Evaluating the impact of an anti-microbial silver-impregnated surgical dressing on wound infections and healing: A randomised clinical trial
Abstract
StopBac is an innovative silver-impregnated antimicrobial dressing specifically designed to reduce surgical site infections and enhance healing. The primary objective of this study was to compare infection healing rate at 30 days after surgery between primarily closed surgical wounds covered with StopBac and those covered with Cosmorpor, a standard surgical dressing. Between 1.3.2023 and 30.4.2023, we conducted a prospective screening of all patients undergoing surgical operations within a single surgical department. Patients were randomised into either the Cosmopor group or the StopBac group. Outcome measures were superficial and deep surgical site infections and healed wounds. Data concerning patient and surgical factors were prospectively collected and analysed. The analysis comprised 275 patients, divided into two groups: 140 patients in the StopBac group and 135 in the Cosmopor group. The StopBac dressing was associated with a reduced rate of infection, with an odds ratio of 0.288 (p < 0.001), and an increased likelihood of wound healing at 30 days after surgery. The odds ratio for healing at 30 days was 4.661 (p < 0.001). StopBac was associated with a lower incidence of surgical wound infections and a higher probability of healing at 30 days after surgery, when compared with standard dressing.
Abbreviations
-
- AIC
-
- akaike information criterion
-
- BMI
-
- body mass index
-
- CDC
-
- center for disease control and prevention
-
- CI
-
- confidence intervals
-
- SD
-
- standard deviation
1 INTRODUCTION
Surgical site infections can be serious complications that significantly prolong hospitalisation, increase treatment costs, and have a detrimental effect on patients' health.1 Preventing surgical site infections is therefore a key priority in surgical practice. One of the approaches to prevent these infections is the use of silver dressings.
Silver dressings have become a popular choice for treating surgical wounds due to their antimicrobial properties. The principle of silver dressings lies in the release of silver ions, which exhibit potent bactericidal activity. Silver binds to bacterial membranes and enzymes, resulting in the disruption of microbial cell structures and inhibition of their growth. Thanks to these attributes, silver dressings are believed to be an effective tool in inhibiting infections.2
Currently, there is limited data on the efficacy of surgical dressings in the prevention of infection in closed surgical wounds. A Cochrane review highlighted the insufficiency of high-quality evidence regarding the choice of wound dressings for such wounds.3
The objective of this study was to compare a new silver dressing called StopBac® (Grade Medical, Prague, Czech Republic) in its ability to prevent surgical wound infections and support healing with a standard surgical dressing Cosmopor® (Hartmann, Heidenheim an der Brenz, Germany). The study was conducted as a single centre, randomised trial, assessing wound healing and infection occurrence for 30 days after abdominal surgeries where the skin incisions underwent primary closure.
2 METHODS
2.1 Study design and patient recruitment
This study was designed as a randomised, single centre not controlled and not registered prospective study. All patients undergoing surgical procedures at a single department of surgery, between March 1, 2023, and April 30, 2023, were screened for eligibility for inclusion in the study. Using block randomisation patients were allocated into two groups. One group included patients treated with the dressing StopBac and the other group included patients treated with the dressing Cosmpor.
2.2 Power analysis
The incidence of surgical site infections following general surgical procedures in the literature ranges from 12% to 22%.4-7 Based on our previous multicentre study, we anticipated an infection rate of approximately 20%.8 We expect a two-thirds reduction in infections in the StopBac cohort, which corresponds to a wound infection rate of 13%. According to the power analysis, 107 patients were needed in each group, resulting in a total of 214 patients (alpha 0.05 and power 0.8). We planned to enrol as many patients as possible, accounting for an approximate dropout rate of 10%.
2.3 Data collection
Data on various parameters including demographics, comorbidities, type of procedure, procedural urgency, wound characteristics, occurrences of wound infection, progress in wound healing, and any adverse event were prospectively collected between March 1, 2023, and April 30, 2023.
2.4 Inclusion criteria
The study included patients with primarily closed skin incisions at the end of the surgery. Patients undergoing surgical revisions and patient with surgical wounds that were not primarily closed were excluded. Informed consent from patients was required for participation in the study.
