First report of glyphosate resistance in an Amaranthus palmeri population from Europe
Subject Editor: Maor Matzrafi Volcani Institute, Ramat-Yishay, Israel
Abstract
The invasive weed Amaranthus palmeri is spreading throughout Spain, with Catalonia being one of the most affected regions. For this species, acetolactate synthase-inhibiting herbicide-resistant populations have been reported, and now glyphosate resistance is also suspected. Glyphosate targets and inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), but A. palmeri has evolved different resistant mechanisms leading to plant survival. One of the most effective is the EPSPS overexpression due to copy number variation (CNV). Gene copies accumulate within the EPSPS cassette that is an extrachromosomal circular DNA displaying unique structural polymorphisms. This study aims to determine the response to glyphosate of a suspected resistant population collected from a roadside and investigate the resistance mechanism involved. The herbicide bioassay confirmed that 40% of the plants survived glyphosate applied at 540 g a.i. ha−1. No known mutations endowing glyphosate resistance were found at EPSPS amongst confirmed resistant plants, while in most of them (70%) specific molecular markers revealed the presence of the EPSPS cassette. All these results indicate that this population is glyphosate resistant and it is very likely that the EPSPS gene CNV is the main resistance mechanism. This is the first case of glyphosate resistance in A. palmeri in Europe whose introduction is likely due to importation of contaminated seed with glyphosate-resistant Palmer amaranth from the Americas. This introduction poses a significant danger to summer crops in our continent.
1 INTRODUCTION
Amaranthus palmeri S. Watson (Palmer amaranth) is the most globally troublesome weed in agricultural lands and the most threatening species in summer row crops such as soybean, cotton or maize (Palma-Bautista et al., 2019). The most remarkable biological traits that explain its invasiveness and competitive abilities are rapid growth, huge reproductive capacity, high genetic and phenotypic plasticity (Chaudhari et al., 2017), but no less so, its proneness to evolve herbicide resistance to several sites of action (Heap, 2022). This species is native to the Sonoran Desert in south-west North America (Ehleringer, 1983), and first it spread across North America, and afterwards, to Central and South America, specifically in Argentina and Brazil (Kaundun et al., 2019). Across the American continent, glyphosate-resistant biotypes are the most frequently reported. Several resistance mechanisms can confer glyphosate resistance in A. palmeri. Although point mutations in the enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene (Dominguez-Valenzuela et al., 2017) and reduced absorption and translocation (Palma-Bautista et al., 2019) have been reported in some biotypes, the most common resistance mechanism is overexpression of the EPSPS (Gaines et al., 2010), target of the herbicide. The overexpression is due to copy number variation (CNV): an extrachromosomal circular DNA (eccDNA), consisting of 72 putative genes and an autonomous replication sequence (Molin et al., 2017), is capable of accumulating multiple copies (from one to thousands) of the EPSPS gene (Koo et al., 2018, 2021). Primers specifically designed to recognise this EPSPS cassette can be used to detect this resistance mechanism (Molin et al., 2018).
Unfortunately, A. palmeri has become an invasive weed in other continents such as Africa, Asia and Europe (EPPO, 2020). In Europe, its presence has been reported in several countries (Roberts & Florentine, 2022), even if as a ‘casual alien’ or at least ‘naturalised’ in non-agricultural habitats in Spain (Recasens & Conesa, 2011) and Italy (Iamonico, 2015). Moreover, in these two countries, the presence of several biotypes resistant to acetolactate synthase inhibitors has already been confirmed (Milani et al., 2021; Torra et al., 2020). Since A. palmeri is a non-native, recently introduced weed and without a long history of herbicide selection pressure in these countries, the introduction of already-resistant biotypes remained the most likely hypothesis.
This research aims to provide a preliminary description of a Spanish A. palmeri population suspected of being resistant to glyphosate and to investigate the resistance mechanism involved through the amplification of some EPSPS cassette markers and EPSPS sequencing. To our knowledge, this is the first report of a European glyphosate-resistant A. palmeri biotype. The confirmation of glyphosate-resistant Palmer amaranth in Spain needs to be properly managed and communicated to farmers and all stakeholders to make them aware and to encourage the adoption of appropriate practices to limit the spread of this troublesome weed within the European Union.
