Tempo and mode of diversification of the red devil spiders (Araneae: Dysderidae) of the Canary Islands
Abstract
The study of adaptive radiations has shed light on our current understanding of evolution. However, previous studies examining the mode in which species diversified, how diversification rates varied, and how ecological specialisation affected these processes have found few different results across different taxa and geographic and ecological systems, showing how complex this process is. To gain a more complete picture of how species evolve, additional model systems that encompass alternative ecological requirements are needed. Here, we present the results of a study aimed at unravelling the diversification mode and evolutionary drivers of the spider genus Dysdera, the red devil spiders, endemic to the Canary Islands. These species exhibit remarkable phenotypic variability in their mouthparts, which has been related to different levels of specialisation in the predation of isopods. We explored patterns of lineage diversification and assessed the role of trophic specialisation as a driver of species diversification. Additionally, we used climatic variables, occurrence data and morphological information to unravel the underlying mode of speciation by means of joint species distribution models and age-range correlation methods. Our results reveal that red devil spiders underwent an early burst of diversification, followed by a slowdown of diversification rates, which is a hallmark of adaptive radiation. We also found evidence that the trophic morphology shaped diversification, with specialist species exhibiting higher rates of diversification. Finally, our analyses suggest that speciation occurred mostly in allopatry, with subsequent secondary sympatry following range expansion.
1 INTRODUCTION
Evolutionary radiations, or periods of accelerated species diversification, have been a prominent feature of life's history on Earth (Linder & Bouchenak-Khelladi, 2017). Among these, adaptive radiations—the diversification of ecologically and phenotypically distinct species from a common ancestor (Futuyma, 1986; Schluter, 2000)—have garnered significant attention due to their importance in shaping the planet's biodiversity (Glor, 2010; Harmon et al., 2010). Not surprisingly, the study of one of these radiations, the Darwin finches in the Galapagos Islands, was seminal for the development of the theory of evolution by natural selection (Darwin, 1859). Indeed, the study of these events offers a unique opportunity to explore the mechanisms of species evolution and the complex relationships among extant species diversity (Givnish & Sytsma, 1997; Losos & Mahler, 2010). While adaptive radiations are observed in various environments, young and isolated geographic regions such as volcanic islands or glacial lakes offer ideal conditions for studying these phenomena (Schluter, 2000) and have been instrumental in advancing our understanding of speciation and other evolutionary processes (Gillespie et al., 2020). As a result, many of the most well-known cases of adaptive radiation have occurred in isolated regions (Cerca et al., 2023; Harmon et al., 2010; Patton et al., 2021), including Tethragnatha spiders in the Hawaiian Islands (Gillespie, 2004), cichlid fishes in the African lakes (Takahashi & Koblmüller, 2011), the anoles lizards in the Caribbean (Losos, 2009) and the aforementioned Darwin's finches from the Galapagos (Lack, 1947), to mention just a few. Hypotheses on what triggers these adaptive radiations have been mainly focused on ecological opportunity, that is, the availability of new ecological resources not previously accessible (see Stroud & Losos, 2016 for a review). Such ecological opportunities may emerge under different circumstances (Simpson, 1953), but the colonisation of oceanic islands, which are usually more species-depauperate, has provided clearer examples (Gillespie et al., 2020; Stroud & Losos, 2016). In this context, specific traits that allow linages to enter new ‘adaptive zones’ (Simpson, 1944) and promote ecological specialisation (Heard & Hauser, 1995), the so-called ‘key innovations’, have also been a central element in the discussion of adaptive radiations, as they have been linked to increased species diversification rates (Losos & Mahler, 2010; Mayr, 1963; Simpson, 1944; Wellborn & Langerhans, 2015).
Despite the importance of adaptive radiations in shaping Earth's biodiversity, our understanding of the detailed underlying mechanisms remains incomplete. While the evolution of ecologically and phenotypically distinct species from a common ancestor is the signature of adaptive radiations (Futuyma, 1986), few other common processes have emerged from studies of these patterns. For instance, exploring associations between lineage and phenotypic diversification has produced mixed results when studying continental adaptive radiations, with asymmetric diversification between these two patterns (see Pincheira-Donoso et al., 2015), and the typical pattern of an early burst followed by a slowdown in linage diversification (Harmon et al., 2010; Phillimore & Price, 2008; Rabosky & Lovette, 2008), has lacked support in cases where secondary factors could have erased this typical signature (Slater et al., 2010). Such inconsistencies suggest that the dynamics of adaptive radiations are more complex than previously thought. Similarly, studies of the macroevolutionary consequences of ecological specialisation on species diversification have also yielded disparate results. Historically seen as an evolutionary dead-end, specialist species, or lineages, have been hypothesised to exhibit lower diversification rates than generalists, evolving from them but not in reverse (Day et al., 2016; Schluter, 2000). However, several studies have shown that both irreversibility and lower diversification rates assumed for specialist species are far from being a general pattern (Nosil, 2002; Nosil & Mooers, 2005).
