An Approach to Controlling Inflammation and Coagulation in Pig-to-Baboon Cardiac Xenotransplantation
Funding: Financial support was provided by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) TRR 127 and — in part — by the Swiss National Science Foundation (CR-SII5_198577), the Bavarian Research Foundation (AZ-1543-22) and the Leducq Foundation (23CVD01).
ABSTRACT
Introduction
Inflammatory responses and coagulation disorders are a relevant challenge for successful cardiac xenotransplantation on its way to the clinic. To cope with this, an effective and clinically practicable anti-inflammatory and anti-coagulatory regimen is needed. The inflammatory and coagulatory response can be reduced by genetic engineering of the organ-source pigs. Furthermore, there are several therapeutic strategies to prevent or reduce inflammatory responses and coagulation disorders following xenotransplantation. However, it is still unclear, which combination of drugs should be used in the clinical setting.
To elucidate this, we present data from pig-to-baboon orthotopic cardiac xenotransplantation experiments using a combination of several anti-inflammatory drugs.
Methods
Genetically modified piglets (GGTA1-KO, hCD46/hTBM transgenic) were used for orthotopic cardiac xenotransplantation into captive-bred baboons (n = 14). All animals received an anti-inflammatory drug therapy including a C1 esterase inhibitor, an IL-6 receptor antagonist, a TNF-α inhibitor, and an IL-1 receptor antagonist. As an additive medication, acetylsalicylic acid and unfractionated heparin were administered. The immunosuppressive regimen was based on CD40/CD40L co-stimulation blockade. During the experiments, leukocyte counts, levels of C-reactive protein (CRP) as well as systemic cytokine and chemokine levels and coagulation parameters were assessed at multiple timepoints. Four animals were excluded from further data analyses due to porcine cytomegalovirus/porcine roseolovirus (PCMV/PRV) infections (n = 2) or technical failures (n = 2).
Results
Leukocyte counts showed a relevant perioperative decrease, CRP levels an increase. In the postoperative period, leukocyte counts remained consistently within normal ranges, CRP levels showed three further peaks after about 35, 50, and 80 postoperative days. Analyses of cytokines and chemokines revealed different patterns. Some cytokines, like IL-8, increased about 2-fold in the perioperative period, but then decreased to levels comparable to the preoperative values or even lower. Other cytokines, such as IL-12/IL-23, decreased in the perioperative period and stayed at these levels. Besides perioperative decreases, there were no relevant alterations observed in coagulation parameters. In summary, all parameters showed an unremarkable course with regard to inflammatory responses and coagulation disorders following cardiac xenotransplantation and thus showed the effectiveness of our approach.
Conclusion
Our preclinical experience with the anti-inflammatory drug therapy proved that controlling of inflammation and coagulation disorders in xenotransplantation is possible and well-practicable under the condition that transmission of pathogens, especially of PCMV/PRV to the recipient is prevented because PCMV/PRV also induces inflammation and coagulation disorders. Our anti-inflammatory regimen should also be applicable and effective in the clinical setting of cardiac xenotransplantation.
1 Introduction
Cardiac xenotransplantation has seen remarkable success in the last few years and is the most promising alternative to human cardiac allotransplantation [1-3]. This success was made possible by important findings in pig-to-baboon experiments [4-6], for example the need for a co-stimulation blockade of the CD40/CD40 ligand (CD40L) pathway [7, 8], continuous cold non-ischemic heart preservation [9, 10], the relevance of growth control [4, 11, 12] or the importance of donor animals free from porcine cytomegalovirus, a porcine roseolovirus (PCMV/PRV) [13] and other pathogens [1, 14].
Another relevant finding is the important role of inflammatory responses and coagulation disorders following xenotransplantation [15, 16]. Without anti-inflammatory approaches, there were relevant inflammatory responses to xenotransplantation which were considered to contribute significantly to the poor survivals reported in some cases [17, 18] after orthotopic cardiac xenotransplantation in non-human primate models [16].
One approach to cope with these inflammatory and coagulatory challenges is the genetic engineering of the organ-source pig, for example via the introduction of transgenes for human thrombomodulin (hTBM), human heme oxygenase-1, human A20, or human CD39 [1, 16]. Besides the genetic modification of the organ-source pig, there are several therapeutic strategies to prevent or reduce inflammatory responses following xenotransplantation, for example, corticosteroids [19, 20], white blood cell filtration [16], anti-complement agents like C1-inhibitors [15] or cobra venom factor [19], IL-6 receptor blockade agents [21, 22], anti-histone antibodies [22], TNF-α inhibitors [23], NF-κB-inhibitors [22], alpha 1-antitrypsin [15], platelet inhibitors [15], or triiodothyronine [15].
