A role for vitamin D and the vitamin D receptor in keloid disorder
Abstract
Keloids are disfiguring fibroproliferative lesions that can occur in susceptible individuals following any skin injury. They are extremely challenging to treat, with relatively low response rates to current therapies and high rates of recurrence after treatment. Although several distinct genetic loci have been associated with keloid formation in different populations, there has been no single causative gene yet identified and the molecular mechanisms guiding keloid development are incompletely understood. Further, although it is well known that keloids are more commonly observed in populations with dark skin pigmentation, the basis for increased keloid risk in skin of colour is not yet known. Because individuals with dark skin pigmentation are at higher risk for vitamin D deficiency, the role of vitamin D in keloid pathology has gained interest in the keloid research community. A limited number of studies have found lower serum vitamin D levels in patients with keloids, and reduced expression of the vitamin D receptor (VDR) in keloid lesions compared with uninjured skin. Vitamin D has documented anti-inflammatory, anti-proliferative and pro-differentiation activities, suggesting it may have a therapeutic role in suppression of keloid fibrosis. Here we review the evidence supporting a role for vitamin D and VDR in keloid pathology.
Abbreviations
-
- 1,25(OH)2D
-
- 1,25 dihydroxyvitamin D
-
- 25(OH)D
-
- 25 hydroxyvitamin D
-
- CTGF
-
- connective tissue growth factor
-
- ECM
-
- extracellular matrix
-
- EMT
-
- epithelial-mesenchymal transition
-
- HTS
-
- hypertrophic scar
-
- ICU
-
- intensive care unit
-
- IGF 1
-
- insulin-like growth factor 1
-
- PAI1
-
- plasminogen activator inhibitor 1
-
- PBL
-
- peripheral blood lymphocyte
-
- PDGF
-
- platelet derived growth factor
-
- RXR
-
- retinoid X receptor
-
- SHH
-
- sonic hedgehog
-
- TBSA
-
- total body surface area
-
- TGF-β1
-
- transforming growth factor beta 1
-
- Tregs
-
- regulatory T cells
-
- UVB
-
- ultraviolet B
-
- VDR
-
- vitamin D receptor
-
- VDRE
-
- vitamin D response element
1 KELOIDS: MORE THAN JUST ‘SCARS’
Keloids are fibroproliferative lesions that can occur in predisposed individuals following an injury to the skin. For reasons that remain incompletely understood, keloids begin as scars that progress to grow beyond the original wound margin, often increasing in size indefinitely and rarely regressing (Figure 1). This unregulated growth distinguishes keloids from hypertrophic scars (HTSs), which are raised but do not extend beyond the boundaries of the original wound.1 Because of their uncontrolled growth, keloids are currently considered to represent a form of benign fibroproliferative tumour rather than a scar, and the terms ‘keloid disorder’ or ‘keloid disease’ are increasingly used in cases of patients presenting with multiple keloid lesions.2-4 Keloids are disfiguring, which may negatively affect body image, and they may restrict range of motion when they occur across joints.1 In addition, keloids can be very itchy and painful; pruritis and/or pain are present in a majority of keloid patients and contribute significantly to impaired quality of life.3, 5-8 Treatment of keloids is notoriously challenging, despite the existence of numerous therapeutic modalities.9, 10 Algorithms for treatment of keloids have been proposed,11, 12 yet there is no standard treatment that is effective for all keloids, and recurrence rates for most common therapies are very high.9, 13 A greater understanding of the pathological mechanisms driving keloid formation is needed to guide development of more effective therapies.
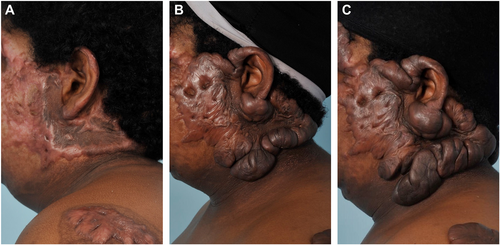
Keloids are characterised by excessive and abnormal deposition of collagen, with an increased ratio of type I to type III collagen compared with normal, uninjured skin.14 Histologically, the presence of disorganised, thick, hyalinised collagen bundles is considered as a distinguishing feature of keloid lesions.4, 15 Other hallmarks of keloids include a thickened, flattened epidermis; increased dermal cellularity; a tongue-like advancing edge in the dermis and increased immune cell infiltration.4, 9, 15 The central region of the keloid may be relatively acellular and hypoxic due to capillary occlusion, whereas keloid margins tend to be well-vascularised and contain metabolically active fibroblasts.2, 4, 16 Keloids tend to occur more often in certain body sites, including the ears, chest, shoulders, upper back and neck.17 It has been proposed that skin tension plays a role in the site-specific nature of keloids.18, 19 However, while skin stretching has been proposed to increase the risk of hypertrophic scar formation,20 the involvement of skin tension in keloid development is less clear, particularly because the earlobe—among the body sites most prone to keloid formation—is an area of low skin tension,4 and other regional differences in skin, including differences in extracellular matrix (ECM) and site-specific gene expression, may be involved.21, 22
The occurrence of keloids is significantly more common in certain ethnic groups, including individuals of African and Asian descent, with 15- to 20-fold greater frequency in dark versus light skinned populations.23-26 Additionally, keloid severity tends to be greater in individuals of African versus European descent.27 The basis for the association of skin pigmentation and scar risk is unknown, and it is as yet unclear whether this is related to skin pigmentation per se, or genetics, or a combination of factors. The occurrence of keloids in Africans with albinism at roughly the same rate as in non-albino Africans contradicts earlier anecdotal reports that albinos do not get keloids, and suggests that factors other than pigmentation, such as genetics, may underlie the greater incidence of keloids in populations of African descent.28 Although most cases of keloids occur spontaneously,26 the predisposition for keloid formation frequently occurs in families, strongly suggesting a genetic component.1, 29 Genetics studies have suggested that keloids can be inherited as an autosomal dominant trait with incomplete penetrance,30 and it is currently believed to be a multigenic disorder.4, 31 Multiple chromosomal loci and even candidate genes have been identified in different populations, however no single causative gene has yet been identified.1, 26, 29, 32-35