The actual drivers of speciation are another potential source of conflict among different adaptive radiation processes. Historically, the widespread view was that speciation was mainly triggered by geographic isolation (Mayr, 1963), that is, obliteration of gene flow coupled with time. However, with the rise of molecular phylogenetics and the use of more sophisticated comparative methods, that allow for quantitative detection of post-speciation range shifts (e.g. age-range correlation methods), it has become evident that the actual mechanisms underlying geographic patterns of species diversification could be more complex. Indeed, several studies have found evidence of sympatric speciation and thus of the potential involvement of natural or sexual selection in the actual speciation process (Bolnick & Fitzpatrick, 2007). Comparative methods assessing the relationship between distribution overlapping and phylogenetic relatedness, are based on the assumption that, following the initial speciation stage, the geographical distribution of species becomes randomised due to their dispersal capabilities (Barraclough & Vogler, 2000). In cases where a species clade undergoes allopatric diversification, the overlap between their ranges would initially be zero. However, over time, as their ranges randomly change, the overlap increases, resulting in a positive correlation between range overlap and older stages in the species' phylogenetic relationships, relative to more recent cladogenetic events (Fitzpatrick & Turelli, 2006). However, it is important to note that these correlations can be misleading when species clades exhibit complex patterns of geographical speciation (Fitzpatrick & Turelli, 2006). For example, the randomisations in post-speciation range shifts may be compromised when species experience secondary contact after the original speciation process, as observed in numerous cases of island clade diversification (e.g. Macías-Hernández et al., 2013; Thorpe et al., 2010).
Given the intricate temporal, ecological and geographic variation in species diversification patterns and processes observed across different species groups, additional model systems that encompass alternative ecological requirements are needed to obtain a more complete understanding of how species emerge and evolve. The Canary Islands form a volcanic archipelago that includes seven major islands and several islets located 100 kilometres to the northwest of the African coast (Figure 1a). The islands are geochronologically arranged, with the oldest ones, Lanzarote and Fuerteventura (15 Mya and 23 Mya, respectively), lying at the easternmost side, and becoming progressively younger towards the western end (from east to west): Gran Canaria (subaerial age 15 Mya), Tenerife (12 Mya), La Gomera (11 Mya), La Palma (1.7 Mya) and El Hierro (1.1 Mya) (Van Den Bogaard, 2013). These islands have been home to a remarkable diversification in the red devil spiders of the genus Dysdera (Figure 1b), with approximately 60 endemic species recorded in the archipelago (Bellvert et al. in prep) out of a total of 300 currently described species (World Spider Catalog, 2024). These species exhibit remarkable phenotypic variation in their cheliceral morphology—the spiders' mouth parts, which has been linked to different levels of trophic specialisation (Řezáč et al., 2008, 2021; Toft & Macías-Hernández, 2017, 2021)—where different cheliceral morphotypes evolved independently several times during the diversification of the group in the islands (Bellvert et al., 2023). Prey preference analyses have shown that species with certain cheliceral types exhibit either a more generalist diet or a specialisation towards preying on isopods, and phylogenetic comparative analysis has identified nine distinct cheliceral morphotypes, where species with cheliceral types A, D and E were classified as generalists, while those with types B, C, F, G and I were identified as specialists (Bellvert et al., 2023). Because of the single colonisation event inferred for these species (but see Adrián-Serrano et al., 2020) and the eco-phenotypic differences observed among relatives, this genus has been suggested to be a case of adaptive radiation in the islands (Bellvert et al., 2023), but this hypothesis has never been formally tested and the exact evolutionary drivers of this diversification remain poorly understood.
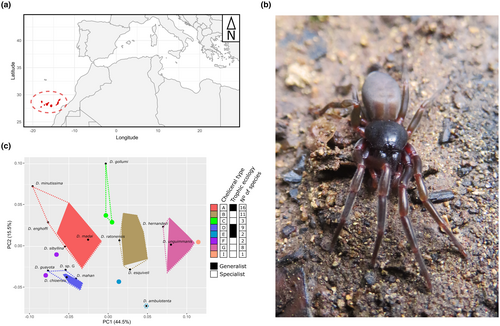
In the present study, we used phylogenetic comparative methods and joint species distribution modelling to elucidate the mechanisms underlying the diversification of Dysdera spiders in the Canary Islands and examine how it has been influenced by trophic specialisation. Our specific objectives were: (1) to test if the temporal pattern of lineage diversification of the group aligns with that expected under a scenario of adaptive radiation, that is an early rapid diversification of lineages with a subsequent deceleration (Glor, 2010); (2) to explore whether trophic specialisation played a role in this diversification; and (3) to investigate whether allopatry followed by secondary overlapping drove the diversification of these species, similarly to other cases of adaptive radiation in oceanic islands. We conducted a comprehensive study by integrating the fully resolved phylogeny of the group with phenotypic data, occurrence records and climatic variables. Our investigation aimed to understand the mode and tempo of the genus' evolution in the archipelago, as well as to identify the factors that potentially played a role in its remarkable diversification.