2.5 Definition of surgical wound infection
The Center for Disease Control and Prevention (CDC) definition was used to classify surgical site infections.9 Superficial and deep surgical wound infections were taken as study outcomes, while organ/space infections were not included in the analysis.
2.6 Definition of wound healing
A healed wound was defined as a closed wound after removal of the sutures. After the stitches are taken out, the wound should be fully closed, and there should be no signs of infection or inflammation.
2.7 Study outcomes
Study outcomes monitored were wound healing within 30 days, wound infection (superficial or deep) and adverse events to the dressings (allergic reactions to silver, skin irritation or discomfort).
2.8 Statistical analysis
Descriptive statistics are expressed as the mean with standard deviation (SD) for normally distributed numeric data and median and interquartile range (IQR) for data that did not follow a normal distribution. For continuous variables, Student's t-test was used and for categorical variables the Chi-squared test was used or, in cases of expected frequencies less than 5, Fisher's exact test. The Bonferroni correction was employed to establish the significance threshold for the p value by dividing 0.05 by the number of factors tested. As 32 factors were tested the p value threshold was set to 0.00156.
To evaluate the impact of the surgical dressing on the outcomes a comparison of two models was performed. The influence of covariates was examined by comparing a null model containing covariates (BMI, age, gender, procedure type, procedure urgency, wound type, and comorbidities) with a model containing covariates and dressing type. The goodness of fit of the second model was compared to the null model using a likelihood ratio test. Furthermore, a sensitivity analysis was conducted, where 80% of the data was randomly excluded, and this process was repeated 1000 times to verify the stability of the results when comparing the two models.
2.9 Ethical statement
This clinical study has been conducted in strict adherence to ethical principles and guidelines outlined in the Declaration of Helsinki. Ethical approval was obtained by the institutional ethical board. All patients consented to participation in the study and data was anonymised.
3 RESULTS
3.1 Patient selection
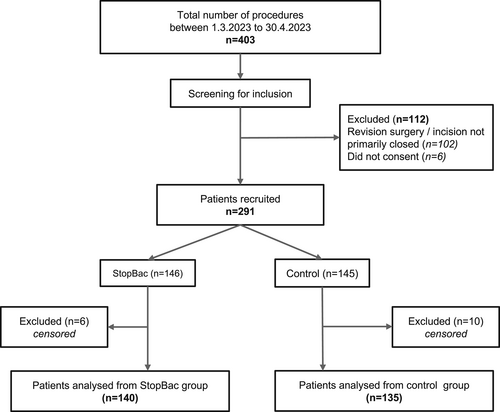
Between March 1, 2023, and April 30, 2023, all patients who underwent surgical procedures at single department of surgery were evaluated for inclusion in the study. During this period, a total of 403 surgical procedures were performed. From these 112 were excluded during the screening process for the following reasons: lack of consent, revision surgery, and surgical wounds not primarily closed at the end of the procedure. After screening, 291 patients were randomly assigned to either the StopBac group (n = 146) or the Cosmopor group (n = 145). Sixteen patients were excluded due to loss of follow-up. In total, 275 patients were included: 140 in the StopBac group and 135 patients in the Cosmopor group. The patient selection flow chart is shown in Figure 1.
3.2 Demographics
The average age was 56.9 years (SD 17.8). There were 133 male patients and 142 female patients. The average BMI was 27.2 (SD 6.2). No significant differences in demographic data were found between the two patient groups (Table 1).