Keloids are only observed in humans, thus there are no animal models of wounds that result in keloids.36 As a result, most preclinical keloid research involves in vitro analyses of keloid-derived cells. A majority of in vitro studies have investigated keloid-derived fibroblasts and generally report higher proliferation rates, reduced apoptosis, increased migration, increased metabolic rates and increased production and decreased degradation of ECM, compared with normal fibroblasts (recently reviewed by Limandjaja et al.4). From a mechanistic perspective, there have been numerous studies aimed at understanding the molecular pathways that drive keloid development. Not surprisingly, many of the same signalling pathways involved in regulating normal wound healing are dysregulated in keloid disorder, as are pathways involved in fibrosis in other organs. Several profibrotic cytokines, including transforming growth factor beta 1 (TGF-β1), platelet derived growth factor (PDGF), connective tissue growth factor (CTGF) and components of insulin-like growth factor 1 (IGF1) and Wnt/β-catenin signalling pathways, are increased in keloid fibroblasts and contribute to excess production and deposition of ECM components.37-41
Abnormalities of keloid keratinocytes have also been described, with aberrant expression of genes involved in migration, adhesion and differentiation, as well as abnormal paracrine interactions with fibroblasts.42-46 Thus, the transcriptional profile of keloid keratinocytes resembles epithelial–mesenchymal transition (EMT),42, 47 a process that is involved in embryonic development and has been associated with fibrosis in other organs as well as with cancer metastasis. In EMT, epithelial cells acquire characteristics of mesenchymal cells, such as decreased adhesion and increased migration, in addition to expression of mesenchymal markers.48 In cancer, these features may promote metastasis of cancer cells. However, keloid keratinocytes exhibit an incomplete EMT, which resembles changes observed in skin during wound healing, suggesting that the EMT-like phenotype of keloid cells reflects a dysregulated, over-healing response.43 During normal wound healing, wounds pass through overlapping phases generally referred to as the inflammatory, proliferative and maturation phases. The proliferative phase of wound healing resembles partial EMT in that cell–cell contacts are reduced and cells become more migratory to allow re-epithelialisation to occur.49 However, once the wound has closed, signals between the epithelium and mesenchyme lead to a reversal of the EMT-like phenotype, and a decrease in cellular proliferation, which together allow the wound to transition from the proliferative to the remodelling phase. This transition appears to be defective in keloids, leading to continued proliferation and incomplete remodelling.43 Keloids display many features reminiscent of EMT, including aberrant expression of genes involved in TGF-β1, Wnt/β-catenin and sonic hedgehog (SHH) signalling.42, 43, 50-53 Previous studies revealed the EMT-like expression profile of keloid keratinocytes, including increased expression of EMT-related genes and expression of biomarkers of EMT, such as increased active β-catenin.42, 43 Further, keloid keratinocytes have increased rates of migration in vitro, and decreased cell–cell contacts in vivo.43, 54 These data are consistent with the notion that keloids represent a pathological over-healing response, with cells stalled in the proliferative phase of wound repair in a perpetual partial EMT-like state. Hypothetically, restoring the appropriate ‘stop signals’ to promote transition to the remodelling phase of healing may suppress keloid formation.
Consistent with over-healing, keloids have been reported to display elevated levels of cytokines, immune cells and immune mediators.55 Tissue injury induces an acute inflammatory response that involves local release of chemotactic factors and recruitment of immune cells, including neutrophils and macrophages, which initially function to reduce invasion of pathogenic microbes. Dysregulation of the inflammatory response can lead to alterations in wound healing, including chronic, non-healing wounds or excessive scarring. Keloids have been shown to exhibit increased numbers of macrophages, T lymphocytes and mast cells, and these cells have intrinsic abnormalities that may contribute to fibrosis.4, 55 For example, macrophages in keloids display higher cytokine expression than normal skin macrophages, including higher TGF-β1 expression, and a shift in macrophage phenotype towards M2 versus M1.56 M2 macrophages promote activation of fibroblasts via production of TGF-β1, which can lead to conversion of fibroblasts to myofibroblasts and increased ECM production. Additionally, regulatory T cells (Tregs) are also elevated in keloids, which may stimulate collagen production in fibroblasts.55 While it is generally accepted that excessive and persistent inflammation contributes to keloid formation, the drivers of this dysregulated inflammation are not fully understood. Interestingly, a recent investigation of wound healing in reindeer, which exhibit fibrotic scarring in back skin but scarless healing in antler skin (‘velvet’), showed that tissue-resident fibroblasts exhibit site-specific differences that can regulate the inflammatory response to drive scarring or scar-free wound healing.57 This suggests that dermal fibroblasts do not simply respond to inflammatory signals, but can actively regulate scar formation by modulating immune cell recruitment and function. However, what initially drives dermal fibroblasts to promote formation of keloids or hypertrophic scars is not yet known.
