Phosphorylation and growth inhibitory activity of all ketohexose analogs
Abstract
Sugars are not only important energy sources and structural components, but they also act as signaling molecules that are involved in specific signal sensing. Among all ketohexose analogs (d-fructose, d-psicose, d-tagatose, d-sorbose, l-fructose, l-psicose, l-tagatose and l-sorbose), only d-psicose inhibited the growth of Arabidopsis roots. Phosphorylation by fructokinase occurred in d-fructose and d-psicose. d-Psicose-induced inhibition was relieved by adding d-fructose. Thus, the inhibition could not be attributed to the toxicity of phosphorylated d-psicose. The phosphorylation process requires ATP. After phosphorylation, d-fructose is metabolized in glycolysis and becomes energy sources and structural components, whereas d-psicose cannot contribute to the energy sources and structural components because it does not get metabolized further. However, d-psicose did not affect ATP level in the Arabidopsis roots, suggesting that the d-psicose-induced growth inhibition may not be related to the starvation of ATP. The phosphorylation of ketohexoses by fructokinase is known to a trigger signal-sensing resulting in growth inhibition. Therefore, d-psicose can be phosphorylated by fructokinase and this process may play a possible role in signal-sensing. This is probably one of the useful model systems for the study of the hexokinase-independent sugar-sensing function and for developing new types of weed-control agents.
Sugars are not only important energy sources and structural components, but they also act as potent signaling molecules. The control of sugar metabolism, growth and development, stress and gene expression has long been thought to be metabolic effects. However, observations of the control of gene expression with partially metabolizable sugars suggest the involvement of specific signal-sensing and transduction mechanisms that do not require sugar catabolism (e.g. Jang & Sheen 1997; Loreti et al. 2001; Koch 2004; Rolland et al. 2006; Rognoni et al. 2007). The first plant sugar sensor identified was the hexokinase1 (HXK1) protein, which acts as a sensor when the phosphorylation process of sugar by HXK1 occurs (Roitsch et al. 1995; Jang & Sheen 1997; Rolland et al. 2006; Hanson & Smeekens 2009). This protein is multifunctional, being both a glycolytic enzyme and a glucose sensor. As a sensor, HXK1 regulates the gene expression involved in many essential processes, including photosynthesis, respiration, starch and sucrose synthesis and degradation, cell cycle regulation and plant growth and development (Jang & Sheen 1994; Smeekens & Rook 1997; Umemura et al. 1998; Pego et al. 2000; Pourtau et al. 2006). In addition, the phosphorylation of sugar by HXK1 was reported to trigger a signal cascade resulting in the growth inhibition (Pego et al. 1999; Rolland et al. 2006).
Fructokinases are also reported to be involved in sugar sensing (Pego & Smeekens 2000; Kato-Noguchi et al. 2005; Rolland et al. 2006). However, the sensing function by fructokinase is still largely unknown. The first step of sugar sensing could be the phosphorylation process of sugars (Roitsch et al. 1995; Jang & Sheen 1997; Rolland et al. 2006; Hanson & Smeekens 2009). The objective of this study was to evaluate the possible involvement of all ketohexose analogs in the specific sugar sensing that results in growth inhibition. Thus, the effects of all ketohexose analogs (d-fructose, d-psicose, d-tagatose, d-sorbose, l-fructose, l-psicose, l-tagatose and l-sorbose) on the growth of Arabidopsis roots were determined. In addition, the phosphorylation of those ketohexoses by fructokinase was analyzed and discussed whether these ketohexoses act as signaling molecules involved in the sensing resulting in growth inhibition.
Materials and Methods
Sugars
d-Fructose and d-mannitol were obtained from Sigma (St Louis, MO, USA), while d-psicose, d-tagatose, d-sorbose, l-fructose, l-psicose, l-tagatose and l-sorbose (Fig. 1) were supplied by Rare Sugar Research Center (Miki, Japan).
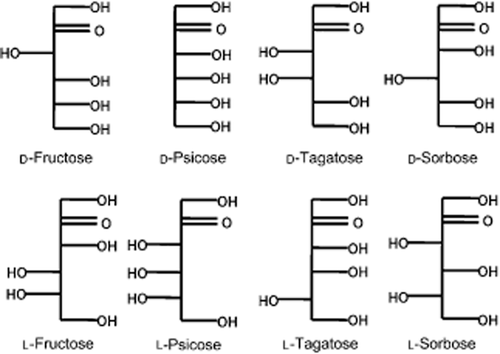
Structure of the ketohexoses.
