A review of the biology, distribution, and management challenges posed by the invasive weed Ziziphus mauritiana L., with special reference to its invasion in Australia
Subject Editor: Stephen Novak, Boise State University, Boise, USA
Abstract
Ziziphus mauritiana is an economically detrimental and environmentally destructive plant in non-native areas where it has escaped cultivation. It forms dense, impenetrable thickets that restrict the movement of livestock across the landscape and has the capacity to alter various ecological functions at the site of invasion, all of which contribute towards land degradation and the reduction of economic profitability. Although there are several management strategies implemented to control Z. mauritiana, it is clear that no single-method approach will effectively control the species in the long-term. Whilst chemical and mechanical methods appear to show promising results, they tend to be restricted to areas that are easily accessible and, even so, can be challenging and laborious to treat evenly across dense thicket areas. Several prospective biological control agents have been identified for Z. mauritiana, although further investigations are required to ascertain the host specificity, and to explore and identify their climatic and environmental suitability of host specific agents for release in non-native regions. Ecological burning alone is not effective in controlling Z. mauritiana and will likely increase its emergence. As such, it could be adopted as part of an integrated management approach to assist other methods for long-term control, but again the development of such an approach requires further investigation. To contribute towards the control of Z. mauritiana, this review explores its biology, distribution and management challenges whilst identifying areas of research that will assist in the long-term and confident control of the species, with an emphasis on its invasion in Australia.
1 INTRODUCTION
Ziziphus mauritiana L. (commonly known as Indian ber, Indian jujube and Chinee apple) is a small tree or large shrub that has been reported to impact negatively on agricultural and native ecosystems in areas where it has escaped deliberate cultivation (Anderson, 1993; Navie, 2004; Pareek, 1983; Parsons & Cuthbertson, 1992). Outside of its cultivated areas, Z. mauritiana can outcompete agricultural and native plant species and form dense, impenetrable thickets that restrict the movement of livestock and wildlife across the landscape (Anderson, 1993; ISSG, 2007; Navie, 2004). Densely infested areas can significantly reduce the carrying capacity of the land, in terms of both crops and pasture, resulting in significant financial costs relating to its management and removal (Anderson, 1993; ISSG, 2007; Navie, 2004; Weeds Australia, 2023). The current management strategies employed to control Z. mauritiana are often limited to the use of herbicides or mechanical methods, which often do not provide effective long-term control and are not applicable to all types of infestations (Grice, 1998; O'Brien et al., 2022; Sellers, 2021; Smith, 1957; Weeds Australia, 2023). Although other methods have been attempted, such as ecological burning or soil cultivation, they are likely to increase the species' emergence within the landscape and require follow-up management, all of which can be costly and laborious (Grice, 1998). As such, this review is designed to explore the biology, distribution and current management of Z. mauritiana with an aim to identify potential research gaps that could assist future environmentally sound management of the species. It is anticipated that this review will assist agricultural departments and communities, land protection officers, land managers and researchers in identifying what are currently the most suitable methods of control and to advise future directions and research opportunities towards the confident control of this invasive species.
2 METHODOLOGY
This review was conducted between April and October 2023, and explored the available global English literature that directly relates to the biology, distribution and management of Z. mauritiana. The literature search was conducted using Google Scholar, utilising the term ‘Ziziphus mauritiana’ plus one or more of the following terms: biology, control, distribution, ecology, impacts, invasive and management. Each paper that was identified during this process that had these key terms within their title and abstract or that were presented as a keyword was then scanned in detail for its suitability for this review.
3 TAXONOMY AND COMMON NAMES
The genus Ziziphus belongs to the Rhamnaceae family which is made up of more than 900 species (Azam-Ali et al., 2006; Maaiden et al., 2020). Although there are approximately 58 accepted species belonging to the Ziziphus genus, there are claims that this number might be much higher, with reports suggesting they range between 86 and 170 individuals, although many of these added species appear to be confused by synonyms and hitherto unresolved names (Azam-Ali et al., 2006; Maaiden et al., 2020). Confusion over the classification of the Ziziphus genus and the species within it, has also arisen due to the high potential for hybridisation of related species and the use of alternative published names for the same species across geographically distinct localities (Azam-Ali et al., 2006; Maaiden et al., 2020). In this regard, there are more than 170 cultivars of Z. mauritiana within India, all of which exhibit slightly different phenotypical variations (Azam-Ali et al., 2006). It has been reported that many of these cultivars may have hybridised with wild-growing populations, ultimately creating greater confusion over the specific species classification (Hocking, 1993). In addition, Z. mauritiana has previously been known as Rhamnus jujuba L, Z. aucheri Boiss, Z. insularis Smith, Z. jujuba L. Gaertn, Z. jujuba Lam, Z. mauritania nom illeg, Z. orthocantha DC, Z. rotundata DC, Z. sonoria Roem and Schult, and Z. tomentosa Poir (Maaiden et al., 2020).