1.1 Vitamin D, vitamin D deficiency and the vitamin D receptor
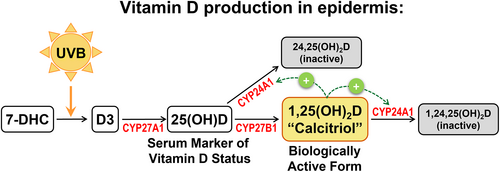
Vitamin D3 (D3) is produced in the skin in response to ultraviolet B (UVB) radiation in sunlight (Figure 2). Epidermal keratinocytes express the enzymes CYP27A1 and CYP27B1 required to convert D3 to 25 hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D and 1,25 dihydroxyvitamin D (1,25(OH)2D), also called calcitriol, the biologically active metabolite, respectively (Figure 2).58 Vitamin D and its metabolites regulate calcium and phosphate homeostasis and thus help regulate musculoskeletal physiology.59 In addition, vitamin D has numerous beneficial effects in the immune system, generally suppressing pro-inflammatory cytokines and stimulating anti-inflammatory cytokines in cells expressing the vitamin D receptor (VDR).60 Vitamin D also has anti-inflammatory activities in skin, and deficiency has been associated with several cutaneous inflammatory diseases.61 Vitamin D has demonstrated anti-fibrotic activities in many cells and tissues, including lung, liver, kidney and skin.62-65 Further, vitamin D has been shown to inhibit EMT in a variety of cell types; this involves induction of target genes involved in cell adhesion and epithelial differentiation, and repression of key inducers of EMT.66-68 Most of the effects of vitamin D are mediated by binding of calcitriol to VDR, a member of the nuclear hormone receptor family of transcription factors. Upon calcitriol binding, VDR translocates to the nucleus where it partners with the Retinoid X Receptor (RXR) and binds vitamin D response elements (VDREs) in target genes to regulate their expression, including a VDRE in the promoter of the VDR gene itself.58 In keratinocytes, calcitriol binding to VDR regulates expression of genes to inhibit proliferation and stimulate differentiation.69 Mutations in genes encoding VDR or CYP27B1, the enzyme that converts 25(OH)D to calcitriol, result in hyperproliferation of basal keratinocytes and defective skin barrier formation, and mice lacking VDR are susceptible to skin tumour formation.70
Circulating 25(OH)D levels <10 ng/mL indicate severe vitamin D deficiency, which may cause skeletal disorders such as rickets.59 Currently, most experts define vitamin D deficiency as <20 ng/mL 25(OH)D, with optimal levels >30 ng/mL suggested for realisation of vitamin D's full benefits.58 Severe vitamin D deficiency is relatively common, and is associated with increased risk of infection, disease and mortality.71 Supplementation with vitamin D is considered relatively safe, with no toxicity observed at daily doses <10,000 IU, which is 5- to10-fold higher than required to increase circulating 25(OH)D levels to 30 ng/mL in most individuals.58 Because of the association of vitamin D deficiency with adverse health outcomes, there have been numerous interventional trials investigating vitamin D replacement or supplementation for various diseases, though results have frequently been disappointing, with many studies failing to show significant benefits. However, these studies are often biased by methodological errors and poor study design: for example, including patients without vitamin D deficiency, using insufficient dosing regimens to achieve beneficial levels, or not monitoring vitamin D supplement intake in control subjects.59, 71-73 Additionally, differences in individual responsiveness to vitamin D supplementation, which may be due in part to genetic differences such as polymorphisms in genes involved in vitamin D related pathways, may confound results of large clinical trials.73 Despite this, several studies that reported no significant decrease in cancer incidence with vitamin D supplementation did show significant reductions in death rates,71 suggesting that vitamin D may serve as an important adjuvant for treatment of disease.