Plant material and treatment
Seeds of Arabidopsis thaliana (L.) ecotype Columbia were surface-sterilized in a 2% (w/v) solution of sodium hypochlorite for 15 min and rinsed four times in distilled water. The seeds were incubated on two sheets of moist filter paper (No. 1; Toyo, Tokyo, Japan) at 4°C for 3 days and at 22°C with a 12 h photoperiod of an additional day to break their dormancy. Thirty seeds were then transferred to 5 cm Petri dishes, each containing two sheets of filter paper moistened with 3 mL of MES buffer (1 mol L−1; pH 6.5) containing d-fructose, d-psicose, d-tagatose, d-sorbose, l-fructose, l-psicose, l-tagatose, l-sorbose or d-mannitol, and grown in darkness at 22°C. The control seedlings were incubated with the MES buffer. After 3 days, the length of the Arabidopsis roots was measured and the percentage length was determined by reference to the length of the control roots. For quantification of ATP, whole roots of the seedlings were harvested, frozen immediately in liquid N2 and stored at −80°C until extraction. This experiment was repeated five times with 30 plants for each determination. Significant differences between the treatment plants and the control plants were examined by Welch's t-test.
Partial purification of fructokinase
Fructokinase was extracted and purified from tomato fruit (20 fruits, obtained from the local market) according to the procedure described by Schaffer and Petreikov (1997) because its purification process is well established. In addition, the amino acid sequences of fructokinase were highly conserved between tomato and Arabidopsis (Pego & Smeekens 2000). The precipitated crude protein fraction was then resuspended in the extraction buffer, desalted on a Sephadex G-25 column (Sigma-Aldrich, St. Louis, USA) and applied to a DEAE-Sepharose column. The column was eluted with the solvent of a 0–0.5 mol L−1 KCl salt gradient. The eluted fractions (5 mL each) were collected and fructose-phosphorylating activity of the fractions was measured as described below. Then, 2 mmol L−1 d-fructose was used for the substrate instead of fructokinase. The most active fraction was desalted and reapplied to the DEAE-Sepharose column, as described above.
Phosphorylation assay
For the assay of ketohexose phosphorylation of fructokinase, the production of NAD was monitored by an enzyme-linked assay (Schaffer & Petreikov 1997). The reaction mixture (1 mL) contained 50 mmol mL−1 HEPES-NaOH (pH 7.5), 1 mmol mL−1 EDTA, 5 mmol mL−1 MgCl2, 20 mmol mL−1 KCl, 0.5 mmol mL−1 NADH, 1 mmol mL−1 ATP, 1 mmol mL-1 phosphoenolpyruvate, two units of lactate dehydrogenase (Sigma-Aldrich), two units of pyruvate kinase (Sigma-Aldrich) and two units of partially purified fructokinase, as described above. Then, the reactions were started by adding 2 mmol mL−1 d-fructose, d-psicose, d-tagatose, d-sorbose, l-fructose, l-psicose, l-tagatose or l-sorbose and incubating at 30°C for 15 min. The NAD production rate was monitored spectrophotometrically at 340 nm. The phosphorylation of the ketohexoses was expressed as a percentage of the phosphorylation of d-fructose. This experiment was repeated five times and four assays for each determination were carried out.
Determination of ATP
The frozen roots of the seedlings as harvested above, were placed in a mortar containing liquid N2 and ground to a fine powder with a pestle. The powdered tissue was homogenized with five volumes of ice-cold 0.4 mol L−1 HClO4 and the homogenate was kept for 30 min in an ice bath, with occasional shaking. Then, the mixture was centrifuged at 30,000 g for 15 min at 4°C and the supernatant was neutralized with 5 mol L−1 K2CO3. The precipitated potassium perchlorate was removed by centrifugation (30,000 g for 5 min) and the supernatant was used for analyzing ATP, which was quantified spectrophotometrically according to the methods described by Bergmeyer (1985). This experiment was repeated five times and four assays for each determination were carried out.