Due to the significant history of its establishment in so many countries, Z. mauritiana is known by a wide range of common names, with the most used English names being Chinee apple, Chinese apple, Chinese fig, Indian Jujube and Jujuba (Pasiecznik, 2007). The species is also known in many countries by regional specific names within Afghanistan (berra), Bangladesh (bozoi, kul), Cambodia (putrea), China (hong tsao, lang tsao, tsao tsao), Ethiopia (abateria, gewa-ortigi), Fiji (baer), India (badari, beri, boroi, ilamda, yelchi), Indonesia (bidara, widara), Kenya (ekalati, olongo), Malaysia (bidara, jujub), Nepal (baer), Pakistan (ber, jujube, kunar), Senegal (dem, djabie, tabi), Spain (azufaifo, jujubier), Sri Lanka (ilanda, masaka), Thailand (ma tan, phusta) Uganda (esiland), Zambia (akasongole, massau) and Zimbabwe (masua) (Azam-Ali et al., 2006; Pasiecznik, 2007).
4 PLANT DESCRIPTION
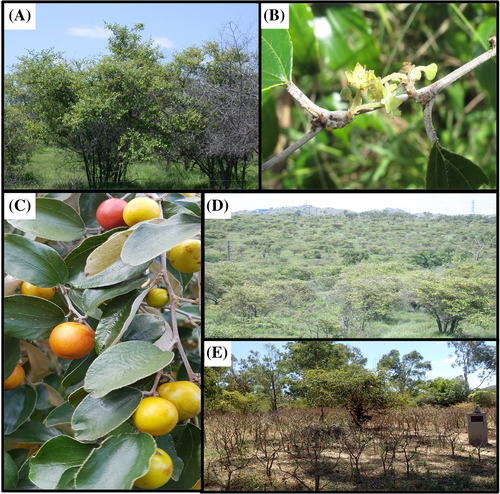
Z. mauritiana is described as a densely branched, small tree or large shrub that commonly grows between 6 and 10 m high (Anderson, 1993; Kleinschmidt & Johnson, 1987; Macoboy, 1982; Navie, 2004; Pareek, 1983; Parsons & Cuthbertson, 1992; Smith, 1957; Wilson, 2000). Large trees contain a single or few stems and are more densely branched compared with juvenile plants, which form a shrub-like appearance (Figure 1) (Anderson, 1993; Kleinschmidt & Johnson, 1987; Navie, 2004; Pareek, 1983; Parsons & Cuthbertson, 1992; Smith, 1957; Wilson, 2000). Each branch grows in a zigzag formation and contains both a leaf and a thorn that extends out on an angle (Kleinschmidt & Johnson, 1987; Pareek, 1983; Parsons & Cuthbertson, 1992; Smith, 1957; Wilson, 2000). These thorns are curved, sharp and grow between 50 and 200 mm in length (Pareek, 1983; Parsons & Cuthbertson, 1992). The leaves are arranged in an alternate pattern, oval to round in shape, and are glossy green in colour with a paler underside, which is covered with fine hairs (Anderson, 1993; Kleinschmidt & Johnson, 1987; Pareek, 1983; Parsons & Cuthbertson, 1992; Smith, 1957). Flowers are small (5–8 mm), arranged in a cyme inflorescence, green to white, hermaphrodite and sometimes emit an unpleasant acidic odour (Azam-Ali et al., 2006; Vashishtha & Pareek, 1979). They form a hypanthium and contain five membranous petals and five triangular sepals and are borne in clusters along the leaf axil (Tel-Zur & Schneider, 2009). Fruits are obovate or oblong, may have a smooth, rough or glossy surface and are pale yellow or green before turning reddish-brown or orange when they mature (Navie, 2004). The fruit capsules contain a singular, hard, oblate stone that can contain one to three elliptical brown flattened seeds that are 6–8 mm long and weigh approximately 46.9 mg (Grice, 1996; Weeds Australia, 2023). Z. mauritiana also has a deep taproot system that can produce new shoots when damaged or under stress (Grice, 1998).