Melanin in pigmented skin blocks UVB, which decreases the amount of UVB reaching keratinocytes and reduces vitamin D3 production,74, 75 contributing to lower circulating levels of 25(OH)D and higher rates of vitamin D deficiency in individuals with darkly pigmented skin.76 In the United States, most Blacks and Hispanics have vitamin D deficiency or insufficiency, with mean circulating 25(OH)D levels of 15 ng/mL and 20 ng/mL, respectively; in contrast, non-Hispanic Whites have mean 25(OH)D levels of 26 ng/mL.59 Non-Hispanic Blacks in the United States are 10 times more likely than non-Hispanic Whites to be vitamin D deficient; Hispanics and other ethnic minorities have 2.5- to 3-fold increased risk.59 The association of lower serum vitamin D levels with darker skin pigmentation has been well-documented in different populations,77-84 but the influence of sun exposure and UVB intensity must be taken into consideration. Africans with dark skin pigmentation living in lower latitudes (e.g., Africa), and thus exposed to more UVB, have higher vitamin D levels than African Americans living in the United States.59, 85 However, serum vitamin D levels are not just influenced by skin pigmentation and solar exposure, as dietary intake is also a major source of vitamin D. Relatively few foods are naturally high in vitamin D, making it difficult to meet the recommended daily allowance without supplementation. Vitamin D fortification of milk and other food sources is one of the most common strategies for increasing circulating 25(OH)D levels, although rates of consumption of fortified foods vary widely among different populations, making it difficult to meet the needs of ethnically diverse populations without fortification of a wider variety of foods.86, 87
1.2 Does vitamin D have a role in keloid disorder?
The high rates of vitamin D deficiency in African Americans, together with the increased incidence of hypertrophic scars and keloids in populations of African descent and the role of vitamin D in regulation of inflammation, led Cooke et al. in 2005 to hypothesise a link between vitamin D and abnormal scar formation.88 Since then, there have been a limited number of clinical studies investigating the potential role of vitamin D in abnormal scar formation (Table 1). In general, studies have shown that patients with keloids tend to have lower circulating vitamin D levels than patients in control groups, and differences in VDR expression have also been observed. In 2013, Yu et al. published a study of Chinese cohorts that compared 261 patients with keloids with a control group comprised of 261 healthy individuals without keloids.89 The authors reported that mean serum vitamin D levels were 15.1 ng/mL for the keloid patients, compared to 20.4 ng/mL for the control group (p < 0.001), and found that a serum vitamin D cut-off of 16.1 ng/mL could discriminate keloid subjects from controls.89 The authors also investigated several single nucleotide polymorphisms in the VDR gene that may modulate expression of VDR. Interestingly, they found that a specific allele of the TaqI polymorphism of VDR, which changes a T to a C located in exon 9 at the 3′ end of the gene but does not change the coding sequence, was closely associated with keloid incidence and carriers of the CC allele of this polymorphism had significantly lower serum vitamin D levels than carriers of other TaqI alleles.89 In a study performed in Indonesia, Damanik et al. enrolled 32 patients with keloids who were ranked according to keloid severity, which was measured using the Vancouver scar scale.90 The authors found a significant negative correlation between 25(OH)D levels and keloid severity, indicating that lower 25(OH)D levels were associated with the most severe keloids.90
| Study authors | Country | Year | Study design | Scar patient population | Control patient population | Main findings |
|---|---|---|---|---|---|---|
| Yu et al.89 | China | 2013 | Case-control | 261 pts (69% female) with keloids; age 19–55 yrs | 261 non-keloid pts (50% female); mean age 20.4 yrs | Keloid pts had significantly lower 25(OH)D versus controls: 15.1 ± 3.4 ng/mL versus 20.4 ± 5.7 ng/mL |
| Damanik et al.90 | Indonesia | 2019 | Cross-sectional analytic | 32 pts (75% female) with keloids; age 16–40 yrs | NA | Pts ranked according to keloid severity; mean 25(OH)D inversely correlated with keloid severity: 12.34 ± 2.61 ng/mL in most severe, 26.95 ± 0.78 ng/mL in least severe |
| El-Hadidi et al.91 | Egypt | 2021 | Case-control | 19 pts (58% female) with keloids; age 6–55 yrs | 20 non-keloid pts (60% female); age 9–53 yrs | Keloid pts had significantly lower 25(OH)D versus controls: 22.79 ± 21.68 ng/mL versus 53.40 ± 32.24 ng/mL; levels of VDR also significantly lower in skin of keloid pts versus controls |
| Ung et al.95 | United Kingdom | 2023 | Cross-sectional population-based cohort study (Biobank) | 972 pts (65.1% female) with excessive scarring (keloid or HTS); mean age 68 ± 8 yrs | 229,106 pts (54.6% female) without excessive scarring; mean age 63 ± 8 yrs | Vitamin D deficiency was ~twice as common in excessive scar cohort vs controls: 5.1% versus 2.4%; note: scar incidence determined based on electronic medical records |
| Correia-Sa et al.106 | Portugal | 2017 | Prospective cohort | 17 females who developed HTS after body contouring surgery; age 20–65 yrs | 46 females who did not develop HTS after body contouring surgery; age 25–60 yrs | Pre-surgery 25(OH)D levels were significantly lower in pts who developed HTS after surgery versus pts who did not develop HTS: 15.46 ng/mL versus 23.52 ng/mL |
| Cho et al.100 | Korea | 2019 | Cross-sectional | 486 burn pts (10% female) with >20% TBSA burns; mean age 37.1 yrs for males, 33.8 yrs for females | NA | Vitamin D deficient pts (mean 25(OH)D 13.3 ng/mL) had longer ICU stay, higher scar melanin, & lower scar elasticity versus non-deficient pts (mean 25(OH)D 24.2 ng/mL); note: all burn scars included (not all diagnosed as HTS) |
| Ince et al.107 | Turkey | 2019 | 2 stage: first stage = observational, second stage = intervention | 84 pts (46% female) with linear HTS; mean age 28.6 yrs | NA | HTS pts had mean 25(OH)D of 16.6 ng/mL; in 2nd stage of study, some pts given vitamin D supplement or vitamin D + surgery or no treatment; vitamin D alone did not improve subsequent scarring |
- Abbreviations: HTS, hypertrophic scars; NA, not applicable; pts, patients; TBSA, total body surface area; yrs, years.