All these approaches showed some anti-inflammatory and anti-coagulatory effect in vitro or in vivo in several xenotransplantation studies. However, it is still unclear, which combination of anti-inflammatory and anti-coagulatory drugs should be used.
To elucidate this, we analyzed data from 14 pig-to-baboon orthotopic cardiac xenotransplantation experiments using 3-fold genetic-modified pigs and a combination of several anti-inflammatory drugs.
2 Material and Methods
2.1 Animals
Hearts from 14 genetically modified piglets at the age of 4 to 7 weeks were transplanted into male captive-bred baboons between April 2017 and October 2021. All piglets were from the same breeding cohort (German Landrace/Large White; blood group 0) and were homozygous for alpha1,3-galactosyltransferase knockout (GGTA1-KO) and hemizygous transgenic for human CD46 (hCD46) and hTBM (Revivicor, Blacksburg, USA and Institute of Molecular Animal Breeding and Biotechnology, Gene Center, LMU Munich, Germany). Fourteen baboons (Papio anubis and Papio hamadryas; blood group A [n = 5], B [n = 7] and AB [n = 2]; German Primate Centre DPZ, Göttingen, Germany) served as recipients. Expression of hCD46 and hTBM was verified post mortem by immunohistochemistry (data not shown). Twelve animals were tested negative, two were tested positive for PCMV/PRV, as published elsewhere [5] or unpublished data [24]. Besides final testing, piglets, littermates, and breeder animals were regularly screened for PCMV/PRV as recommended [25] at the Free University Berlin and the Paul-Ehrlich-Institut. The study was approved by the Government of Upper Bavaria. All animals were cared for and treated in accordance with the Guide for the Care and Use of Laboratory Animals (German Legislation for the Welfare of Laboratory Animals and US National Institutes of Health).
2.2 Anesthesia, Surgical Procedure, and Heart Preservation
After sedation, induction of anesthesia, and endotracheal intubation of the animals [26], surgery was conducted as published in detail elsewhere [4].
In brief, after median sternotomy of the donor animal, the aorta was cross-clamped and antegrade non-ischemic preservation commenced immediately; continuous perfusion with 8°C oxygenated, hyperoncotic solution containing albumin, hormones, nutrients, and erythrocytes [9, 10] was provided by an extracorporeal heart preservation system (University of Lund, Sweden) consisting of a pressure- and flow-controlled roller pump, an O2/CO2 exchanger, a leukocyte filter, and a cooler/heater unit. During storage, the heart was preserved the same way and the perfusion pressure kept at 20 mmHg.
After median sternotomy in the baboon recipient, extracorporeal circulation was installed and started. Explantation of the recipient's native heart and xenotransplantation followed the techniques of Lower and Shumway [27]. The donor heart was intermittently perfused for 2 min every 15 min during implantation.
2.3 Immunosuppression
Immunosuppressive therapy was based on CD40/CD40L co-stimulation blockade [7], as described in detail elsewhere [4]. For induction therapy, all animals received B cell depleting anti-CD20 ab (Mabthera; 19 mg/kg body weight [bw], days −7, 0, 7 and 14; Roche Pharma, Basel, Switzerland), anti-thymocyte globulin (ATG, thymoglobulin; 5 mg/kg bw, days −2 and −1; Sanofi, Paris, France), and either a therapy with a mouse/rhesus chimeric anti-CD40 IgG4 monoclonal antibody (anti-CD40 Mab; 50 mg/kg bw; mouse/rhesus chimeric IgG4 clone 2C10R4, NIH Non-human Primate Reagent Resource; Mass Biologicals, Boston USA; courtesy of K. Reimann) (n = 8) or a combination therapy with anti-CD40 Mab (50 mg/kg bw) and a humanized PASylated anti-CD40L antigen-binding fragment (anti-CD40L PAS-Fab [28]; 20 mg/kg bw; XL-protein, Freising, Germany) (n = 6).