2 MATERIALS AND METHODS
2.1 Plant material
In 2021, an A. palmeri population (TA) was found on a roadside near Tarragona harbour (41°6′N 1°13′E) and suspected of being glyphosate resistant, since it survived application with glyphosate, one of the most frequently used herbicides for roadside weed control in that area. Mature seeds were collected from at least 20 female plants by the Unit of Weed Science of the Plant Protection Service of the Generalitat de Catalunya, Spain, and the whole plant herbicide assays were performed at the University of Leida, Spain (41.629°N, 0.598°E). A susceptible (S) A. palmeri population from Georgia (USA, 32°09′N 85°52′E) was also used (Bravo et al., 2017). Seeds of the TA and S populations were sown in aluminium trays with peat and placed in a greenhouse at 33/20°C day/night temperatures and a 16/8 h photoperiod. After 4 days, seedlings were transplanted into plastic pots (8 × 8 × 8 cm3) filled with a mixture of silty loam soil and peat (1:1 by volume).
2.2 Herbicide bioassay
When the seedlings reached the three- to four-leaf stage (BBCH 13–14; Hess et al., 1997), the EPSPS inhibitor glyphosate (Roundup®, SC, 360 g a.i. L−1, Bayer Cropscience), HRAC/WSSA Group 9, was applied at the field rate of 540 g a.i. ha−1 (minimum registered dose). A total of 25 replicates (one pot with one seedling) were included. This experiment was conducted twice in the same conditions. Herbicide was applied using a precision bench sprayer with a boom equipped with two flat fan 110° opening nozzles (Hardi ISO LD-110-02), operating at a forward speed of 0.9 m s−1 and delivering 200 L ha−1 at a pressure of 215 kPa. Pots were maintained in the greenhouse at 33°C during the day (16 h light) and 20°C at night (8 h) and regularly watered. Four weeks after treatment, the glyphosate survival rate (% in respect to untreated control) was evaluated for each pot and population. Plants without green tissues were classified as dead.
2.3 TAP-IVS domain sequencing and EPSPS amplification cassette detection
2.3.1 DNA extraction
Genomic DNA (gDNA) was extracted from leaf punches sampled from plants of the TA population having survived at 540 g a.i. ha−1 of glyphosate and from untreated plants of the S (10 and 2 plants, respectively). DNA extraction was performed as previously described (Milani et al., 2021) using a cetrimonium bromide (CTAB) protocol (Aras & Cansaran, 2006). In brief, 2–3 fresh leaves were collected from each plant, left to air-dry in paper bags and leaf punches of about 1 cm2 were transferred into a 2-mL tube (with two disposable stainless-steel beads of 4 mm diameter) and ground to a paste at room temperature with a TissueLyser II (Qiagen, Hilden, Germany), with no buffer. CTAB protocol was then used to extract gDNA. The quantity and quality of gDNA were determined by gel electrophoresis and analysis with a Nanodrop (Thermo Fisher Scientific, Waltham, MA).
2.3.2 EPSP mutations at TAP domain
The pair of primers EGF (5′-ATGTTGGACGCTCTCAGAACTCTTGGT-3′) and EGR (5′-TGAATTTCCTCCAGCAACGGCAA-3′) (Gaines et al., 2010) was used to amplify a 195-bp fragment of EPSPS containing the conserved domain where all described mutations of this gene have so far been observed (TAP-IVS) (Perotti et al., 2019).
Polymerase chain reaction (PCR) was performed using GoTaq® G2 Hot Start Polymerase (Promega, Madison, WI) in a 25 μL mixture including 5 μL of 5X Green GoTaq Flexi Buffer, dNTPs mix (0.2 mM each), MgCl2 (1.5 mM), forward and reverse primers (0.2 μM), 0.125 μL GoTaq DNA Polymerase (5 U μL−1) and 10 ng of gDNA. Amplification conditions: 2 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 58°C, 20 s at 72°C; 5 min at 72°C. PCR products were purified using NucleoSpin® Gel and PCR Clean-up kit (Macherey-Nagel GmbH & Co., Duren, Germany) following the manufacturer's instructions, and the F strands were Sanger-sequenced by BMR Genomics (Padova, Italy). The sequences were edited with FinchTV 1.4.0.