In 2021, El Hadidi et al. performed a case-control study in Egypt that compared 19 patients with keloids with a matched control group and found that vitamin D deficiency was significantly more prevalent in the keloid group compared with controls (68% vs. 10%, respectively).91 Additionally, the mean serum 25(OH)D level was significantly lower in the keloid patients (22.8 ng/mL) compared with controls (53.4 ng/mL).91 Patients in the study had keloids ranging in duration from 1 to 50 years, and there was a negative correlation between the duration of the keloid and serum 25(OH)D level.91 The authors observed no difference in representation of different skin phototypes determined using the Fitzpatrick scale—a scale used to describe skin pigmentation levels—between the keloid and control groups, and no correlation with serum 25(OH)D levels and skin phototype.91 However, the sample size may have been too small, and the level of ethnic diversity of participants too low, to detect any associations with skin pigmentation. El Hadidi et al. also measured levels of VDR protein in tissue extracts from keloid lesions, non-lesional skin of keloid patients and normal skin of controls. They found that VDR protein levels were significantly lower in the keloid patients, in both the lesional keloid tissue samples and the non-lesional ‘normal’ skin biopsies of keloid patients, compared with normal skin of non-keloid control patients.91 Interestingly, despite this finding, there was no statistically significant correlation between serum 25(OH)D levels and VDR tissue levels, although it is not clear whether the sample size was large enough to detect such a correlation.
In 2017, our laboratory examined VDR expression and found it was reduced in 24 keloid lesions compared with normal skin samples from 24 unrelated non-keloid donors.92 Because liganded VDR acts as a transcription factor, we measured nuclear localisation of VDR and found significantly lower VDR nuclear localisation levels in keloid epidermis compared with epidermis of normal skin.92 Further, we observed significantly lower VDR levels and rates of nuclear localisation in normal skin of African American and Black patients compared with normal skin of non-Hispanic White patients, suggesting the possibility that VDR may be involved in the increased keloid susceptibility in patients with dark skin pigmentation.92 Also in 2017, Gong et al. examined expression of VDR in peripheral blood lymphocytes (PBLs) of 236 Chinese keloid patients compared with 219 control patients without keloids.93 They reported significantly reduced levels of VDR mRNA in PBLs of the keloid patients compared with controls, in addition to significantly increased levels of mRNA for the gene encoding plasminogen activator inhibitor 1 (PAI1),93 which was previously shown to be increased in keloid fibroblasts compared with normal skin fibroblasts.94 A limitation of both of these studies is that the vitamin D status of patients was not measured, thus it was not possible to examine any potential correlations between serum vitamin D levels and VDR expression.
Recently, Ung et al. analysed comorbidities present in patients with excessive scarring who participated in a large United Kingdom Biobank study.95 Out of over 230,000 participants with linked primary care records, 972 individuals reported having excessive scarring (keloids or hypertrophic scars). Vitamin D deficiency was reported at about twice the frequency in the excessive scar cohort compared with the non-scar control group.95 Excessive scarring was also found to be significantly associated with atopic eczema,95 which has also been shown to be associated with vitamin D deficiency.96, 97 Interestingly, in this UK Biobank study, subgroup analysis only showed a significant association between excessive scarring and vitamin D deficiency in Asian participants.95 Limitations of this study included the relatively low number of participants reported to exhibit excessive scarring, which was identified based on electronic medical records and included patients with a diagnosis of keloid or hypertrophic scarring, and the fact that most of the participants reported White ethnicity, which may make the findings less generalisable to other ethnic groups that display elevated risks for excessive scar formation.95 Additionally, the rates of vitamin D deficiency in both the excessive scarring group and the unaffected control group (5.1% and 2.4%, respectively) were much lower than rates of vitamin D deficiency in the United States (24.6% for the years 2001–2018),98 which has a more ethnically diverse population.