Immunosuppression was maintained with mycophenolate mofetil (CellCept; 40 mg/kg bw, daily, started on day −2; Roche Pharma, Basel, Switzerland), methylprednisolone (urbasone soluble; 10 mg/kg bw, daily, tapered down; Sanofi, Paris, France), and either anti-CD40 Mab (50 mg/kg bw once weekly) or the combination therapy of anti-CD40 Mab (50 mg/kg bw once weekly, reduced to 30 mg/kg bw after 1 month) and anti-CD40L PAS-Fab (20 mg/kg bw every 4 days, reduced to 10 mg/kg bw after 2 months).
All animals received a therapeutic regime to slow xenograft overgrowth, which was described in detail elsewhere [4, 5]. Methylprednisolone was tapered down quickly and additional antihypertensive drugs (enalapril [Enahexal; Hexal, Holzkirchen, Germany] and metoprolol tartrate [Beloc; AstraZeneca, Cambridge, United Kingdom]) as well as the mTOR inhibitor temsirolimus (Torisel; Pfizer, New York, USA) were added.
2.4 Anti-Inflammatory and Additive Medication
All animals received an anti-inflammatory therapy including an C1 esterase inhibitor (Berinert; CSL Behring, King of Prussia, USA), an IL-6 receptor antagonist (RoActemra; Roche Pharma, Basel, Switzerland), a TNF-α inhibitor (Enbrel; Pfizer, New York, USA) and an IL-1 receptor antagonist (Kineret; Swedish Orphan Biovitrum, Solna, Sweden).
The C1 esterase inhibitor was administered at a dosage of 17.5 U/kg bw as an intravenous short infusion at the day of xenotransplantation and at postoperative day (POD) 1, 7, and 14. Also as an intravenous short infusion, 8 mg/kg bw of the IL-6 receptor antagonist were given at the day of xenotransplantation followed by monthly applications from POD 7 on. The TNF-α inhibitor was administered weekly starting from POD 5 as a subcutaneous bolus at a dosage of 0.7 mg/kg bw. The IL1-receptor antagonist was given as a subcutaneous or intravenous bolus at a dosage of 1.3 mg/kg bw every day beginning from the day of xenotransplantation (for an overview of the application intervals of the anti-inflammatory medication see also Figure 1).
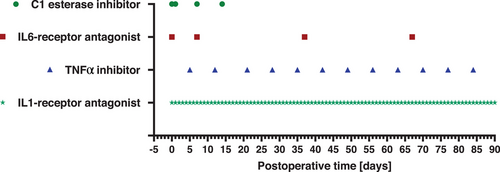
The additive medication consisted of acetylsalicylic acid (Aspirin; Bayer, Leverkusen, Germany) and unfractionated heparin (Heparin-Natrium-25000-ratiopharm; Ratiopharm, Ulm, Germany). Acetylsalicylic acid was administered daily beginning at POD 3 at a dosage of 40 mg intravenous or 100 mg per os. Heparin was given as a continuous intravenous infusion beginning in the first postoperative week at a dosage between 1000 and 5000 I.E./day. Furthermore, ganciclovir (Cymevene, Roche Pharma, Basel, Switzerland), cefuroxime (Cefuroxim; Hikma Pharmaceuticals, London, United Kingdom), and epoetin beta (NeoRecormon 5000; Roche Pharma, Basel, Switzerland) were administered.
2.5 Laboratory Tests
Blood samples were taken from baboon recipients prior to xenotransplantation, regularly during each experiment and before euthanasia. Leukocyte counts, C-reactive protein (CRP) levels, platelet count, fibrinogen levels, and prothrombin time were measured by the Institute of Laboratory Medicine (University Hospital, LMU Munich, Munich, Germany).
2.6 Cytokine Secretion Profile
Systemic cytokine and chemokine levels (IL-8, IL-10, Eotaxin, MCP-1, MIF, IL-12/Il-23, MDC, RANTES, INF-γ and IL-6) were quantified on sera of the animals before transplantation and at several timepoints after transplantation. For cytokine and chemokine detection, an Invitrogen ProcartaPlex NHP Cytokine and Chemokine Panel, 29plex-assay (ThermoFisher Scientific Inc., Waltham, USA) and a Luminex 100/200 analyzer (Luminex Corporation, Austin, USA) were used. Standards and samples were prepared according to the manufacturer's instructions and cytokine concentrations were calculated by Xponent software version 3.1 (Luminex Corporation, Austin, TX, USA). The individual cytokine and chemokine measurements were summarized for the following periods: POD −7 to –1, POD 0, POD 1, POD 2 to 5, POD 6 to 10, POD 11 to 20, followed by further 10-day periods. Then fold increases compared to the period POD −7 to −1 were calculated for each period.