2 METHODS
2.1 Specimens and data collection
All specimens used came from field campaigns conducted by the authors and other colleagues and are stored at the Centre de Recursos de Biodiversitat Animal of the Universitat de Barcelona (CRBA) and Departamento de Zoología de la Universidad de La Laguna, Tenerife, Canary Islands collection (DZUL). Individuals were captured by active searching under rocks, logs and tree barks. The captured specimens were preserved in 95% EtOH and stored at −20°C at the former institutions. All specimens were collected following institutional and governmental regulations and the permits for all species captured were granted by the local authorities of each island or by the governing body of each natural reserve. Phenotypic data for the present study came from Bellvert et al. (2023). We used the statistical environment R (R Core Team, 2023) to conduct all subsequent analyses.
2.2 Time calibrated phylogeny
We inferred a time calibrated phylogenetic tree of Canarian endemic species and selected outgroups using mitogenomic data from Bellvert et al. (2023). We adjusted the taxon sampling and calibration points. We included only Dysderidae representatives, with the outgroup species Opopaea cornuta Yin & Wang, 1984 (Oonopidae) used to root the tree. We used a combination of 2 fossil calibrations and 5 biogeographic events to constrain nodes in the calibration information. The Baltic amber fossil Dasumiana emicans Wunderlich, 2004, has been classified as a member of the Harpacteinae (Bellvert et al., 2023). This information was used to establish a lognormal prior distribution for the Harpacteinae crown node, with an offset of 43 million years ago (Mya), which aligns with the earliest age of the Baltic amber (Magalhaes et al., 2020). Additionally, a hyperprior was set for the mean of the lognormal distribution, with a uniform distribution ranging between 0.01 and 40 Mya. The fossil Burmorchestina acuminata, identified as a member of the Onopidae subfamily Orchestininae, from Burmese amber was used to constrain the tree root. It was assigned a uniform prior ranging from 98.17 Mya, the presumed age of the Burmese amber and 164 Mya, the earliest evidence of a Synespermiata species, the major clade to which Dysderidae family belongs (Magalhaes et al., 2020). The biogeographic information included the Hercynian split of the Iberian plate into present-day major western Mediterranean islands, dated at 33–25 Mya (Rosenbaum et al., 2002; Schettino & Turco, 2006). The information was used to set a normal distribution prior to the node splitting the Iberian and the island species of the genus Parachtes Alicata, 1964, with a mean of 29 Mya and a standard deviation of 2.5 (Bidegaray-Batista & Arnedo, 2011). The remaining biogeographic constraints were provided by the estimated emergence of the younger Canarian Island in species with sister populations on more than one island. Specifically, the species Dysdera gomerensis Strand and D. silvatica Schmidt have sister lineages in La Gomera and El Hierro, respectively. The earliest subaerial age of El Hierro, the younger island, has been estimated at 1.2 Mya (Carracedo & Day, 2002). We defined a uniform prior ranging from 0.1 to 1.5 Mya to the two nodes splitting the former species pairs, to account for both earlier splits and later colonisation of the youngest island. Similarly, the species D. calderensis Wunderlich, 1987 and D. silvatica have sister lineages in La Gomera and La Palma. The earliest subaerial age of La Palma has been estimated at 2 Mya (Carracedo & Day, 2002), and we defined a uniform prior ranging from 0.1 to 2.5 Mya to both nodes.
We inferred the time-stamped calibrated phylogeny under a Bayesian uncorrelated relaxed molecular clock approach as implemented in BEAST v2.7.6 (Bouckaert et al., 2019). The concatenated data matrix was partitioned by gene, and the best evolutionary model for each gene partition was selected with PartitionFinder 2 (Lanfear et al., 2017). Individual log-normal clocks were defined for each gene, and the tree prior was set to the Birth-Death model.
We run three independent chains under selected priors for 100 million generations, sampling every 10,000 generations. Convergence among runs and correct mixing of the chains was monitored with TRACER v.1.7.1 (http://tree.bio.ed.ac.uk/software/tracer/). The burn-in was removed (10%) and the runs were combined with the help of the BEAST accompanying programs LOGCOMBINER and TREEANNOTATOR.
