
CONFLICT OF INTEREST STATEMENT
None of the authors have a conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supporting Information of this article.