4.1 Life cycle
The life cycle of Z. mauritiana begins with the development of vegetative buds or the germination of its seeds during summer to autumn (Table 1). Leaf and shoot development can occur all year round, although the most active growth period generally occurs from early spring to late autumn (Gupta, 1993; Krishna et al., 2018). In some cases, the species may shed its leaves and enter a dormancy period during extremely hot summer days as an adaptive mechanism to prevent desiccation (Awasthi & More, 2009; Krishna et al., 2018; Macoboy, 1982). During late autumn and winter, the species will drop its leaves and become less active or dormant for a short period before quickly reproducing new shoots and leaves in early spring (Gupta, 1993; Krishna et al., 2018). Flowering occurs during spring and early summer, although the timing of flowering becomes more variable in plants that are subjected to pruning or other horticultural practices (Krishna et al., 2018; Tel-Zur & Schneider, 2009). The emergence of the inflorescence and subsequent flowering is also influenced by the availability of water (Krishna et al., 2018). Fruits develop from spring to early summer, during which time fruits ripen, seeds mature and the fruits disperse (Gupta, 1993).
| Life cycle event | Autumn | Winter | Spring | Summer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active growth period | |||||||||||||
| Flowering | |||||||||||||
| Fruitification | |||||||||||||
| Germination | |||||||||||||
| Least active growth period | |||||||||||||
5 ECONOMIC IMPORTANCE
5.1 Detrimental effects
Outside of its native regions and areas where it is not purposely cultivated, Z. mauritiana can quickly become a troublesome weed. It has caused great concern and impact to the agricultural industry and natural environment within a range of countries such as Australia and Fiji (Anderson, 1993; Bebawi et al., 2002; ISSG, 2007). Its formation of dense, impenetrable stands restricts and prevents the movement of livestock and native wildlife across the landscape (ISSG, 2007). This issue has been commonly observed in parts of Northern Australia, where dense thickets of Z. mauritiana have restricted the movement of livestock such as Bos indicus L. (domesticated cattle) and limited their access to food resources, stockyards and water (Anderson, 1993; Bebawi et al., 2002; ISSG, 2007). These impacts can result in the death, dehydration or malnutrition of the species if left unattended. Z. mauritiana also competes with agricultural and native plant species and has the capacity to alter species assemblages at the site of invasion (Weber, 2003). Beyond its natural habitats and in locations where it is not intentionally grown, Z. mauritiana has the potential to rapidly transform into a major weed (Dhileepan, 2017; Grice, 1998; O'Brien et al., 2022). Research has also shown that dense infestations of Z. mauritiana can provide suitable habitat for a range of pest animal species such as Sus scrofa L. (feral pig), which can further increase the economic and environmental impacts associated with the species (Grice, 1996; Ward-Fear et al., 2016).
5.2 Beneficial effects
Z. mauritiana can provide a range of benefits when it is cultivated and managed appropriately to avoid its spread into unwanted areas (Krishna et al., 2014). In some cases, Z. mauritiana can be an economically important fruit crop in areas with water deficiencies or poor soil conditions (Hocking, 1993; Krishna et al., 2014; Muhammad et al., 2022). Its fruits are rich in fat, fibre and protein, and contain a range of inorganic substances and elements such as calcium, chlorine, magnesium, phosphorus, potassium and sodium (Cheng et al., 2000; Mojtaba et al., 2016; Muhammad et al., 2022; Thanatcha & Pranee, 2011). These fruits are commonly processed into a range of products such as alcoholic beverages, flour, juice or paste, and, in some cases, used as a source of fodder for livestock (Hocking, 1993; Latiff, 1991). The fruits, leaves and stems of Z. mauritiana have also been reported to contain various phytochemical properties and possess several medical benefits, and constituents which can be extracted and used as anti-diarrhoeal, antidepressant, antimicrobial, antioxidant, hepatoprotective and immunomodulatory formulations (Khare, 1995; Kirtikar & Basu, 1994; Latiff, 1991; Prakash et al., 2021). In terms of forming dense thickets, Z. mauritiana is also commonly used (i) for erosion control, (ii) as a hedge row plant on farmlands and (iii) to provide habitat for some native species, although it is more commonly used by pest animals such as Capra hircus L. (Feral goat) and S. scrofa, which can also become invasive across the landscape if they are not managed appropriately (Gupta, 1993; Ward-Fear et al., 2021).
6 GEOGRAPHIC DISTRIBUTION
6.1 Native regions
Due to the long history of cultivation and dispersal of Z. mauritiana, the species occupies a large geographical area (Hooker, 1875; Macoboy, 1982; Pareek, 1983; Parsons & Cuthbertson, 1992). Whilst it is generally reported that Z. mauritiana is native to the Indo-Malaysian region, which extends from the Indian subcontinent to Southeast Asia, Malaysia and Western Indonesia; and it has also been reported to be native to East Africa (Gupta, 1993; Navie, 2004; Pareek, 1983; Parsons & Cuthbertson, 1992). In these regions, Z. mauritiana is often found growing as a horticulturally important plant or a wild growing plant that has escaped cultivation (Anderson, 1993; Jamadar et al., 2009; Navie, 2004). It can be found growing across a wide range of regions from arid and semi-arid landscapes to areas with altitudes of up to 1500 m (Gupta, 1993; Hocking, 1993).