Results
Effects of eight ketohexoses on root growth of Arabidopsis
Effects of the eight ketohexoses on the root growth of Arabidopsis were determined (Figs 1, 2). Only d-psicose inhibited the root growth of Arabidopsis. Like other metabolizable sugars (Rook et al. 1998; Baskin et al. 2001), d-fructose increased the root growth slightly (significantly at P < 0.05). The other ketohexoses did not affect the root growth of Arabidopsis. d-Psicose inhibited the growth at concentrations >3 mmol L−1 and the level of inhibition increased with an increasing concentration (Fig. 3). In contrast, d-mannitol showed no effect on the growth of the Arabidopsis roots at ≤100 mmol L−1 (Fig. 3). The growth inhibition of d-psicose was relieved by the addition of d-fructose to the medium (Table 1), which is consistent with results that HXK1-involved growth inhibition was relieved by adding metabolizable sugars (Pego et al. 1999; Baskin et al. 2001).
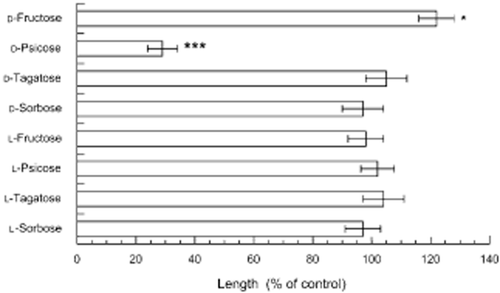
Effects of ketohexoses on the growth of the Arabidopsis roots. Means ± standard errors from five independent experiments with 30 plants for each determination are shown. The control root length was 12 ± 2.1 mm. *P < 0.05; ***P < 0.001.
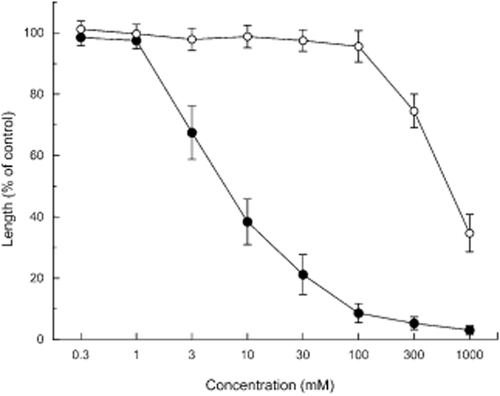
Effects of d-psicose (●) and d-mannitol (○) on the growth of the Arabidopsis roots. Means ± standard errors from five independent experiments with 30 plants for each determination are shown. The control root length was 12 ± 2.1 mm.
| Ketohexose | Root length (% of control) |
|---|---|
| d-Fructose | 121 ± 7.1 |
| d-Psicose | 34 ± 5.7 |
| d-Psicose + d-fructose | 97 ± 7.9 |
- Seedlings were incubated with 30 mmol L−1 d-psicose in the presence and absence of 30 mmol L−1 d-fructose for 3 days. The control root length was 12 ± 2.1 mm (mean ± standard error).
Phosphorylation of ketohexose and the ATP level
The phosphorylation of eight ketohexoses by fructokinase was determined. d-Fructose and d-psicose were phosphorylated by fructokinase but the phosphorylation rate of d-psicose was 34 ± 4.1% (mean ± standard error) that of d-fructose. The other ketohexoses were not phosphorylated.
Concentrations of ATP in the d-psicose-treated and d-fructose-treated seedlings and control seedlings were determined. There was no significant difference in the ATP levels between these ketohexose-treated roots and the control roots (Table 2). Concentrations of ATP in the other ketohexose-treated roots were also not significantly different from that of the control roots (data not shown).
| Ketohexose | ATP concentration (pmol per root) |
|---|---|
| d-Psicose | 1070 ± 160 |
| d-Fructose | 1210 ± 170 |
| Control | 1180 ± 190 |
- Seedlings were incubated with 30 mmol L−1 d-psicose or d-fructose. Means ± standard errors from five independent experiments with three assays for each experiment are shown.
Discussion
Among all ketohexose analogs (Fig. 1), only d-psicose inhibited the growth of the Arabidopsis roots (Fig. 2). The inhibition was not related to osmotic effects because d-mannitol, which was used as an osmotic regulation agent, showed no effect on the Arabidopsis roots at a concentration of ≤100 mmol L−1 (Fig. 3).