2.3 Species linage diversification
To investigate the diversification mode in the Dysdera spiders from the Canary Islands, we used the time-calibrated phylogeny, following the removal of non-Canarian species and the endemic D. lancerotensis, as it has been shown to correspond to an independent colonisation not directly related to the rest of the Canarian species (Adrián-Serrano et al., 2020). To obtain a first view of how diversification proceeded across the history of the group, we extracted the branching times with the function branching. times from the R package ‘ape’ (Paradis & Schliep, 2019) and fitted different variable-rate and constant-rate models to this data using the function fitdAICrc from the R package ‘laser’ (Rabosky & Schliep, 2013) considering 100 shift points and 6 different models: pure birth, birth-death, an exponential and a logistic variant of density-dependent and speciation rate models, pure birth with a single rate shift and pure birth with two rate shifts. To visualise the number of lineages through time and obtain a summary of diversification rates we used a linage-through-time plot produced with the function ltt from the R package ‘phytools’ (Revell, 2012). To consider phylogenetic uncertainty we repeated these analyses over 1000 trees obtained from the posterior distribution from the study of Bellvert et al. (2023). As mentioned before, previous work has delimited, based on geometric morphometric methods, several cheliceral morphotypes that have evolved independently several times during the diversification of these species in the archipelago (Bellvert et al., 2023), and which are hereby hypothesised to have potentially driven species diversification, specifically through their effect on the species trophic specialisation, where certain cheliceral morphologies are associated with generalist predators (cheliceral types A, D and E), while other morphologies exhibit varying degrees of specialisation in preying on isopods (cheliceral types B, C, F, G and I). However, not all the Dysdera diversity from the Canary Islands was represented in the aforementioned study, which only included 40 out of a total of 57 currently recognised species (Bellvert et al., 2023). Such undersampling could lead to a bias when analysing evolutionary rates (Pybus & Harvey, 2000) that could compromise the final results of the study. Some of the unrepresented species were due to lack of any specimen to obtain morphometric data; and had to be excluded from the study. However, other species were previously excluded due to an insufficient number of samples for statistical analysis or because they belonged to different ecological regimes (see Bellvert et al., 2023). To mitigate the effect of the undersampling on the diversification analyses conducted here, we used a Linear Discriminant Analysis (LDA) to classify the species with unknown cheliceral affiliation that were not examined in the previous study, but for which morphometric data were available, into the already established cheliceral morphotypes. We used the function prep.lda from the R package ‘RRPP’ (Collyer & Adams, 2018, 2021) to prepare the geometric morphometric data used in Bellvert et al. (2023) study and the function lda from the R package ‘MASS’ (Venables & Ripley, 2002) to run the discriminant analysis. For subsequent analyses, we pruned the complete phylogeny of the Dysdera spiders from the Canary Islands, including the species with cheliceral type information from Bellvert et al. (2023) and those that we could recover from the discriminant analyses. We used the function drop. tip from the R package ‘ape’ (Paradis & Schliep, 2019) to remove all specimens without cheliceral type information from the phylogeny.
2.4 State-dependent diversification models
To test if tropic specialisation as represented by different cheliceral morphotypes increased or decreased the rate at which Dysdera species diversified, we fitted a BiSSE model where diversification and extinction rates varied freely between two distinct states of trophic strategy using the function make.bisse from the R package ‘diversitree’ (Fitzjohn, 2012). Binary states were obtained from Bellvert et al. (2023) which link the different cheliceral morphologies to generalist or specialist trophic strategies. We compared this model to a null model with equal diversification and extinction rates by extracting the loglikelihood of each model through the function find.mle and comparing both with the function anova. Furthermore, to test and visualise differences in diversification rates between trophic strategies, we used Bayesian inference to calculate the posterior distribution of our parameters with the function mcmc from the R package ‘coda’ (Plummer et al., 2006).
However, it could be the case that cheliceral morphotype by itself, capturing the evolutionary influences of pressures other than or in addition to trophic strategy, could have affected diversification rates. To test if the evolution of different cheliceral morphotypes, rather than their trophic strategy, has an impact on the diversification rates in the Dysdera species, an optimal approach would be to use a multi-state speciation and extinction model like MuSSE (Fitzjohn, 2012). Unfortunately, in our data, some cheliceral morphotypes were represented only by one or two species, and the resulting MuSSE model would probably not be trustworthy. Instead, to investigate which cheliceral type, or combination of cheliceral types, may have had the greatest influence on Dysdera diversification, we ran independent BiSSE models for all possible combinations of the eight cheliceral morphotypes, dividing them into two distinct groups. From these models, we calculated the percentage of times that each cheliceral type appeared in the pairwise cheliceral combination that was inferred to exhibit significantly different diversification rates when compared to a null model with equal rates between states. We represented the percentage of times that each cheliceral morphotype appeared in significant pairwise combinations with significantly different diversification rates using a spider chart with the function radarchart from the R package ‘fmsb’ (Nakazawa, 2023). Finally, to examine which cheliceral combination maximised change in diversification rates, we ran a BiSSE model delimiting the binary state with the cheliceral types that showed a higher percentage of occurrence in the previous combinations. We compared a model with different diversification and extinction rates with a null model with equal rates, with the binary state representing each of the three cheliceral types with the highest percentages of occurrence on the one hand and the rest of the types on the other. Next, we also examined a model with the binary state defined as the five cheliceral types with the highest percentages and the two cheliceral types that have not appeared a single time in these different combinations that significantly increase diversification rates (see results).
Note that a trait–state-dependent model with different diversification rates that performs better than a null one, does not necessarily mean that the examined trait is the main driver of the diversification of the group, as an unmeasured factor could have a stronger impact on the species' diversification (Beaulieu & O'Meara, 2016). To account for this possibility, for all previous models with the binary state delimited based on the cheliceral percentages in which the different-rates model performed better than the null, we fit three additional ‘hidden rate’ models (Beaulieu & O'Meara, 2016): one in which there is an influence on diversification rates of an unaccounted trait but not of the cheliceral type (CID-2), a four-level hidden rate model (CID-4), which will allow testing for more complex diversification processes (Revell & Harmon, 2022), and a full model were both the hidden state and our cheliceral trait affect the Canarian Dysdera spider diversification. We used the hisse function from the R package ‘hisse’ (Beaulieu & O'Meara, 2016) to fit these models. We calculated the AIC of each model with the AIC function to test which performed best.