6.2 Introduced regions
As previously noted, Z. mauritiana has been widely introduced to several countries around the world due to its agricultural and horticultural importance and reported pharmaceutical properties (Oshima et al., 2015; Paudel et al., 2023; Prakash et al., 2021; Wunderlin et al., 2021). As a consequence, the species can now be found growing as a cultivated crop or a wild-growing plant across Africa, Asia, North and South America and Oceania (Figure 2). In particular, Z. mauritiana has now been reported as an invasive plant and has spread across several regions within Australia, Egypt, Fiji, Kenya, Iran, Italy, Malaysia, Mozambique, Myanmar, Papua New Guinea, Philippines, Senegal, Spain, Syria, the USA, Zambia and Zimbabwe (Oshima et al., 2015; Pareek, 1983; Weeds Australia, 2023; Wunderlin et al., 2021). The exact year of introduction into these countries is often unknown, although it is believed that the species was introduced into many of these countries during the late 1800s to early 1900s (Weeds Australia, 2023).
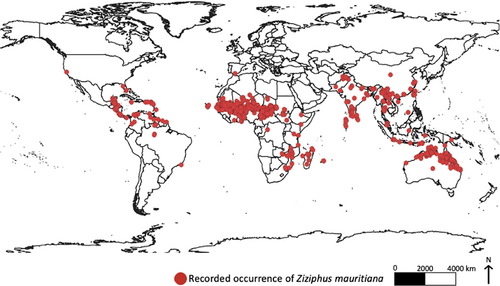
With reference to Australia, Z. mauritiana was purposely introduced into several mining towns across the Northern Territory, Western Australia and Queensland, as a cheap and easy-to-grow food resource (Anderson, 1993; Calvert, 1999; Kleinschmidt & Johnson, 1987). Soon after its introduction, the species was reported to quickly and uncontrollably spread and infest an area of approximately 200 000 ha (Grice, 1998; Hussey et al., 1997; Weeds Australia, 2023). Although Z. mauritiana is mostly restricted to tropical Australia or along waterways, its spread to other regions has been facilitated by a wide diversity of animals that feed on its fruit and unintentionally disperse its seeds across the landscape (Grice, 1996; Hussey et al., 1997; Parsons & Cuthbertson, 1992; Smith, 1957; Weeds Australia, 2023; Wilson, 2000).
Within Ethiopia, Fiji, Zambia, Zimbabwe and several countries across South-East Asia, Z. mauritiana commonly invades agricultural landscapes, roadsides and disturbed areas (Mungate et al., 2018). It is believed that many of the waste products of the fruit, which include its inedible seed, are thrown out, and often establish along roadsides and wastelands (Grice, 1996; Mungate et al., 2018). Z. mauritiana has been reported as one of the earliest domesticated fruit trees around the world and was commonly introduced to several remote mining towns as a cheap and easy-to-grow food resource (Mungate et al., 2018; Paudel et al., 2023).
7 HABITAT AND CLIMATE REQUIREMENTS
Z. mauritiana can be found growing across a wide range of habitat types such as arid to semi-arid regions, marginal farming landscapes, riparian zones and topical or subtropical landscapes (Pareek, 1983; Usman et al., 2023). The species has the capacity to withstand extreme diurnal annual temperatures, high evaporation rates and highly variable precipitation events (Pareek, 1983). It can be found growing across a wide range of soil types such as alluvial soil, clays, gravels and sandy loam soils, with optimum growth more commonly observed within deep sandy to loamy soils (Hocking, 1993; Pareek, 1983; Smith, 1957). In most cases, Z. mauritiana can withstand areas with seasonal waterlogging, moderate levels of salinity and a soil pH between 5.5 and 8.5 (Hocking, 1993; Pareek, 1983; Smith, 1957; Usman et al., 2023). The maximum shade temperature that Z. mauritiana can withstand is 49°C, with the minimum ranging between −5 and 13°C (Hocking, 1993; Kaaria, 1998). On a local scale, Z. mauritiana is commonly found growing in areas with at least 200–300 mm of annual rainfall and will readily establish within disturbed agricultural areas, floodplains, grasslands, open woodlands, roadsides and a wide range of semi-arid to subtropical areas (Grice, 1998; Kleinschmidt & Johnson, 1987; Navie, 2004; Pasiecznik, 2007; Usman et al., 2023; Weeds Australia, 2023).