2.7 Statistics
Data collection and analyses were performed with Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 9.0 (GraphPad Software Inc., Boston, MA, USA). Kaplan–Meier curves were plotted for analysis of survival. Data are presented as group means ± standard deviations (SD) if not indicated otherwise.
3 Results
Here we present the results of an anti-inflammatory and anti-coagulatory therapeutic regimen to prevent inflammatory responses and coagulation disorders after pig-to-baboon cardiac xenotransplantation using donor pigs with the same genetic modifications. In the current study, we only present data of inflammation and coagulation markers. Other data, for example, causes of death, myocardial histological findings, and hemodynamic data have not been subject of these analyses and have been published elsewhere [4, 5] or are so far unpublished data [24].
3.1 Study Population, Survival Times, and Observation Period
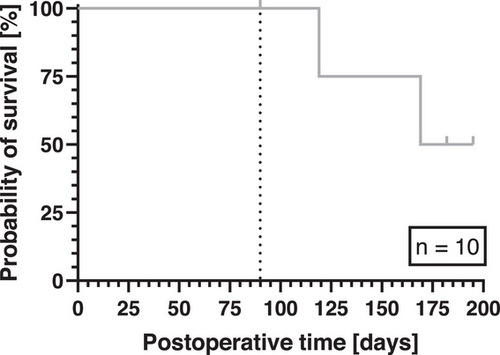
The entire study population consisted of 14 pig-to-baboon orthotopic cardiac xenotransplantation experiments. Two experiments (#17494 and #17492) were terminated after 15 and 27 days respectively, because the donors were tested PCMV/PRV-positive [13] and the recipients presented with signs of multiorgan failure [5]. Two experiments (#16950 and #17353) failed early due to technical reasons [24]. Consequently, only experiments with survival times >30 days were included, which led to a total of 10 experiments (Table 1).
| Experiment | ID | Recipient blood-group | Donor blood-group | Survival (days) | Co-stimulation blockade | Causes for euthanasia | Reference |
|---|---|---|---|---|---|---|---|
| 1 | #17186 | B | 0 | 90 | anti-CD40 Mab | study endpoint | 4, 5 |
| 2 | #17290 | B | 0 | 90 | anti-CD40 Mab | study endpoint | 4, 5 |
| 3 | #17482 | B | 0 | 90 | anti-CD40 Mab | study endpoint | 5 |
| 4 | #17491 | B | 0 | 182 | anti-CD40 Mab | study endpoint | 4, 5 |
| 5 | #17493 | B | 0 | 195 | anti-CD40 Mab | study endpoint | 4, 5 |
| 6 | #17494 | B | 0 | 15 | anti-CD40 Mab | multiorgan failure (PCMV/PRV) | 5 |
| 7 | #17492 | B | 0 | 27 | anti-CD40 Mab | multiorgan failure (PCMV/PRV) | 5 |
| 8 | #16701 | A | 0 | 90 | anti-CD40 Mab | study endpoint | 5 |
| 9 | #16956 | A | 0 | 90 | anti-CD40 Mab + anti-CD40L PAS-Fab | study endpoint | 24 (unpublished data) |
| 10 | #16935 | A | 0 | 90 | anti-CD40 Mab + anti-CD40L PAS-Fab | study endpoint | 24 (unpublished data) |
| 11 | #16950 | AB | 0 | 1 | anti-CD40 Mab + anti-CD40L PAS-Fab | technical failure (pulmonary stenosis) | 24 (unpublished data) |
| 12 | #17353 | AB | 0 | 1 | anti-CD40 Mab + anti-CD40L PAS-Fab | technical failure (insufficient perfusion) | 24 (unpublished data) |
| 13 | #17012 | A | 0 | 120 | anti-CD40 Mab + anti-CD40L PAS-Fab | recalcitrant pleural effusions | 24 (unpublished data) |
| 14 | #17020 | A | 0 | 170 | anti-CD40 Mab + anti-CD40L PAS-Fab | graft failure (humoral rejection) | 24 (unpublished data) |
- Note: Four experiments were excluded from further data analysis: #17494 and #17492, because the donor animals were PCMV/PRV-positive; #16950 and #17353, because they had to be stopped within the first 24 h due to technical failures. Data already published [4, 5] or unpublished data [24].
- Abbreviations: anti-CD40 Mab, chimeric anti-CD40 monoclonal antibody; anti-CD40L PAS-Fab, humanized PASylated anti-CD40L antigen-binding fragment; PCMV/PRV, porcine cytomegalovirus/porcine roseolovirus.