A small number of studies have examined associations between vitamin D status and hypertrophic scarring. Two studies from Korea examined a large cohort of burn patients with relatively large total body surface area (TBSA) burns (mean ~36% TBSA) and examined scars in wounds after closure.99, 100 The vitamin D status of patients was measured and they were divided into vitamin D deficient (<20 ng/mL 25(OH)D) and non-deficient groups.99, 100 The vast majority of patients were vitamin D deficient (420 out of 486 participants, or 86.5%).100 Vitamin D deficiency is relatively common in burn patients, due in part to the nutritional demands and metabolic derangements of burn injury, as well as prolonged hospitalisation, which limits UV exposure.101 Cho et al. reported that patients with vitamin D deficiency had increased scar pigmentation, decreased epidermal barrier function in scar tissue, and lower scar elasticity and greater scar rigidity compared to non-deficient burn patients.99, 100 A limitation of this study is that the scars were evaluated immediately upon discharge from the hospital to a rehabilitation unit, so the scars were relatively young and no follow-up was reported; thus, it is not known how many of those burn scars may have progressed to form hypertrophic scars or keloids. Interestingly, although there was no difference in percent TBSA burned between the groups, the patients with vitamin D deficiency had a significantly longer length of stay in the intensive care unit (ICU) compared to the non-deficient group.100 Additionally, a more recent study from this group reported a significantly increased length of time to wound healing in burn patients with vitamin D deficiency compared to a non-deficient patient group.102 The risk of hypertrophic scar formation is significantly increased in burn wounds that take longer to heal,103, 104 thus it is possible that vitamin D deficiency may indirectly increase hypertrophic scar formation if it delays time to healing. Further, the synthesis of vitamin D in burn scars is reduced compared with uninjured skin, which may contribute to vitamin D deficiency.105 The relationships between vitamin D status, wound healing and scar formation are complex, particularly in the burn population, making it difficult to tease apart any potential cause-and-effect relationships.
There have been two studies that reported associations between vitamin D deficiency and hypertrophic scar formation in surgical patients. In the first, Correia-Sa et al. examined hypertrophic scar formation in a cohort of 62 patients in Portugal undergoing body-contouring surgery.106 Scars were evaluated at 6 months after surgery, and hypertrophic scars—defined as scars elevated above or beyond the original wound boundaries—were observed in 27% of patients. The patients who developed hypertrophic scars had significantly lower circulating 25(OH)D levels compared to patients who did not develop hypertrophic scars (15.46 vs. 23.52 ng/mL, respectively).106 In another study conducted in Turkey, Ince et al. reported on 84 patients presenting to their outpatient clinic with existing linear hypertrophic scars resulting from prior surgeries on the trunk or extremities.107 They found that these patients were all vitamin D deficient, with mean 25(OH)D levels of 16.6 ng/mL. The authors further studied 50 of these patients in a trial to examine whether vitamin D supplementation could improve scar outcomes. They divided patients into three groups: patients with no further medical or surgical treatment (group 1), patients with vitamin D replacement only (group 2) and patients who underwent surgical scar excision after vitamin D replacement (group 3).107 Although precise circulating 25(OH)D levels after vitamin D replacement were not reported in this study, the authors stated that vitamin D levels in patients in groups 2 and 3 were above 25 ng/mL after 1 month of oral supplementation with 2000 U/day vitamin D. However, vitamin D supplementation alone (group 2) had no significant effect on severity of hypertrophic scarring, which was only improved in group 3 (vitamin D and surgical excision).107 A limitation of this study is the lack of a fourth group receiving only surgery without vitamin D replacement, which may have revealed whether vitamin D can serve as an adjunct to improve the results of surgical scar therapy.
1.3 Vitamin D and keloid disorder: possible mechanisms
A large and growing body of evidence supports a role for vitamin D in inflammation and modulation of immune function, and vitamin D deficiency has been associated with increased systemic levels of inflammatory markers such as interleukin-6 (IL-6).108, 109 Vitamin D has been shown to inhibit production of numerous proinflammatory cytokines and increase production of anti-inflammatory mediators in cells expressing the VDR, including many different immune cell populations.110 Preclinical studies have demonstrated that vitamin D administration can reduce the occurrence or slow the progression of many different immune-related diseases.73, 111 This is consistent with epidemiological evidence of associations between vitamin D deficiency and immune-related diseases and disorders, including type 1 diabetes, multiple sclerosis, psoriasis, rosacea, rheumatoid arthritis, respiratory infections, cardiovascular disease, systemic lupus erythematosus, inflammatory bowel disease and sepsis, in addition to keloid disorder.60, 73, 89, 91, 108, 110, 112-114 Given the widely accepted role of both local and systemic inflammation in keloid disorder,4 it is reasonable to speculate that vitamin D deficiency may contribute to a pro-inflammatory state that is permissive to, or actively promotes, development of keloids.
Vitamin D is a potent regulator of the innate immune system, stimulating the antimicrobial activities of macrophages and monocytes via production of antimicrobial peptides such as cathelicidin.113 Other antimicrobial proteins regulated by vitamin D include two members of the S100A family, psoriasin (S100A7) and koebnerisin (S100A15), which have been found to be overexpressed in peripheral blood mononuclear cells of patients with psoriasis, as well in psoriatic skin lesions, and were proposed to induce expression of pro-inflammatory cytokines.115 Treatment of psoriatic lesions with a vitamin D analogue decreased expression of psoriasin and koebnerisin.116 In addition to increased rates of vitamin D deficiency in psoriasis patients, reduced expression of VDR has been identified in psoriatic lesions, similar to what we and others have observed in keloid lesions.91, 92, 117 Interestingly, psoriasin and koebnerisin were found to be reduced in keloid lesions compared with normal skin, and koebnerisin was reduced in serum of keloid patients compared with controls, where its levels were inversely correlated with vitamin D levels.91, 118 Treatment of fibroblasts in vitro with koebnerisin led to a decrease in expression of collagen, and the combination of psoriasin and koebnerisin synergised to reduce proliferation of fibroblasts, suggesting that although they may be pro-inflammatory in some contexts, they have anti-fibrotic activities in dermal fibroblasts.118 Thus, the decreased expression of koebnerisin observed in keloids91 may be involved in dysregulated ECM production and increased fibroblast proliferation compared with normal skin.