8 POPULATION DYNAMICS
8.1 Growth and development
Z. mauritiana can live for over 20 years as a wild-growing plant, although research suggests that the species may live for longer in some regions (Grice, 2002). It can reproduce asexually through the development of vegetative reproduction from its underground root system and sexually with the development of its seeds (Anderson, 1993; Pareek, 1983; Weber, 2003). The root system of Z. mauritiana contains a substantial storage of non-structural carbohydrates and is highly drought resistant with the capacity to exhibit osmotic adjustments when soil water moisture is low (Pareek, 1983). These characteristics allow the species to easily produce new shoots and increase its longevity and fitness when soil moisture is low (Arndt et al., 2000; Pareek, 1983).
Temperature plays an important role in the development of Z. mauritiana as it can help to regulate the development and production of its flowers, fruits, roots and stems (Gupta et al., 2015; Mendes et al., 2017). The optimum temperature for its growth and development ranges between 22.2 and 25.4°C, although the development of its fruits will begin to decline significantly when daily temperatures reach above 35°C (Azam-Ali et al., 2006; Meghwal et al., 2007). When temperatures exceed this level, Z. mauritiana will often undergo a period of dormancy to help reduce any damage to the plant and minimise potential water loss (Azam-Ali et al., 2006; Meghwal et al., 2007; Nath & Bhargava, 2000). The maturation of its fruit is also strongly influenced by climatic and weather conditions such as rainfall and temperature, although this has been observed to vary amongst diverse plant populations/cultivars, and requires further investigation to understand the influence of different climatic zones and geographical locations on the growth and development of the species (Hall et al., 2016). This issue is of increasing importance given the effects of ongoing climate change.
8.2 Seed development and germination
Z. mauritiana produces thousands of short-lived flowers each year, which produce a sticky nectar to attracts a high diversity of insects from the orders Coleoptera, Diptera, Hymenoptera and Lepidoptera (Pareek, 1983; Vashishtha & Pareek, 1979). These flowers then produce fleshy fruits that usually contain one to three seeds, but sometimes may contain none (Pareek, 1983; Vashishtha & Pareek, 1979). Mature plants can produce 5000–10 000 fruits each year, although this is strongly influenced by environmental factors such as rainfall and temperature (Calvert, 1999; ISSG, 2007). Within Australia, Z. mauritiana has been observed to start producing fruits when it reaches a height of 1 m, a maturity which may take up to 8 years, although this is highly dependent on the surrounding environmental conditions (Calvert, 1999; ISSG, 2007; Weeds Australia, 2023). Seeds are short-lived within the soil and survive for approximately 2 years before their germination begins to significantly decline (Bebawi et al., 2016; Calvert, 1999; Grice, 1996; ISSG, 2007). A study by Grice (1996) shows that germination is significantly reduced at 6 months (31%) and again at 12 months (20%). Further research by Bebawi et al. (2016) investigated the seed longevity and persistence of Z. mauritiana seeds under a range of soil types, pasture covers and burial depths. The survival of Z. mauritiana seeds was greatly influenced by burial depth, duration and soil type, with surface seeds persisting for longer periods than those buried deeper within the soil (Bebawi et al., 2016). It was also observed that no viable seeds were present at 18–24 months in any of the burial trials, which emphasises the short-lived nature of the seeds (Bebawi et al., 2016). This study also showed that Z. mauritiana seeds were capable of germinating in a range of alternating light and dark temperature regimes from 12/16°C to 36/47°C, with optimal germination occurring at 27/33°C (Bebawi et al., 2016). Seed germination also appeared to cease at the extreme temperature ranges of 6/11°C and 40/52°C, suggesting that temperatures that are too cold or too warm will significantly influence the germination of the species (Bebawi et al., 2016). Although germination may be restricted within these temperature ranges, it still allows germination across a wide range of temperature zones, explaining its ability to grow and expand beyond its current global distribution and establish itself in a wide range of climatic regions (Bebawi et al., 2016).