Mean survival in these 10 experiments was 120 days; maximum survival was 195 days (Table 1 and Figure 2). Six out of 10 experiments were deliberately terminated when the predetermined period (set by the regulatory authorities) of 90 postoperative days was reached. Analyses of inflammation and coagulation parameters were limited to this period (Figure 2).
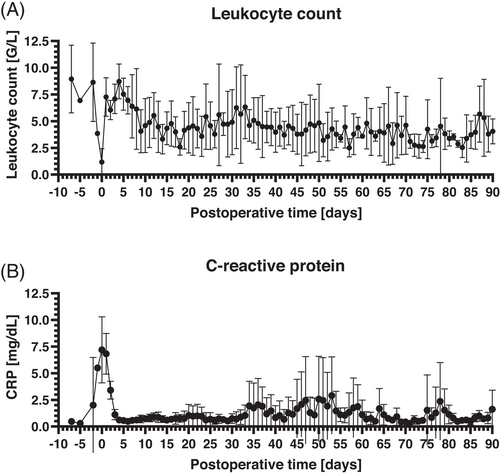
3.2 Leukocyte Counts and CRP Levels
Analyses of leukocyte counts showed a relevant perioperative decrease (min. 1.172 ± 1.254 G/L at the day of xenotransplantation) followed by an increase in the early postoperative period. Afterwards, leukocyte counts remained consistently within normal ranges till the end of the observation period (mean 4.200 ± 0.870 G/L between postoperative Day 7 and Day 90; Figure 3A). Comparable to the leukocyte counts, CRP was elevated in the perioperative period (max. 7.2 ± 3.1 mg/dL at the day of xenotransplantation) but subsequently dropped to normal ranges within five postoperative days. Afterwards there were three peaks of CRP levels after about 35, 50, and 80 postoperative days, respectively. After each peak, CRP levels dropped to normal ranges (Figure 3B).
3.3 Systemic Cytokine and Chemokine Levels
The analyses of systemic cytokine and chemokine levels revealed different patterns. IL-8 (Figure 4A), IL-10 (Figure 4B), Eotaxin (Figure 4C), MCP-1 (Figure 4D), and MIF (Figure 4E) increased about 1.5- to 2.5-fold in the perioperative period. Within the first postoperative week, all these markers decreased to levels comparable to the preoperative values or even lower and stayed at these levels until the end of the observation period. IL-12/IL-23 (Figure 4F), MDC (Figure 4G), RANTES (Figure 4H), and INF-γ (Figure 4I) showed no relevant increase in the perioperative period. IL-12/IL-23 and MDC (Figure 4F,G) decreased in the perioperative period and stayed at these low levels until the end of the observation period. RANTES levels (Figure 4H) were stable in the perioperative period compared to the preoperative values until about 60 postoperative days and showed also a decrease in the following course. INF-γ (Figure 4I) dropped down in the perioperative period and presented with an increasing course afterward, ending with levels about 1.5-fold higher at the end of the observation period.
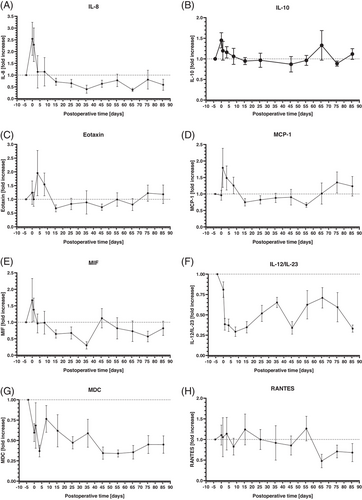
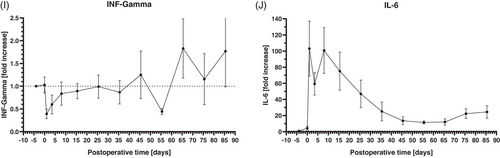
Compared to the other cytokines and chemokines, IL-6 presented a different dynamic (Figure 4H), increasing more than 100-fold after surgery. Following the first postoperative week, IL-6 levels declined, but never returned to preoperative levels. The lowest postoperative IL-6 levels after about 55 postoperative days were still about 10-fold higher compared to the preoperative values.