| StopBac | Cosmopor | p value | |
|---|---|---|---|
| (n = 140) | (n = 135) | ||
| Age | 54.9 (±17.7) | 58.9 (±17.7) | 0.06552 |
| Sex ratio (male:female) | 67:73 | 66:69 | 0.960 |
| Body mass index | 26.8 (±6.6) | 27.5 (±5.9) | 0.4325 |
| Comorbidities | |||
|
60 (42.9%) | 57 (42.2%) | 1.000 |
|
21 (15.0%) | 25 (18.5%) | 0.535 |
|
44 (31.4%) | 43 (31.9%) | 1.000 |
|
13 (9.3%) | 12 (8.9%) | 1.000 |
|
14 (10.0%) | 19 (14.1%) | 0.393 |
|
4 (2.9%) | 8 (5.9%) | 0.342 |
|
12 (8.6%) | 18 (13.3%) | 0.283 |
|
20 (14.3%) | 15 (11.1%) | 0.543 |
| Type of procedure | |||
|
20 (14.3%) | 21 (15.6%) | 0.900 |
|
6 (4.3%) | 4 (3.0%) | 0.750a |
|
6 (4.3%) | 11 (8.1%) | 0.281 |
|
21 (15.0%) | 29 (21.5%) | 0.216 |
|
21 (15.0%) | 17 (12.6%) | 0.687 |
|
9 (6.4%) | 12 (8.9%) | 0.5886 |
|
17 (12.1%) | 6 (4.4%) | 0.03684 |
|
11 (7.9%) | 12 (8.9%) | 0.9274 |
|
17 (12.1%) | 11 (8.1%) | 0.3704 |
|
12 (8.6%) | 12 (8.9%) | 1.000 |
| Urgency | |||
|
95 (67.9%) | 85 (63.0%) | 0.4676 |
|
45 (32.1%) | 50 (37.0%) | |
| Type of wound | |||
|
64 (45.7%) | 59 (43.7%) | 0.8306 |
|
44 (31.4%) | 41 (30.4%) | 0.9527 |
|
24 (17.1%) | 26 (19.3%) | 0.7653 |
|
8 (5.7%) | 9 (6.7%) | 0.9383 |
| Wound infection | |||
|
11 (7.9%) | 31 (23.0%) | 0.000921 |
|
3 (2.1%) | 9 (6.7%) | 0.123 |
|
14 (10.0%) | 40 (29.6%) | 0.00006047 |
| Wound healed | |||
|
132 (94.3%) | 105 (77.8%) | 0.00008319 |
|
8 (5.7%) | 30 (22.2%) | |
- Note: For continuous variables, Student's t-test was used (age and body mass index) and for categorical variables, the chi-square test (or Fisher's exact test in cases of expected frequencies less than 5) was used. The Bonferroni correction was employed to establish the significance threshold for the p value by dividing 0.05 by the number of factors tested. As 32 factors were tested, the p value threshold was set to 0.00156.
- a Fisher test.
3.3 Comorbidities
Among the overall patient population, 117 (42.5%) had arterial hypertension, 46 (16.7%) had diabetes mellitus, 87 (31.6%) had a history of malignancy, 25 (9.1%) had peripheral artery disease, 33 (12.0%) had ischemic heart disease, 12 (4.4%) had chronic renal insufficiency, and 35 (12.7%) were active smokers. No significant differences in comorbidities were observed between patients in the StopBac and Cosmopor group (Table 1).
3.4 Types of surgery
Hernia repair was performed on 41 patients (14.9%), gastric procedures on 10 patients (3.6%), small bowel procedures on 17 patients (6.2%), procedures on the large intestine or rectum on 50 patients (18.2%), appendectomy on 38 patients (13.8%), cholecystectomy on 21 patients (7.6%), hepatopancreatobiliary procedures on 23 patients (8.4%), mastectomy on 23 patients (8.4%), vascular procedures on 28 patients (10.2%), and other procedures on 24 patients (8.7%). The type of surgery did not significantly differ between the two patient groups (Table 1).
3.5 Urgency of the procedures
There were 95 urgent surgeries (34.5%) and 180 elective surgeries (65.5%) performed. The urgency of the procedures did not significantly differ between the patient groups (Table 1).
3.6 Type of wound
The types of wounds, classified according to the CDC classification, were as follows: clean wounds (n = 123, 44.7%), clean-contaminated wounds (n = 85, 30.9%), contaminated wounds (n = 50, 18.2%), and infected-contaminated wounds (n = 17, 6.2%). Type of wound did not significantly differ between the patient groups (Table 1).
3.7 Surgical wound infection
Surgical wound infection occurred in 40 patients (15.5%, [95% CI 10.9%–19.2%]). Out of these cases, 31 were classified as superficial infections, and nine were deep infections. It is important to note that infections of organs/spaces were not included in the analysis, as the focus was specifically on skin wounds, where the dressing was expected to have an impact. Among patients in the StopBac group, 14 infections occurred (10.0% [95% CI: 3.9%–12.6%]). In the Cosmopor group, there were 40 wound infections (29.6% [95% CI: 22.6%–37.8%]). The infection rate in the StopBac group was significantly lower than the Cosmopor group (p = 0.00006047), odds ratio: 0.265 (CI 0.126–0.532) (Table 1).