The woody endocarp that surrounds the seed often imposes self-dormancy and will prevent the seed from germinating (Grice, 1996). However, once this endocarp is removed, often by a disturbance event or animals eating the fleshy layers, germination will increase by over 56% (Grice, 1996). This germination can also be increased to around 70%–90% when seeds are consumed and excreted by livestock and pest animals such as S. scrofa, due to their digestive system breaking down the endocarp (Grice, 1996). On the other hand, seeds that are ingested and excreted by native animals like wallabies in Australia tend to only increase in germination by 7%–46%, suggesting that the chemical reactions and contents of livestock digestion may play a more important role in the spread and increased germination of this species than with native mammals (Grice, 1996). Research by Wijesooriya et al. (2020) also highlights that germination is significantly increased (>53%) when the surrounding seed coat is completely removed. Seed germination is also increased when seeds are subjected to a priming method (Sodimu et al., 2020). A study in Nigeria showed that when Z. mauritiana seeds are scarified or soaked in sulphuric acid or hot water for 20 min, their germination is significantly higher when compared with seeds with no pre-treatment (Sodimu et al., 2020). Greenhouse experiments have also shown that increased salinity reduces seed emergence, germination and the growth of a range of seedling organs such as its leaves, stems and roots (Bhatt et al., 2008). The influence of salinity has also been studied by Ramoliya et al. (2004) who identified that salt concentrations of 10.0 dSm−1 were detrimental to the emergence of Z. mauritiana, although these conditions can be alleviated under heavy rainfall conditions, which have been shown to increase the emergence and establishment of the species. Research by Nagaraju et al. (2017) also showed that seeds exposed to radiation (32 kV and 9 mA for 12 s) will not influence germination success with up to 85%–100% germination being retained after this level.
8.3 Dispersal mechanisms
The distribution and dispersal of Z. mauritiana were initially facilitated by human-induced actions in regions where it was purposely introduced to be cultivated (Maruza et al., 2017; Pareek, 1983; Pareek et al., 2009). However, on a more local scale, the dispersal of fruits and their seeds is facilitated by animals (zoochory) that feed on the fruit and later dispose of the seeds, and water (hydrochory) where seeds are transported by the flow of water (Grice, 1996). Common bird species around the world that feed on Z. mauritiana include Aprosmictus erythropterus Gmelin (red-winged parrots), Calyptorhynchus banksii Latham (red-tailed black cockatoo), Casuarius casuarius johnsonii (cassowary), Platycercus adscitus Latham (pale-headed rosellas) and Scythrops novaehollandiae Latham (channel-billed cuckoos) (Grice, 1996; Smith, 1957). Several animals within Australia that have been observed as a key disperser of Z. mauritiana, include the native Dromaeus novae-hollandiae Latham (Emu) and Macropus agilis J.E. Gray (Agile wallaby), in addition to several livestock and pest animal species such as Camelus dromedarius L. (Camel), C. hircus and S. scrofa (Grice, 1996). Livestock also play a key role in dispersing Z. mauritiana seeds across the landscape. A study in Australia found an average of 17 Z. mauritiana seed endocarps within the dung of B. indicus during fruiting season (Grice, 2002). As a result, livestock such as B. indicus have the potential to disperse large quantities of seed across the landscape and their movement into fields previously free of Z. mauritiana should be monitored for any new populations of this weed emerging.
9 MANAGEMENT STRATEGIES
9.1 Mechanical/physical control
One method that is often used to remove large infestations of Z. mauritiana is the mechanical bulldozing of the top 25 cm of soil (Smith, 1957; Weeds Australia, 2023). The removal of at least 25 cm of soil will reduce the species' chance of re-establishing from its extensive root system and from its shallow seed bank (Weeds Australia, 2023). Although this method may be useful for reducing the above-ground biomass, there is still a chance that the species may regrow from seeds or remnant roots, thus ongoing or integrated approaches are required for long-term success (Weeds Australia, 2023). It is also important to note here that this form of control may not be appropriate in all situations and, in addition, is highly destructive to the top layer of soil with an immediate requirement for the disposal of large volumes of root-infested soil and biomass. In this regard, this method may only be suitable for level agricultural fields, easy-to-access terrain with no rocky outcrops, areas where there are no cultural or heritage significance, or in areas with native vegetation that may be impacted by this method. Whilst research by Grice et al. (1999) shows that the complete removal of Z. mauritiana plants from the environment is the most effective control method for the species, as noted earlier, if some roots are missed or fragments remain within the soil, the plant is likely to re-establish. As a consequence, a combination of methods, which may include a follow-up of herbicide treatment or physically removing the germinated plant, would be required to prevent re-establishment of the species (Weber, 2003).
9.2 Ecological burning
The use of fire as a control technique for Z. mauritiana can be implemented to help control the species in some situations, but research has shown that Z. mauritiana can rapidly sprout and regenerate following a moderate fire event (Grice, 1997; Weber, 2003). In this respect, cooler or less intense fire events will only cause mortality to standing plants less than 50 cm tall (Grice, 1997). On the other hand, whilst more intensive fires have been known to kill larger plants and also any seeds that may be present on the surface of the soil, seeds within the soil are often protected and may not be killed (Grice & Brown, 1996). Nevertheless, since Z. mauritiana has a relatively short seed longevity (Bebawi et al., 2016), frequent intense fire events over several years may help to reduce the soil seed bank, although the efficacy of this approach requires further investigation. Fire has also been shown to increase and trigger seed germination events, although this is a highly variable factor, and it may critically depend on the type and intensity of the fire and the surrounding environmental conditions (Grice, 1997). In this regard, further investigations should examine how the use of fire in combination with other integrated methods could be used to effectively control the species. We expect that information of this sort will provide land managers with more confidence in their aim to exhaust the soil seedbank and reduce the likelihood of the Z. mauritiana regenerating over time. Combinations with burning that could be examined are the use of herbicides, biological control, or mechanical and physical methods.