3.4 Coagulation Parameters
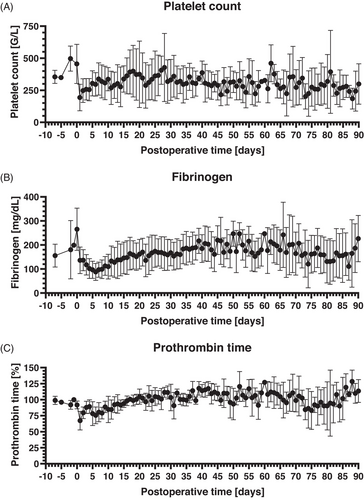
The number of platelets increased in the days before xenotransplantation (max. 497 ± 98 G/L 2 days before xenotransplantation) followed by a sharp decrease at the day of surgery with the lowest count at the first postoperative day (193 ± 104 G/L). Subsequently, the platelet count increased continuously during the first postoperative week and reached normal ranges until the end of the observation period (mean 308 ± 52 G/L between postoperative day 7 and postoperative day 90; Figure 5A). Fibrinogen levels presented with a course comparable to the platelet count. After a preoperative increase (max. 265 ± 88 mg/dL at the day of xenotransplantation), there was a sharp decrease in the perioperative period with the lowest level at postoperative day 6 (88 ± 35 mg/dL). Afterwards, fibrinogen levels were constantly increasing for about 10 postoperative days and remained within normal levels until the end of the observation period (mean 171 ± 32 md/dL between postoperative day 7 and postoperative day 90; Figure 5B). Prothrombin time as a further marker for the coagulation system presented with only a slight decrease in the perioperative period and then leveled within normal ranges again (mean 102 ± 10% between postoperative day 7 and day 90; Figure 5C).
4 Discussion
4.1 Anti-Inflammatory and Anti-Coagulatory Effectivity of the Therapeutic Regimen
Previous analyses of the experiments of the current study group [4, 5] already proved that our therapeutic regimen enables consistent survival after pig-to-baboon orthotopic cardiac xenotransplantation. The current analyses highlight the anti-inflammatory and anti-coagulatory outcome with no relevant inflammatory response or coagulation disorders in all animals of the study group, whether treated with anti-CD40 Mab alone or the combination of anti-CD40 Mab and anti-CD40L PAS Fab. The anti-inflammatory drug regimen with a C1 esterase inhibitor, an IL-6 receptor antagonist, a TNF-α inhibitor, and an IL-1 receptor antagonist should also be applicable in a future clinical setting. Based on the existing data, expression of one complement and one coagulation pathway regulatory protein (hCD46 and hTBM) seems to be sufficient and there is no clear evidence for a requirement to include other proteins, like hEPCR, hCD47, or hHMOX1, although they may turn out to be beneficial [1]. We believe that our drug-based approach offers a relevant advantage over genetic modifications in that the medication can be adjusted at any time if necessary, whereas this might not be possible in the case of genetic modifications. It is furthermore of note, that in the current study group, steroids were tapered down quickly to reduce xenograft overgrowth [4]. In a future clinical setting, donor pigs with smaller body and organ size should be used, for example Auckland Island pigs [1] or animals with growth hormone receptor deficiency [6, 12]. This could allow the temporary or continuous application of higher steroid doses, for example as part of the anti-inflammatory approach. Consequently, it may enable the reduction or even withdrawal of drugs of the current anti-inflammatory regimen.
4.2 Intermittent Increases of CRP and Course of Leukocyte Count
In previous pig-to-baboon organ transplantation studies, CRP levels were elevated for several months after transplantation, indicating a persistent inflammatory state [19, 21, 22]. Furthermore, CRP deposition was detected in the xenografts after pig-to-baboon kidney xenotransplantation [29]. In the current study group, there was a relevant increase in CRP-levels in the perioperative period. This is well-explainable by procedural factors, for example surgical trauma and CPB. In contrast to the previous data [19, 21, 22, 29], CRP levels presented with a marked decrease to nearly normal levels within the first postoperative week. Afterwards, mean CRP levels increased intermittently after about 35, 50, and 80 postoperative days. These mean elevations were caused by high CRP peaks in the individual animals #17186, #17290, #17493, and/or #16701. In these periods, at least one of these animals showed clinical signs of (bacterial) infection, like elevated temperature or reduced activity and food intake. In two of these animals, #17186 and #17493, bacteria could be detected in blood culture samples in phases of elevated CRP levels. Due to the clinical signs of infection, all the above-mentioned animals received calculated or test-appropriate antibiotic treatment. This led to a decrease in both clinical signs of infection as well as CRP levels. We therefore interpret the later intermittent CRP elevations as a sign of the (bacterial) infections that occurred and not as indicators of a general inflammatory response to xenotransplantation.