3.8 Wound healing within 30 days
Surgical wounds fully healed within 30 days in 237 patients (86.2%, [95% CI: 81.6%–89.8%]). In the StopBac group, the wound fully healed in 132 patients (94.3%, [95% CI: 89.1%–97.1%]), while in the Cosmopor group, 105 patients achieved complete healing (77.8% [95% CI: 70.1%–84.0%]). The healing rate was significantly higher in the StopBac group (p = 0.00008319, odds ratio: 4.689 (CI 1.995–12.349) (Table 1).
3.9 Logistic regression analysis: effect of dressing on infection and healing within 30 days
To compare the impact of dressing type on infection and healing within 30 days after surgery, logistic regression was used. The logistic regression analysis revealed a statistically significant effect of the StopBac dressing on infection (estimate = −1.33, standard error = 0.339, p = 8.47 × 10−5) and healing within 30 days (estimate = 1.55, standard error = 0.419, p = 2.14 × 10−4).
3.10 Comparison of model fit
To assess the impact of covariates, we compared a null model containing covariates (BMI, age, gender, procedure type, procedure urgency, wound type, and comorbidities) with a model containing covariates and dressing type. We compared the fit of the second model with the null model using the likelihood ratio test and found that in both cases, including the dressing type significantly improved the fit. For the rate of wound infection, the p-value was 1.67 × 10−4 (d.f. = 205). To quantify the improvement in fit, we calculated the change in Akaike Information Criterion (AIC), which was −12.16. For the healing rate, the p-value was 2.25 × 10−5 (d.f. = 205) with a change in AIC of −15.97.
3.11 Sensitivity analysis
We performed a sensitivity analysis by randomly selecting 80% of the data and repeating the likelihood ratio for both infection and healing after 30 days. All results exceeded the threshold value of less than 0.05. The median p-value was 0.0007 for infection and 0.0001 for healing. A histogram of the negative logarithm (base 10) of p-values for the sensitivity analysis of wound infection is shown in Figure 2 and for wound healing in Figure 3.
3.12 Adverse events
None of the patients experienced allergic or other adverse reactions to the dressings.
4 DISCUSSION
Surgical site infections are the third most common incidence, after catheter-associated urinary tract infections and central line-associated bloodstream infections.10 Surgical site infections are a leading cause of morbidity and mortality after surgical procedures, they lengthen hospital stay, are a significant cause of rehospitalisation and reoperation, and significantly increase the number of postoperative outpatient visits.11
In this randomised clinical trial, we compared the effect of the silver-based antimicrobial surgical dressing StopBac® (Grade Medical, Prague, Czech Republic) with a standard surgical dressing, Cosmopor® (Hartmann, Heidenheim an der Brenz, Germany) on preventing superficial and deep wound infection in primarily close surgical wounds and rate of wound healing at 30 days. The dressing StopBac was associated with a reduced rate of infection, with an odds ratio of 0.288 (p < 0.001), and an increased likelihood of wound healing by 30 days after surgery, with an odds ratio of 4.661 (p < 0.001). These results remained significant using a comparison of two models, one using all the covariates and one with the covariates plus the type of dressing. Doing this showed a significant effect of StopBac in the prevention of wound infections and rate of healing. Our results were confirmed even further by our sensitivity analysis, which showed that by randomly removing 80% of the data and repeating the analysis 1000 times, the results remained significant each time.