9.3 Chemical control
Chemical control of Z. mauritiana involves the use of herbicides to reduce or prevent the growth of the species. One method that has been reported to be successful is the combination of a cut stump application which involves cutting the plant at the base stump and directly applying registered herbicides within 15 s (ISSG, 2007; Weeds Australia, 2023). This method has been observed to provide up to 90% success rates when used correctly (ISSG, 2007; Weeds Australia, 2023). Although this method can be expensive and time-consuming, it can be applied at any time of the year and is a useful method in controlling small or isolated populations before they become too difficult to control using this method. Further research has also shown that herbicides containing fluroxypyr (1% solution) or triclopyr (5% solution) can be used to successfully control Z. mauritiana using the basal bark method (Sellers, 2021; Weeds Australia, 2023). Applying a herbicide directly to the actively growing plant via a foliar spray has also been observed as a successful method to control Z. mauritiana (Sellers, 2021; Weeds Australia, 2023). Herbicides commonly used within Australia to control Z. mauritiana either contain or include a combination of the active constituent's aminopyralid, picloram or triclopyr (Grice, 1998; Weeds Australia, 2023). Herbicides consisting of glyphosate can also be used to control the species, although like most herbicides, careful consideration is needed to reduce the impact on surrounding native species and any potential unintended pollution to the soil or surrounding waterways (Sellers, 2021). To minimise these impacts and improve the efficacy of herbicide control, an integrated approach would be more suitable for dense infestations, which may include ecological burning or mechanical control, followed by herbicide application. Future research would be required to investigate the effectiveness of combinations of various management options for the management of Z. mauritiana in Australia and other global localities where the species is listed as invasive.
Another novel approach to controlling Z. mauritiana is the use of stem-implantation of herbicides to help control established and emerging populations (O'Brien et al., 2022). Stem-injection of various synthetic herbicides as capsules resulted in high mortality when plants were treated with (i) aminopyralid (37.5 mg/capsule) combined with metsulfuron-methyl (30 mg/capsule) and (ii) metsulfuron-methyl (330 mg/capsule) and picloram (1000 mg/capsule) (O'Brien et al., 2022). These methods showed continued effectiveness 8 months after the implementation of drill and fill methods using the herbicide Tordon® (200 g/L triclopyr, 100 g/L picloram and 25 g/L aminopyralid) (O'Brien et al., 2022). This method can limit the exposure of herbicide to the environment and the applicant. Further findings from this research also suggest that 100% mortality could be achieved within 15 months after application (O'Brien et al., 2022). Although this method may be effective in controlling isolated or small patches of the species, it may not be economically suitable or successful as a single-use method to control larger infestations.
9.4 Biological control
Biological control can be a cost-effective and long-term weed management strategy that has the potential to be used to control Z. mauritiana (Dhileepan, 2017). Field surveys and literature searches conducted by Dhileepan (2017) identified over 133 phytophagous insect species, 9 phytophagous mite species and 12 plant pathogens that were found on Ziziphus species within its native region. A majority of these species are leaf-feeders, which account for 72% of the identified species, whilst others feed on all parts of the plant or exclusively on its bark, fruit, seeds, or stems (Dhileepan, 2017). Prospective biological agents that may be suitable for Australian include phytophagous insects such as Aubeus himalayanus Voss (a seed-feeding weevil), Phyllodiplosis jujubae Gover & Bakhshi (a leaf-galling midge), Phyllonorycter iochrysis Meyrick (a leaf-feeding gracillariid moth), Platypria erinaceus Fabricius (a leaf-mining chrysomelid beetle), Silvestriola jujubae Chandra (a stem-galling midge), Synclera univocalis Walker (a leaf-folding crambid moth), Aceria cernuus Masse (a gall-forming eriophyid mite) and Larvacarus transitans Ewing (a gall-mite); and a fungal pathogen Pseudoidium ziziphi J.M Chen & C.C Wang (Balikai, 2009; Dhileepan, 2017; Kalaichelvan et al., 2004; Nizamani et al., 2015). Of the identified agents, A. himalayanus, A cernuus and L. transitans are considered the most suitable for the Northern regions of Australia (Dhileepan, 2017). Although these prospective biological agents have the potential to control Z. mauritiana, further investigation would be essential to ascertain if they are highly host specific and do not pose any risks to any non-target species including the conspecific Z. jujuba, a prospective crop in Australia, and the two closely related Australian native species Z. oenoplia L. Mill (jackal jujube) and Z. quadrilocularis F.Muell (mardarrgu) (Dhileepan, 2017; Grice, 2002). Further studies are also needed to ascertain the climatic suitability of any prospective agents for establishment in the arid northern Australian environment. The value of this information will help to improve the targeted control of Z. mauritiana and ensure that resources are not directed towards agents that may be unsuccessful in this climate regime.