After a decrease in the perioperative period, leukocyte counts stabilized rapidly within the first postoperative days and were stable at normal or slightly reduced levels until the end of the experiment, also in the periods of CRP-increase mentioned above. We assume the lack of relevant increases in periods of (bacterial) infections is to be seen as effect of the adequate immunosuppression based on CD40/CD40L co-stimulation blockade.
4.3 Courses of Cytokines and Chemokines
Increased levels of several cytokines and chemokines (for example IL-6, IL-8, IL-12, INF-γ, and MCP-1) have been observed in different xenotransplantation models [16, 20, 30]. In pig-to-baboon orthotopic cardiac xenotransplantation experiments, the increase of IL-6, IL1β, TNF, INF-γ, and CXCL9 was seen as an indicator of a “cytokine storm” following CPB [16]. In our current study there was also an increase of several cytokines and chemokines in the perioperative period. However, with fold changes of a maximum of about 2.5 this increase was lower than in the studies mentioned above and so, in our opinion, does not meet the criteria of a “cytokine storm.” Furthermore, in our study group, the increase was not persistent, but regressed within the first postoperative week.
Although the inflammatory response to CPB has been bluntly reduced since the first CPB approaches, there is still a relevant systemic inflammatory response to CPB in the human cardiac surgery setting [31-33]. We assume, that the perioperative cytokine and chemokine elevations were in part caused by CBP. Interestingly, there were no relevant changes in cytokine levels when animals of the study group showed clinical signs of infection and intermittent CRP elevations (see above). In other pig-to-baboon cardiac xenotransplantation experiments, there were significant increases of TNF, IL-1β, CXCL9, INF-γ, and IL-8 when the animals were either treated for a positive blood culture or when a leukocytosis of unknown cause developed [16]. We regard this as a sign of effectiveness of our targeted anti-inflammatory drug therapy.
In contrast to all other cytokines, IL-6 levels increased to a much greater extent (100-fold) and persisted at least 10-fold higher throughout the whole observation period. We assume that this might have been caused by the treatment with the anti-IL-6 receptor antibody tocilizumab. In rheumatoid arthritis and Castleman disease patients, tocilizumab treatment increased serum levels of IL-6 and soluble IL-6R [34]. As one possible reason for this increase, tocilizumab might stimulate the production of IL-6 if its blockade of IL-6 signaling inhibits a negative feedback effect of IL-6 on IL-6 production [34]. Another possible explanation for the increase in serum IL-6 after tocilizumab administration is that tocilizumab may inhibit the clearance of IL-6 from serum [34].
4.4 Coagulation Dysregulation
Several studies suggest that inflammatory responses to xenotransplantation precede activation of coagulation [29, 35, 36]. In the current study, we administered acetylsalicylic acid according to its clinical indication; as a preventative against vascular disease, which is associated with a reduction in myocardial infarction and stroke [15, 37]. Furthermore, acetylsalicylic acid down-regulates several proinflammatory cytokines [38] and pathways [39]. Heparin was only given prophylactically as low-dose therapy to prevent clot formation at the central venous catheter, but not as specific therapy aiming at coagulation disorders. We therefore assume that the acetylsalicylic acid may have played a role regarding the avoidance of coagulation disorders and inflammatory responses in the current study group. With the currently existing data, we cannot clearly answer whether future patients after cardiac xenotransplantation should also receive acetylsalicylic acid. However, we assume that these patients will probably have other indications for acetylsalicylic acid anyway, which is independent from the xenotransplantation setting. Following human cardiac allotransplantation, the role of antiplatelet therapy is unclear and current guidelines do not include recommendations for its routine use [40].
4.5 Further Anti-Inflammatory Factors
In addition to the targeted anti-inflammatory drug therapy, other factors also play a role in the sufficient control of inflammation and coagulation disorders in xenotransplantation.