StopBac is a unique surgical dressing that utilises the sol–gel process and contains silver cations. Its outer active zone, an antibacterial nanolayer is impregnated with a colloid solution containing isopropyl alcohol, 3-(trimethoxysilyl)propyl methacrylate, benzoyl peroxide, demineralised water, nitric acid, and trace quantities of silver nitrate. The solution is deposited on a pad through spray atomization, resulting in a polymer nanolayer matrix that offers prolonged and controlled release of silver ions. This controlled release of silver ions effectively prevents infections, making it highly suitable for wound healing. The dressing is composed of a porous material that facilitates fluid drainage away from the wound while simultaneously creating a barrier against bacteria. The main features of the StopBac dressing are compared with Cosmopor dressing in Table 2.
| Feature | Cosmopor | StopBac |
|---|---|---|
| Material | Non-woven fabric backing, hypoallergenic adhesive | Non-woven fabric backing, hypoallergenic adhesive |
| Active agents | No | Polymer nanolayer matrix with a patented solution containing AgNO3 |
| Purpose | To cover and protect wounds, and to help keep them moist | To prevent and treat surgical site infections |
| Available sizes and shapes | Variety of sizes and shapes | Two types: Standard and waterproof Aquastop Variety of sizes |
| Antimicrobial properties | No | Yes |
Typically, StopBac dressings are applied immediately after the final skin suture and remain in place for 48–72 h. When in contact with the wound, the dressing's nanolayer matrix releases silver ions. These silver ions are highly reactive and bind to various cellular components of bacteria, disrupting their metabolic processes, DNA replication, and cell wall formation.12 The antibacterial properties of silver dressings are tied to the rate and quantity of silver ion release from the dressing. Recent advancements in nanotechnology have further improved this by encapsulating silver into nanoparticles, significantly increasing the available surface area for silver dissolution and thereby enhancing its antimicrobial efficacy. Additionally, silver nanoparticles have demonstrated anti-inflammatory properties during the wound healing process, further supporting the overall healing process.13, 14
StopBac dressings offer a safe and highly effective solution for preventing and treating surgical site infections. No allergic or adverse events related to the dressing were observed in multiple studies, including the current study on 140 patients, as well as our group's previous investigations conducted by Oliverius (2020) with 32 patients and Lawrie (2019) with 109 patients.8, 15 Additionally, an observational study conducted by Stryja et al. (2020) involving 21 patients reported no allergic reaction and excellent patient tolerance to the dressing.16
This is the first study to compare the silver-ion antimicrobial dressing StopBac with a standard dressing in the form of a randomised trial. In our first pilot study, we evaluated the ability of StopBac to prevent surgical site infections in a cohort of 32 patients undergoing abdominal surgery, although the study was limited by its cohort size and the inclusion of various types of procedures only one surgical site infection occurred. Furthermore, the study showed a clear economic benefit of using the StopBac dressing, less dressing changes was needed (one to two), compared with standard dressings (two to three).15 Our pilot study was followed by a multicentre observational controlled trial.8 Data was collected and analysed from four surgical departments in the Czech Republic. Overall data from 218 patients were analysed. StopBac was associated with a lower incidence of incisional surgical site infections and a higher incidence of primary healing when compared to standard non-silver dressing. One of the weaknesses of the study was its non-randomised nature. This current study confirms the results of the previous studies in the form of a randomised clinical trial.
Several other studies in the literature have investigated the potential benefit of silver-impregnated dressings to standard wound dressings. One clinical trial investigated the effect of silver impregnated dressings in colorectal cancer surgery. The dressing Aquacel was compared with the silver dressing Ag Hydrofiber and no difference in the incidence of surgical site infections was noted.17 A different study, which investigated two silver dressings and one standard dressing on healing after sternotomy, reported that the silver dressings were easier to remove and more comfortable for the patients, but the investigators were unable to prove a significant reduction in wound infections.18 A meta-analysis of four randomised controlled trials concluded that silver-impregnated surgical dressings had no additional benefit to standard dressing in reducing SSI. However, the quality of evidence was very low as there was a high risk of bias and imprecision within the included studies imprecision,19 which further indicates the need for continued research in this field.
5 STUDY LIMITATIONS
Even though this is the first randomised trial investigating the new surgical dressing StopBac, it has several limitations. These include the small cohort size, lack of registration, no control group and non-blinded design.
6 CONCLUSION
In this randomised clinical study, we demonstrated that the surgical dressing StopBac was associated with a lower incidence of wound infections and a higher rate of wound healing compared to a standard surgical dressing.
ACKNOWLEDGEMENTS
This research was supported by the Charles University Research program ‘Cooperatio—Cardiovascular Science’.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interests to declare.