Vitamin D has been shown to display protective effects against endothelial dysfunction, including stimulation of nitric oxide production, inhibition of oxidative stress and suppression of proinflammatory cytokine production in endothelial cells.108 Vitamin D deficiency has been associated with abnormal microvascular endothelial function, which is more prevalent in African Americans compared with other ethnic groups and may contribute to increased risk of cardiovascular disease in this population.119 Endothelial dysfunction has also been found to be associated with dark skin pigmentation, independent of race or ethnicity.109 It was proposed that vitamin D deficiency in darkly pigmented individuals may be a predisposing factor that increases risk of endothelial dysfunction.109 Endothelial dysfunction has also been implicated in keloid disorder; Ogawa and Akaishi proposed that increased vascular permeability caused by endothelial dysfunction may increase local inflammation by allowing increased levels of inflammatory cells and soluble mediators into the tissue.120 A study of vascular function in keloid patients found that they exhibited significantly lower reactive hyperaemia index values and higher augmentation index values, indicators of endothelial function, compared with non-keloid control patients.121 Additionally, two recent single-cell RNA-sequencing studies of keloids identified expansion of vascular endothelial cell populations in keloids and dysregulation of pathways related to angiogenesis, and suggested mesenchymal activation of endothelial cells in keloids.122, 123 Thus, endothelial dysfunction may be one of the avenues that links vitamin D deficiency with keloid pathology.
EMT is another potential mechanism linking vitamin D signalling to keloid disorder. As mentioned above, the process of EMT has been implicated in keloid, as keloids and keloid-derived keratinocytes exhibit features of EMT, including decreased cell adhesion and abnormal epidermal differentiation, as well as expression of markers of EMT such as activated β-catenin.42, 43, 47 In addition to a role in cancer metastasis, EMT has also been implicated in organ fibrosis. As in skin wound healing, processes related to EMT are involved in the repair programme of many different tissue types and EMT is thought to be a source of fibroblasts that take part in tissue regeneration.66 However, dysregulation of this process can lead to fibrosis. Vitamin D signalling via binding to VDR has been shown to inhibit EMT in cells of the kidney, liver, prostate, lung and other organs.66, 124-127 The level of VDR in a cell has been found to modulate the expression of EMT-related genes, with increased VDR levels associated with reduction in key EMT genes and, conversely, silencing of VDR is associated with EMT induction.66 For example, VDR was reduced in a mouse model of chronic kidney disease, and VDR knockdown in cultured kidney cells promoted β-catenin activation and TGF-β1-induced EMT.124 In the mouse chronic kidney disease model, administration of vitamin D restored VDR expression in kidney cells and reduced markers of EMT.124 Further, VDR expression was found to be decreased in diseased tissue from patients with Crohn's disease, and vitamin D deficiency was associated with decreased VDR expression and increased intestinal fibrosis in a mouse Crohn's disease model.128 In the mouse Crohn's model, dietary intervention to increase vitamin D serum levels caused an increase in VDR expression and an accompanying reduction in fibrosis, indicating that vitamin D can regulate the development of intestinal fibrosis by modulating intestinal VDR expression.128 In at least some types of human cancers that undergo EMT, the transcription factors SNAIL1 and SNAIL 2, considered master regulators of EMT, reduce expression of VDR and thereby block the effects of vitamin D treatment on epithelial differentiation. These findings suggest a complex feedback loop balancing the activities of vitamin D signalling via VDR and EMT in regulating cell fate.66 Given the reduction of VDR observed in keloids, it is reasonable to speculate that reduced VDR expression, possibly resulting from or exacerbated by vitamin D deficiency, contributes to the EMT-like phenotype of keloid cells that promotes fibrosis.
Although we do not yet know whether reduced VDR expression in keloid disorder—reported in PBLs, non-lesional skin and keloid lesions of keloid patients91-93—is due to vitamin D deficiency, the observed reduction of nuclear VDR in normal epidermis of African Americans compared with non-Hispanic Whites92 suggests that VDR expression in skin may be related to vitamin D status. However, it is not clear whether vitamin D replacement therapy can restore VDR expression to normal levels. In a rat model, vitamin D deficiency was shown to reduce levels of VDR protein in skin, and supplementation with dietary vitamin D increased skin expression of VDR.129 In humans with atopic dermatitis, clinical studies have demonstrated reduction in severity of symptoms upon vitamin D supplementation, which was also shown to increase expression of VDR in atopic skin lesions.130, 131 Thus, it is hypothetically possible that vitamin D supplementation may increase VDR expression and nuclear localisation in keloid lesions, although this has not yet been demonstrated in patients with keloids.