2.3.3 EPSPS cassette
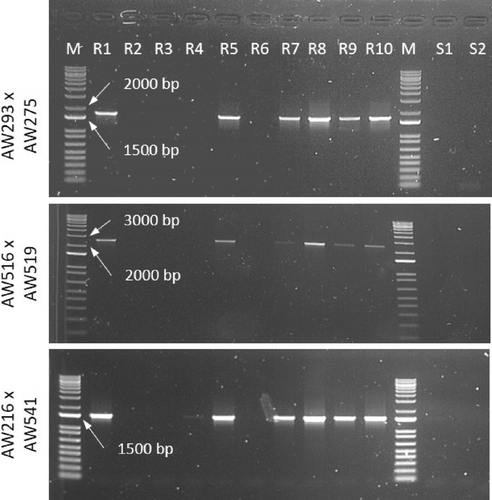
The presence of the EPSPS cassette was investigated as described recently by Molin et al. (2018) on the same 10 plants analysed for mutations (see Section 2.3.2), plus two plants of the susceptible control. Three primer pairs were used: AW293 × AW275, AW516 × AW519 and AW216 × AW541. The expected size of the amplicons was 1757, 2532 and 1544 bp, respectively. PCRs were performed as described for the EPSPS sequencing with the Promega reagents, but with a final volume of 15 μL and increasing the elongation time up to 2′30″. Amplicons were purified and sequenced as described above. The specificity of amplifications was firstly checked by Sanger-sequencing three amplicons of a single sample. Since a few samples did not amplify with the above-mentioned protocol, a second attempt was made with different conditions. These PCRs were performed using Advantage 2 Polymerase Mix (Takara Bio, Otsu, Shiga, Japan), in a 15 μL mixture including 1.5 μL of 10X Advantage 2 PCR Buffer, dNTPs mix (0.2 mM each), forward and reverse primers (0.2 μM), 0.3 μL Advantage 2 Polymerase Mix and 6 ng DNA. Amplification conditions: 1 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 58°C, 3 min at 68°C; 3 min at 68°C.
3 RESULTS
3.1 Herbicide bioassay
At the minimum recommended field dose of 540 g a.i. ha−1, the S biotype was totally controlled, no plants survived except the untreated plant on the right (Figure 1A). Conversely, 10 out of 25 treated plants of the TA population survived and had a phenotype similar to the untreated plant on the right (Figure 1B).

3.2 EPSPS gene mutation
Amplified EPSP fragments were obtained with primers EGF and EGR for both the susceptible and resistant plants, apart from the R2 sample. Sanger sequencing revealed that none of the sequences had a point mutation at the TAP domain.
3.3 EPSPS amplification cassette detection
None of the tested primer pairs specific for the EPSPS cassette gave amplification bands with susceptible samples, as expected (Figure 2, Samples S1 and S2). Six samples of the population TA showed robust amplification with two primer pairs, AW293 × AW275 and AW216 × AW541, and, except for sample R7, also with the primer pair AW516 × AW519. Sequencing confirmed that the amplifications were specific.
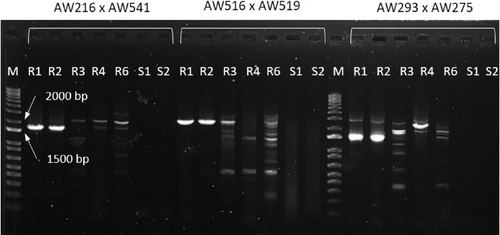
The four samples that did not give any amplification band by using the Promega reagents (namely, R2, R3, R4, and R6) were also amplified with the Takara Bio reagents, together with a positive control (Sample R1) and the two susceptible samples (S1 and S2). Only Sample R2 gave bands of the expected size (Figure 3).
4 DISCUSSION
This research provides the first evidence of a glyphosate-resistant biotype of A. palmeri in Europe. No amino acid substitution was found in the EPSPS codons known to confer glyphosate resistance in A. palmeri (Thr102, Arg103 and Pro106, called TAP) (Perotti et al., 2019) and other Amaranthus species (Heap, 2022). So, there is no evidence of target site resistance, but we cannot exclude the presence of mutation endowing resistance in other part of the EPSPS gene, not considered in this study. Conversely, TSR by means of mutation has seldom been found, for example, in a single population from Mexico (Dominguez-Valenzuela et al., 2017) and one from Argentina (Kaundun et al., 2019).