2.5 Time-dependent diversification models
We tested if diversification rates varied through time in the radiation of the Dysdera spiders from the Canary Islands. We used the function make.bd.t from the R package ‘diversitree’ (Fitzjohn, 2012) to fit a model where diversification rates were an arbitrary function of time. We compared the time-dependent model to a null with a constant diversification rate fitting our data with the function make.bd. We obtained the maximum likelihood of each model with the function find.mle and compared them with the anova function. We also used the mcmc function to run a Bayesian MCMC analysis for our time-dependent model and plot the posterior distribution through time of speciation (λ) to see the general tendency that this parameter has.
2.6 Species overlap and age-range correlation
Finally, to unravel the main speciation pattern of these species in the islands—that is allopatric versus sympatric—we analysed how the overlap between species distribution ranges changed over time. We used age-range correlation methods (ARC), which seek to establish statistical relationships between species-pairs geographic overlap and the time since their divergence (Fitzpatrick & Turelli, 2006). For the pairwise measurements of the niche overlap between species, we used joint species distribution modelling (jSDM) to obtain the residual patterns of occurrence between species, as they capture more complex interactions than other co-occurrence metrics could show (Pollock et al., 2014). Those residuals are features that influence the occurrence between species that are not explained by environmental variables. We decided to use these residuals, instead of the species-shared environmental responses, because they explain better the difference between allopatric and sympatric species pairs (see results). We used all the species for which we had sufficient morphometric data from Bellvert et al. (2023) (N = 40), and we considered the first two principal components of each species as the trait value for the model to also test for competitive exclusion in the biotic interactions captured by the residual correlations. For the species' occurrence, we built a presence matrix for each species for each locality where Dysdera spiders have been collected in the Canary Islands during the last 50 years. Finally, for the model explanatory variables, we selected the three different climatic predictors from Patiño et al. (2023) that we hypothesise may have a greater impact on the species' potential distribution, namely the highest temperature of any month, to include the potential effect of aridity; the accumulated precipitation over 1 year, to take into account the effect of the more humid areas; and elevation, which has an influence on temperature but also on the vegetation present in the island. We ran the model with the function jSDM_binomial_probit from the ‘jSDM’ R package (Clément & Vieilledent, 2022), with 10,000 iterations with a burn-in of 5000 and a thinning rate of 20. We extracted the latent variables of the model and the correlation of the response matrix with the get_residual_cor and get_enviro_cor functions respectively. For the ARC analysis we used the function age.range.correlation from the R package ‘phyloclim’ (Heibl & Calange, 2018). An observed negative slope to more distant relationships would point to a sympatric diversification, while a positive one would be indicative of an allopatric diversification (Fitzpatrick & Turelli, 2006). We ran the model with 999 iterations to test for statistical significance.
3 RESULTS
3.1 Best macro-evolutionary candidate model
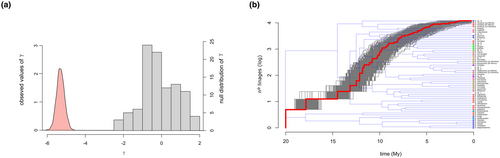
The gamma statistic exhibited a negative value (−5.35 ± 0.18), being significantly smaller than the expected under a birth-death process which would have a normal distribution around 0 (Figure 2a), which suggests rejection of the hypothesis of constant rate in the diversification of the group in favour of decreasing diversification through time (Pybus & Harvey, 2000). Accordingly, the LTT plot showed a clear hump-shaped increase in the number of lineages with a posterior stabilisation (Figure 2b). Finally, when contrasting different candidate diversification models, the density-dependent speciation model (DDL) showed the best performance with an AIC of −37.76 ± 5.06, followed by the pure-birth model with two rate shifts (yule 3 rates, AIC = −33.73 ± 5.41), the pure-birth with one shift (yule 2 rates, AIC = −33.65 ± 5.79), the exponential variant of the density-dependent speciation model (DDX, AIC = −18.89 ± 4.50), the pure-birth model (AIC = −6.62 ± 4.59) and the birth-death model (AIC = −4.62 ± 4.59).