9.5 Preventative strategies
It is claimed that preventing the spread and establishment of Z. mauritiana is one of the most successful and cost-effective measures to reduce its economic and environmental impact (Grice, 1998). However, with Z. mauritiana occupying large areas and, in some cases, remote and difficult-to-access regions, the early detection of this species in new areas may be a challenge. One strategy that could be more widely used for the detection of this species is the use of unmanned aerial surveillance using drones (Roslim et al., 2021). The use of this technology would improve the response time and identification of emerging populations and allow land managers to quickly control the species before it establishes an uncontrollable spread pattern. When moving cattle from an infested area to an area with no Z. mauritiana, it is advisable to keep cattle within paddocks until all the feed (including seeds of Z. mauritiana) from the infested properties are excreted (Grice, 1998). As such, the careful monitoring of cattle paddocks is recommended to be regularly conducted in order to identify any emerging Z. mauritiana plants. These should then be controlled in the early stages of their growth to facilitate improved control. If monitoring and early detection protocols, in addition to general farmland biosecurity measures, are put in place, then the spread and impact of Z. mauritiana can be reduced in regions where it can become seriously invasive and widespread, and consequently difficult to control. Ecological restoration of an invaded site must also be considered upon completing a control measure for Z. mauritiana. This will help to prevent the re-establishment of the species or other invasive plants from establishing at the control site. For example, methods that may assist in the prevention of re-establishment include (i) introducing native plants at a large-scale to suppress emerging weeds, (ii) improving soil conditions and limit disturbed or bare soil and (iii) ensuring follow up treatments are conducted at each site to eliminate large infestations from redeveloping (Young & Hamerlynck, 2022). For greater confidence in using native species to suppress Z. mauritiana, more localised investigations will be required to find suitable species for specific regions.
10 CONCLUSION
Z. mauritiana has the potential to cause significant, long-lasting negative impacts on agricultural and natural environments in regions where it has escaped cultivation and has not been appropriately managed. The current global literature indicates that no single method can effectively control an infestation of Z. mauritiana in the long-term and the success of a certain method may depend on the specific climatic or environmental conditions of that region. This suggests that searching for a general method of control will not be successful, and considerable thought should be given to the environment and context of the infestation before attempting to institute control and mitigation strategies. A potential approach to address this issue is to classify the nature of the infestation into three levels: (i) areas currently not infested but at risk from surrounding known infestations, (ii) infestations in areas that are easily assessable (such as agricultural land or open landscapes) and (iii) infestations in difficult to access areas. The first level will require preventative control measures such as good farm hygiene, early detection and rapid response protocols, and continued ecological restoration and maintenance of the site to minimise disturbance and potential establishment. This stage will also benefit from directed herbicide use or cut-and-paint methods to physically remove newly emerging plants. The second level will benefit from chemical control, mechanical control or the removal of the top 25 cm of the soil. As mentioned, these methods alone will not be effective in the long-term control of the species, thus an integration of methods may be necessary for several years. The third level will require a more complex and dynamic approach. In most cases, mechanical control will be unavailable, and, although ecological burning may be useful in reducing the above-ground biomass, it will need to be followed up with other methods such as biological or chemical control. Although this approach is certainly promising, it would nevertheless require further investigation in extensive and remote regions where Z. mauritiana has become an established invader. Thus, the overall impact and spread of Z. mauritiana can possibly be reduced if ongoing management activities appropriate to the infested area are conducted. To assist in this work, it is recommended that the research gaps identified as a result of this literature review are explored further.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Michael McBain for creating Figure 2. Open access publishing facilitated by Federation University Australia, as part of the Wiley - Federation University Australia agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
This paper serves as a comprehensive review, where in the authors have synthesised information from previously published materials. It is important to note that the authors did not conduct any reanalysis of the data presented in those papers. All the relevant papers utilised in this review are listed in the reference section, ensuring transparency and accessibility for readers seeking additional details.