One of these factors is the cold non-ischemic heart preservation with continuous perfusion (CNIHP) [9, 10]. After orthotopic cardiac xenotransplantation with ischemic preservation, graft failure within 24–48 h has been reported in a range of 40%–60 % [17, 18, 41, 42]. This graft failure, which was independent of immune organ rejection, has been defined as perioperative cardiac xenograft dysfunction (PCXD) [17, 41]. We assume that PCXD is mainly caused by ischemia-reperfusion injury [10], which is known to induce inflammatory responses in organ allotransplantation [43]. With the use of CNIHP, ischemia-reperfusion injury and PCXD could be reliably prevented [5, 10]. These findings indicate an anti-inflammatory effect of CNIHP, whose exact reasons are not fully understood. The avoidance of myocardial ischemia improves postoperative graft function and so systemic circulation. We assume that this reduces cytokine release and inflammatory response via at least two mechanisms: a reduced cytokine release from the graft because of no ischemia-reperfusion injury on the one hand and a reduced systemic cytokine release duo to sufficient hemodynamics on the other hand. Furthermore, CNIHP may wash out cells ex vivo that are sources of proinflammatory cytokines, dilute those cytokines and diminish white blood cell function by cooling [16], so having a relevant anti-inflammatory effect after xenotransplantation.
Another aspect is the inflammatory response associated with cardiopulmonary bypass (CPB). Several studies carved out a systemic inflammatory response to CBP in the human setting [31-33]. This inflammatory response is for example caused by blood contact with the CBP circuit components, ischemia-reperfusion injury, heparin-protamine interactions, and surgical trauma [44-47]. Despite these detrimental reactions, it is evident that most sick patients, some of them in end-stage heart disease, survive operations using CPB and improve after a relatively short course of postoperative intensive care [48].
Furthermore, a sufficient immunosuppression based on co-stimulation blockade of the CD40/CD40L pathway [7] is essential to control inflammation and coagulation disorders in xenotransplantation. When no immunosuppressive therapy was administered, increases in several cytokine levels have been reported in previous xenotransplantation experiments [29].
Last but not least, it has to be mentioned that it is crucial to prevent the transmission of PCMV/PRV to the recipient. Despite of the host virome that may have an impact the presence of PCMV/PRV has been shown to reduce drastically the survival time of pig hearts and kidneys when the xenograft was infected [49]. Furthermore, infection of the recipient with PCMV/PRV induced consumptive coagulopathy [50] and increased the expression of IL-6 and the level of tissue-type plasminogen activator—plasminogen activator inhibitor type 1 complexes after pig-to-baboon cardiac xenotransplantation [13]. There are contradictory opinions whether the clinical symptoms often described after transplantation of pig organs into nonhuman primates and called systemic inflammation in xenograft recipients (SIXR) may also be associated with the transmission of PCMV/PRV [51, 52]. Therefore, we excluded the PCMV/PRV-positive animals from the current analyses.
4.6 Limitations
There is no control group in this study; variations in study design, therapy, and other factors may have influenced the results of groups we compared our data to. To comply with the principles of the 3Rs [53], we decided not to include a control group, were we would expect significant inflammatory responses. For the same reasons, we have not yet conducted experiments using fewer anti-inflammatory drugs. Therefore, we cannot prioritize the different anti-inflammatory agents, and future studies should investigate whether a reduction or even withdrawal of drugs from the current regimen is possible.
We only used GGTA1-KO pigs in the current study. Regarding the clinical application of xenotransplantation, the use of double- (DKO) or triple-knockout (TKO) pigs [1] would also be interesting. However, testing these pigs in baboon models is complicated by a significant difference in the innate immune response between humans and non-human primates [1]. Consequently, all our pig-to-baboon studies are conducted with GTKO donor pigs, and we intend to reserve the use of TKO pigs for human xenotransplantation [1].
5 Conclusion
Our preclinical experience with the anti-inflammatory drug therapy proved that controlling of inflammation and coagulation disorders in xenotransplantation is possible and well-practicable. In combination with the well-established prerequisites—genetic modifications of the donor pigs, CD40/CD40L co-stimulation blockade, continuous non-ischemic heart preservation as well as virological and microbiological safety of the donor organs—our anti-inflammatory regimen should also be applicable in the clinical setting of cardiac xenotransplantation.
Acknowledgments
The authors thank the German Primate Center and the Walter Brendel Centre of Experimental Medicine for support and provision of facilities.
Conflicts of Interest
Bruno Reichart, Eckhard Wolf, Paolo Brenner, Matthias Längin, and Jan-Michael Abicht are founding members of XTransplant GmbH. Michaela Gebauer is an employee, Uli Binder and Arne Skerra are shareholders of XL-protein GmbH. David Ayares is chief executive officer and chief scientific officer of Revivicor Inc. The other authors have no conflicts of interest to disclose.