3.2 BiSSE state-dependent models
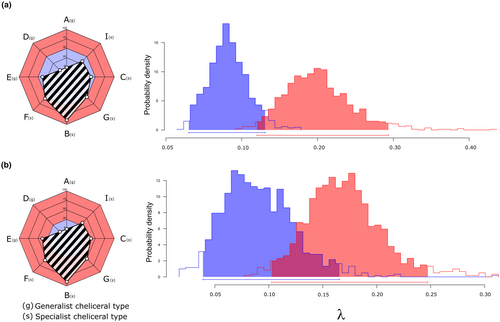
The results from the discriminant analysis and the cheliceral type adscription can be found in the supplementary material and Table S1. The BiSSE model with trophic strategy as the binary state (specialist vs. generalist) with free diversification rates (fr), performed poorly compared to the null model with equal rates between states (null), without significant differences between them (AICfr = 364.05, AICnull = 362.81, p = .25). However, the Bayesian MCMC analysis disagreed with the previous one and showed that the diversification rate of specialist species is higher than that of generalist ones (p = .046). When examining all pairwise combinations of binary states among cheliceral types, we found that for 17 out of 254 possible pairwise combinations, the model with free diversification rates performed better as compared to the null model with equal rates between states (Table S2 of the supplementary material). From all these 17 different cheliceral combinations, type B (specialist) was among the cheliceral types that exhibited a higher diversification rate in 89% of the cases, type F (specialist) in 55% of the cases, type G (specialist) in 50% of the cases, type C (specialist) and E (generalist) in a 39%, type I (specialist) in 33% of the cases, while types A and D (both generalists) were never part of the higher diversification rate sets. When creating groups of several cheliceral types to generate the binary state trait for rate comparisons depending on those percentages, we found that, when comparing the cheliceral types that appeared at least 50% of the time in the cheliceral combinations that showed an increase in diversification rates against the other ones (i.e. B, F and G vs. C, E, I, A and D), the BiSSE model with free transition rates exhibited better performance than the null (AICfr = 366.23 and AICnull = 368.23, p-value = .049), with the Bayesian MCMC analyses showing that stateBFG have higher diversification rates compared to stateICFAD (Figure 3a, p-value = .014). However, when combining the cheliceral types that appeared less than 50% of the time in the previous combinations (stateBFGCE), against the cheliceral types that do not appear a single time in the state with an increase in diversification rates (stateAD), the model with free transition rates does not perform better than the null model (AICfr = 373.82 and AICnull = 371.28, p-value = .48), with the Bayesian MCMC analyses not showing differences in the diversification rates between stateBFGCE and stateAD (Figure 3b, p-value = .066).
3.3 Hidden state results
When testing for a hidden state character responsible for differences in diversification rates among Canarian Dysdera species, we found that models accounting for one or more hidden characters, or a combination of a hidden character and the cheliceral morphotypes, performed poorly compared to the model focusing solely on differences in diversification rates based on cheliceral types (stateBFG vs. stateCEIAD, BISSE AIC = 366.22, CID AIC = 368.23, HISSE CID-2 AIC = 373.56, HISSE CID-4 AIC = 377.39, HISSE+BISSE AIC = 391.99).
3.4 Effect of time on species diversification
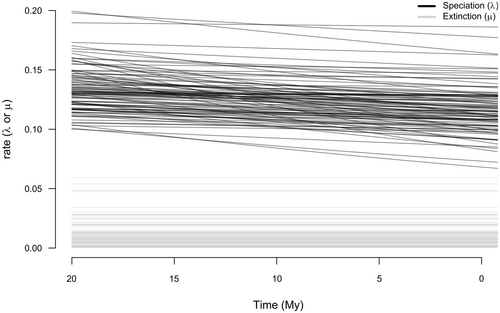
When investigating temporal variation in diversification rates, we found that the time-dependent model performed better than the null with no variation in diversification rates (AIC = −21.23 and AIC = 22.12 respectively, p-value <<.01). When sampling the posterior distribution using Bayesian inference, we observed a slight decrease in the diversification rate of the group through time (Figure 4, lambda. a = 0.0069 ± 0.0073).
3.5 Age-range correlation
jSDM showed varying correlations between different species pairs, both in terms of their correlation with the environmental variables and their residual correlations. However, upon examining species pairs that occur on the same island, we found that they generally exhibited positive correlations with both residuals and environmental factors. Conversely, for species pairs that occur on different islands, the correlation with the residuals tends to be neutral but with a bigger disparity in the environmental correlations, with both positive and negative values (Figure 5a). We opted to utilise residual correlations for conducting the ARC analyses, as they provide better discrimination between species pairs with distributions on the same or different islands. It is important to note that having a distribution on the same island does not necessarily imply a sympatric distribution, as the species may occupy different regions within that island. However, the residual correlations offer the clearest representation of what an allopatric distribution could be (i.e. two species on separate islands), and because of that, we believe they will contribute more significantly to the ongoing analysis.
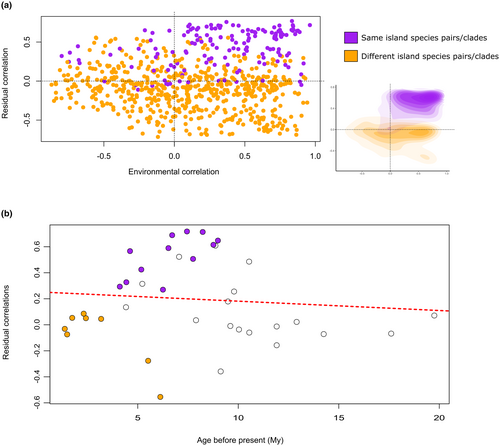
The ARC analysis revealed a decrease in the overlap since species divergence (intercept = 0.253; slope = −0.007), indicative of sympatric speciation with a subsequent loss of contact between species. However, this result was not statistically significant with a p-value of .366, showing no significant association between range overlap and time since species divergence. It should be noted, however, that these values were obtained by fitting the results in a linear model, but our ARC plot clearly exhibited a hump-shaped pattern, with low overlap values in the early splits, a subsequent increase in overlapping followed by a decrease towards the present (Figure 5b).