Since 7 out of 10 plants that survived the glyphosate treatment at 540 g a.i. ha−1 had robust PCR amplification of the EPSPS cassette markers, the main resistance mechanism of TA population is the overexpression of EPSPS due to copy number variation, according to previous research (Molin et al., 2018). A recent paper (A. Shimono et al., 2020) showed that Japanese A. palmeri plants having the marker for the EPSPS cassette also have EPSPS overexpression, thus indicating that this marker can be used as evidence of the resistance mechanism presence. A peculiar characteristic of the EPSPS cassette is its non-mendelian pattern of inheritance (Koo et al., 2021), apparently due to an unpredictable extensive somatic mosaicism for EPSPS copy number, which leads to a variable transmission of copies from one generation to the next, but also to different shoots on the same plant (Giacomini et al., 2019). This happens because most of the eccDNAs are extrachromosomal elements that are randomly anchored to the chromosomes at mitotic metaphases and therefore randomly inherited by daughter cells. Therefore, tissues where the frequency of cells having the eccDNAs with the EPSPS cassette is low, could result negative to PCR amplification targeting the cassette. This might be the reason why a few plants of the TA population that survived the glyphosate treatment resulted negative to the EPSPS amplification. Since a single plant resulted negative with one Taq polymerase, but positive with a different Taq polymerase, it is also possible that different plants might have specific polymorphisms or chromatin structure that cause lower primer efficiencies and limit the power of the assay.
Despite the limitations of the assay, our data suggest that EPSPS overexpression is the main resistance mechanism in this Spanish population. However, considering that in 3 out of 10 plants neither the EPSPS cassette nor mutations were found, we cannot exclude the presence of another resistance mechanism, possibly non-target-site mediated.
EPSPS gene amplification has been reported as the most common glyphosate resistance mechanism in A. palmeri in the United States (Gaines et al., 2010) and has recently been found to be the resistance mechanism in a multi-resistant population from the Republic of South Africa (Reinhardt et al., 2022).
The TA population comes from a roadside near the commercial harbour in Tarragona. Therefore, it is likely that this population resistant to glyphosate was originated from Palmer amaranth contamination in imported grain in the port. Herbicide-resistant weed seeds have been reported as contaminants in commercial grain landing ports throughout Japan (Y. Shimono et al., 2010; A. Shimono et al., 2020) and Amaranthaceae was found to be the most frequent weed family (Ikeda et al., 2022). The small size of the population and the limited area invaded indicate a recent unintentionally introduction of A. palmeri seeds. The finding of an EPSPS cassette through EPSPS-specific marker assays indicates that the eccDNA-based mechanism found in the Spanish population is the same mechanism known to confer glyphosate resistance in US populations. Additional sequencing and comparison of the EPSPS cassette from glyphosate-resistant Palmer amaranth populations from the United States and Spain would be needed to definitely confirm that the eccDNA-based mechanism have occurred because of a recent introduction in Spain.
In summary, this is the first occurrence and demonstration of resistance to glyphosate in the troublesome weed A. palmeri in Europe. The molecular data suggest that the EPSPS gene amplification is the resistance mechanism in this roadside population, although other mechanisms that remain to be investigated may also exist.
A. palmeri is establishing in Spain and is becoming a threat to agriculture. There is a need to raise awareness of the menace among all the stakeholders and encourage them to continually monitor its spread and undertake prevention and management actions. Management strategies should combine non-chemical control with herbicide treatments, always mixing glyphosate with other modes of action to restrict this invasive weed species and prevent it from spreading along roadsides and ultimately infesting cultivated areas.
AUTHOR CONTRIBUTION
All authors contributed equally to this study.
ACKNOWLEDGEMENTS
This work has been supported by the Spanish State Research Agency, Spain (AEI) and the European Regional Development Fund, EU (ERDF) through the projects AGL2017-83325-C4-2-R and PID2020-113229RB-C42. 2021-2024. Joel Torra acknowledges support from the Spanish Ministry of Science, Innovation, and Universities (grant Ramon y Cajal RYC2018-023866-I). This project has received funding from the European Union's H2020 research and innovation program under Marie Sklodowska-Curie grant agreement No 801586. The authors are grateful to Alison Garside for revising the English text. The authors also thank two anonymous reviewers for their helpful comments on the draft of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are available on request from the corresponding author.