In addition to its actions in epidermal keratinocytes, vitamin D has also been shown to decrease fibrotic activity in fibroblasts. Anti-fibrotic activities of vitamin D treatment in fibroblasts include reducing collagen synthesis, decreasing collagen gel contraction and/or inhibiting cell proliferation.132-136 For example, treatment of dermal fibroblasts from scleroderma patients with calcitriol decreased proliferation and collagen production133; similar effects were observed upon calcitriol treatment of bone marrow fibroblasts.132 Additionally, vitamin D has been shown to counteract the profibrotic effects of TGF-β1 on collagen production and alpha smooth muscle actin expression.125, 136-138 Zhang et al. found that keloid-derived fibroblasts expressed functional VDR and responded to vitamin D treatment with a dose-dependent reduction in cell proliferation.136 Additionally, calcitriol treatment was able to inhibit TGF-β1-induced increases in expression of type I collagen, fibronectin and alpha smooth muscle actin in keloid fibroblasts.136 Recently, Mamdouh et al. performed a clinical trial to investigate the efficacy of vitamin D injection for reduction of keloid severity, which was rated using the Vancouver Scar Scale and ultrasound.139 Forty patients with keloids 1–5 cm in size were enrolled and keloids were injected with 200,000 IU vitamin D per 1 cm lesion; injections were performed weekly for up to 3–4 sessions. The authors reported that 2 months after conclusion of the treatment regimen, there were significant reductions in mean Vancouver Scar Scale ratings and scar height measured using ultrasound.139 Minor side effects including temporary pain, redness, swelling and tenderness were observed in approximately half of patients, and a majority of patients expressed satisfaction with the results.139 However, limitations of the study include the lack of a control group for comparison, and relatively short follow-up period.139
2 CONCLUSIONS
As detailed above, there have been several clinical and in vitro studies suggesting involvement of vitamin D signalling and the VDR in the development of keloids, and some evidence suggesting a role in HTS development as well. Clinical studies have demonstrated increased rates of vitamin D deficiency in patients with keloids, with reduced circulating 25(OH)D levels in keloid patients compared with non-keloid control patients. Additionally, reduced protein levels and nuclear localisation of VDR have been observed in keloid lesions compared with normal skin, and PBLs of keloid patients have lower VDR mRNA levels than controls, further implicating vitamin D signalling in keloid disorder. The anti-fibrotic, anti-proliferative and anti-inflammatory activities of vitamin D are consistent with a putative role in suppression of keloid fibrosis (Figure 3), and at least one small clinical trial suggests that vitamin D might have some benefits as a keloid therapy. The reduced expression and nuclear localisation of VDR observed in normal skin of African Americans compared with non-Hispanic Whites, and the increased rates of vitamin D deficiency in ethnic populations that also exhibit higher rates of keloid formation, suggest the possibility that vitamin D deficiency may be involved in the increased risk of keloids in populations with dark skin pigmentation. However, genetics are also known to play a role in keloid risk, with different susceptibility loci identified in different populations.26, 29-31, 34 Thus, the basis of the increased risk in skin of colour populations remains unclear, but likely involves a combination of factors that may include vitamin D deficiency (Figure 3).
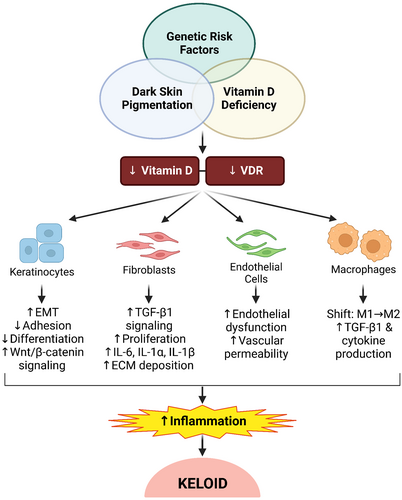
There are still several questions that must be answered before we can fully understand the role of vitamin D and VDR in keloid pathology. For example, we do not yet know whether the levels of VDR expression and nuclear localisation in skin or scar tissue are correlated with patients' circulating 25(OH)D levels, or whether vitamin D supplementation can increase VDR expression and nuclear localisation in skin. It is not clear whether improving patient vitamin D status can suppress abnormal scar formation; given the fact that not all keloid and HTS patients are vitamin D deficient, it is unlikely that vitamin D supplementation alone can serve as a therapy to treat keloids or prevent their occurrence. However, it is possible that maintaining optimal vitamin D levels may reduce the risk of keloid formation in predisposed individuals, and vitamin D supplementation may serve as a useful adjunct to existing keloid therapies to improve their efficacy. Additionally, there may be therapeutic benefit to local administration of vitamin D to keloid tissue. The study by Mamdouh et al. suggests that injection of vitamin D into keloids may help reduce their severity,139 but larger, well-controlled studies are needed, with longer follow-up periods, to determine if vitamin D injections have similar efficacy to other injectable drugs, such as triamcinolone and 5-fluorouracil.10 Finally, understanding how vitamin D signalling through VDR interacts with other signalling pathways to modulate wound healing may help us to elucidate the molecular mechanisms underlying keloid formation, which could help in design of more targeted and effective therapies.
ACKNOWLEDGEMENTS
This work was supported by grant 72005-CIN-21 from Shriners Hospitals for Children.
CONFLICT OF INTEREST STATEMENT
The authors have no financial conflicts of interest related to this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