4 DISCUSSION
In this study, we have focused on understanding how species diversification varies across time and space, and how it is influenced by ecophenotypic traits and trophic specialisation. Moreover, we have provided evidence that the diversification of Dysdera spiders in the Canary Islands, where previous studies have highlighted eco-phenotypic variation (Bellvert et al., 2023), constitutes a case of adaptive radiation. Evidence derived from modelling speciation and extinction lends support to the hypothesis that trophic specialisation and associated morphological evolution shaped the diversification of these species in this archipelago, highlighting the importance of ecological innovation in driving species' diversification. Finally, we have found evidence that the speciation of the Dysdera species in the Canary Islands had been mainly driven by geographic isolation with a posterior secondary contact.
4.1 The diversification mode of Canarian Dysdera spiders
An early increase in species diversification, followed by a decrease in net speciation rates towards the present is the trademark of an adaptive radiation process (Glor, 2010; Losos & Mahler, 2010; Phillimore & Price, 2008). Declining diversification rates through time are generally interpreted as the results of early occupation of vacant niches until their saturation, which leads to the slowdown of cladogenetic events (Gavrilets & Vose, 2005; Schluter, 2000). Such a pattern has been usually described by means of linage-through-time plots (Nee et al., 1992; Pybus & Harvey, 2000), with density-dependent models describing this asymptotic accumulation of lineages through time (e.g. Linder & Bouchenak-Khelladi, 2017; Pincheira-Donoso et al., 2015). The early increase in a number of lineages and the corresponding negative γ values revealed for the Dysdera species from the Canary Islands points towards a rapid filling of ecological niches and a deceleration of speciation rates over time, as also seen with the time-dependent model of diversification. Interestingly, the Canary Islands may have also presented additional episodes of ecological opportunity as new islands emerged over time, which can be reflected in diversification pulses in the LTT plot (Pincheira-Donoso et al., 2015). Such pulses can be identified in Figure 2b. Multirate variants of the pure birth model lend further support to this observation, as they exhibited overlapping AIC values when accounting for the phylogenetic uncertainty, however, their mean values have been slightly higher than the density-dependent model. Obviously, undiscovered species could compromise these results, as incompleteness in the taxon sampling could mirror a similar pattern as the density-dependent speciation (Rabosky & Lovette, 2008). However, given the solid taxonomic ground established by a series of modern taxonomic treatments of the group (e.g. Arnedo et al., 2000; Arnedo & Ribera, 1996, 1997, 1999; Bellvert et al., 2023; Macías-Hernández et al., 2010), we doubt that the number of undiscovered species may actually affect our main conclusions.
4.2 Evolutionary consequences of ecological specialisation
The ecological and evolutionary consequences of species specialisation have been examined repeatedly over the years. Current consensus would claim that the evolution of traits that reduce the breadth of species trophic or ecological niches negatively affects their diversification rates (Vamosi et al., 2014), resulting in the so-called evolutionary dead-ends (Cope, 1896). However, this cannot be taken as a general rule, as many studies have found mixed support for it (Day et al., 2016), or directly contradicted the link between specialisation and evolutionary dead-ends (Zenil-Ferguson et al., 2022). Our results reveal that, globally, specialist and generalist Dysdera species exhibit similar diversification rates. Nevertheless, pairwise rate comparisons of different cheliceral morphotypes and their combination into rate-coherent groups identified specific sets of morphotypes that exhibit significantly higher diversification rates (Figure 3a,b). Interestingly, although trophic specialisation does not seem to be directly associated with an increase in diversification rates in our studied species, we found a significant increase when combining cheliceral morphotypes that have been previously linked to specialist trophic strategies (Bellvert et al., 2023). The combination of phylogenetically unrelated species with cheliceral morphotypes B, G, and F appears to exhibit elevated species diversification rates. In contrast, other cheliceral morphotypes, including some associated with a specialist diet (such as C and I), do not display this acceleration in diversification. However, the rare evolution of cheliceral type F across the Canarian Dysdera diversification, where this specific morphotype has only appeared in two independent occasions, makes its contribution to the inferred diversification dynamics questionable. Indeed, the increase in diversification rates attributed to cheliceral type F could be an artefact due to the close relatedness of these species to those with cheliceral types B and G. In fact, in continental Dysdera this cheliceral type (F) has also evolved multiple times across different species groups and geographic regions, presumably independently. Whether the diversification of cheliceral morphotypes has followed coherent evolutionary pathways in Canarian and continental Dysdera is an intriguing avenue for future inquiry. Here, this morphotype is generally carried by single species with no close relatives exhibiting the same feature, which also reinforces the idea that most likely this cheliceral morphotype is not a key innovation responsible for the increase of diversification rates in Dysdera species.
In a previous study, we found transitions from generalist cheliceral morphotypes to specialist ones to be irreversible in Canarian Dysdera (Bellvert et al., 2023), supporting the hypothesis that prey specialisation is an evolutionary dead-end (Day et al., 2016). However, the high diversification rates found with the specialist cheliceral morphotypes B and G, do not support the idea of an evolutionary dead-end, suggesting instead that trophic specialisation in Dysdera species may constitute a key innovation rather than a limiting factor in their diversification. On the other hand, the cheliceral morphotypes C and I, and most likely also the cheliceral type F, would better fit into the definition of a dead-end, being an irreversible character that has evolved from a generalist state and that is also accompanied by a decrease in species diversification rates (Figure 3c). Put together, these observations suggest that the evolutionary dead-ends in Dysdera spiders would not be so dependent on trophic specialisation per se, but rather related to specific morphotypes.
A point of relevance here is that, for complex diversification processes, BiSSE models are known to frequently correlate neutral traits with higher diversification rates (Beaulieu & O'Meara, 2016; Rabosky & Goldberg, 2015). In spiders, it has already been observed that, although ecologically important, some traits may not be the primary driving force behind species diversification (Fernández et al., 2018). However, our analyses provide strong evidence of the relevance of morphotype differentiation for species diversification: when we used HiSSE to test whether differences in diversification rates might be explained by other unaccounted factors, we found that BiSSE models performed better than the CID-2 or CID-4 models. This indicates that cheliceral morphology indeed plays a strong role in the evolutionary history of these species.
4.3 Secondary contact following allopatric speciation
The controversy of how generalised allopatric modes of speciation are compared to sympatric ones, especially in the context of adaptive radiations, is still a hot topic nowadays (see Bolnick & Fitzpatrick, 2007). One of the major concerns about deciphering speciation with extant sister taxa has been the range shifts experienced by species that could blur present-day patterns (Losos & Glor, 2003), which were confronted by methods that account for post-speciation range shifts like ARC methods. The ARC analysis performed with the Dysdera spider species from the Canary Islands showed a non-significant negative correlation, related to a sympatric mode of speciation. However, the relationship between age and the residual correlations has been shown to be non-linear (Figure 5b). Previous scepticism had already been raised in the past about these methods when analysing complex patterns of geographical modes of speciation (Fitzpatrick & Turelli, 2006). Sympatric patterns are generally explained not as the result of selection-driven speciation, but as secondary contact following population expansion once the overlapping lineages fully diverged genetically (Hudson et al., 2011). Our results of the ARC analysis would better fit under an allopatric speciation followed by a secondary sympatry, making the correlation presented in this test misleading if a more complex pattern that the Dysdera species may have been involved. The posterior decline in the species overlap observed could be a response to the increase of extinction rates expected over time under the general dynamic model of oceanic island biogeography (Borregaard et al., 2017). This pattern of allopatric speciation aligns with what would be expected in a volcanic archipelago, where intra-island geographical isolation is likely less significant for these species compared to isolation resulting from colonising two separate islands (Mittelbach & Schemske, 2015). This would match with the observed in our ARC analysis, where cladogenetic events within species pairs in different islands are more recent than the ones with species in the same island (Figure 5b). For this reason, we argue that the colonisation of different islands has been the main, or one of the main, speciation force(s) during the diversification of the Dysdera species in the Canary Islands.
5 CONCLUSIONS
The diversification of the Dysdera species in the Canary Islands has been previously suggested to be a case of adaptive radiation. Our results unambiguously support this claim by recovering an early burst of species diversification with a posterior slowdown in their speciation, a pattern usually interpreted as the stamp of an adaptive radiation process. The integration of available morphometric data and SSE models provides evidence that the different cheliceral morphotypes exhibited by Canarian Dysdera played a role in shaping diversification dynamics in this group, probably by increasing rates due to multiple instances of trophic specialisation. The use of jSDM approaches provided more refined information on species associations than the use of climatic variables alone, and when used with ARC methods, enabled us to propose that inter-island allopatric speciation is the general pattern of diversification among these species, with sympatry being the results of secondary contact between them.
ACKNOWLEDGEMENTS
We want to thank Dr. Martina Pavlek for reviewing the original version of this work and for her constructive feedback, which has improved the quality of the manuscript. A.B. was funded by an individual PhD grant BES-2017-080538 from the Ministerio de Economía, Industria y Competitividad of the Spanish government. A.K. is supported by a Ramón y Cajal research grant co-funded by the Spanish State Research Agency and the European Social Fund (RYC2019-026688-I/AEI/10.13039/501100011033). This study was supported by project grants CGL2012-36863 and CGL2016-80651-P from the Spanish Ministry of Economy and Competitivity, PID2019-105794GB-I00 from the Spanish Ministry of Science and Innovation and 2017SGR83, 2021SGR689 from the Catalan Government (M.A.).
CONFLICT OF INTEREST STATEMENT
All authors of the present manuscript declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data and R scripts that support the findings of this study are openly available as supporting information and on Github at https://github.com/AdriaBellvert/Tempo-and-Diversification-Dysdera.