Fructokinase has been reported to have high substrate specificity (Renz & Stitt 1993; Pego & Smeekens 2000). In support of this view, only d-fructose and d-psicose were phosphorylated. The phosphorylation was partially inhibited by the orientation of the hydroxyl on C-3 (Fig. 1). Two other ketohexoses, d-tagatose and d-sorbose (the orientation of the hydroxyl on C-4), were not phosphorylated, indicating that trans-positioning of the hydroxyl on C-4 is a requirement for the reaction. The four other ketohexoses, l-fructose, l-psicose, l-tagatose and l-sorbose (the orientation of the hydroxyl on C-5), also did not react, indicating that trans-positioning of the hydroxyl on C-5 is a requirement for the reaction (Fig. 1). Therefore, the trans-positioning of the hydroxyl on both C-4 and C-5 is a requirement for the phosphorylation.
d-Fructose after phosphorylation is metabolized in glycolysis and becomes energy sources and structural components, while d-psicose is not metabolizable after phosphorylation (Myers & Matheson 1994; Matheson & Myers 1998). Therefore, d-psicose does not contribute to the energy sources for ATP production and structural components. The concomitant accumulation of phosphorylated d-psicose in the seedlings may have occurred with the phosphorylation. However, the d-psicose-induced inhibition was relieved by adding d-fructose to the medium (Table 1). This result suggests that the growth inhibition may not be caused by the toxicity of the accumulation of phosphorylated d-psicose and d-psicose, nor by their interruption in fructose metabolism. It is also possible that ATP starvation causes the growth inhibition because the phosphorylation process of d-psicose requires ATP. However, d-psicose did not affect the ATP level in the Arabidopsis roots (Table 2). Therefore, the d-psicose-induced growth inhibition may not be related to the starvation of ATP nor to the toxicity of phosphorylated d-psicose.
It has become apparent that sugars act as physiological signals to repress or activate the plant genes involved in many essential processes (Jang & Sheen 1994; Smeekens & Rook 1997; Umemura et al. 1998; Pego et al. 2000; Rolland et al. 2006; Rognoni et al. 2007). In general, when sugar concentrations in plants increase, the genes involved in photosynthesis and in the mobilization of carbohydrates are repressed and the genes required for storing carbon metabolites for further use are induced (Koch 1996).
The HXK1 protein is known to be a sensor when the phosphorylation process occurs and the HXK1 sensing signal controls several gene expressions, including the genes involved in seed germination and seedling growth (Jang & Sheen 1997; Rolland et al. 2006; Hanson & Smeekens 2009). The hexokinase substrate, d-mannose, was phosphorylated by HXK1 and inhibited the growth of Arabidopsis roots (Pego et al. 1999; Baskin et al. 2001). In the present experiments, d-psicose also inhibited the root growth of Arabidopsis (Fig. 2). Thus, the phosphorylation process of d-psicose by fructokinase may induce some signaling in the growth inhibition of the Arabidopsis roots. It also was reported that d-psicose caused an up-regulation of many defense-related genes in rice (Kano et al. 2011).
Sucrose is a major form of carbohydrate that is transported by the vascular system and is cleaved by either invertase or sucrose synthase; free d-fructose is a product of both reactions. d-Fructose then is phosphorylated by fructokinase because its affinity for d-fructose is much higher than that of hexokinase (Renz & Stitt 1993; Pego & Smeekens 2000). Although d-fructose and d-glucose have similar molecular structures and are highly interconvertible in glycolysis, many plant cells respond differently to d-fructose and d-glucose with respect to uptake, conversion, growth and respiration (Gertlowski & Petersen 1993; Krook et al. 2000). In addition, the suppression of fructokinase activity has been shown to disturb the sugar metabolism required for the proper development of xylem and to reduce water transport (German et al. 2003). The gin2 mutants of Arabidopsis are insensitive to d-glucose but are still sensitive to d-fructose and sucrose (Rolland et al. 2006). It was also reported that the genes encoding the chloroplast triose phosphate translocator were repressed by sucrose, but activated by d-fructose (Knight & Gray 1994). Therefore, all available data suggest that, although the sensing function by fructokinase is still largely unknown, fructokinases may trigger a signal cascade and act as sugar-sensing proteins (Pego & Smeekens 2000; Kato-Noguchi et al. 2005; Rolland et al. 2006; Li et al. 2011). The present results indicate that, among all ketohexose analogs, d-fructose and d-psicose were phosphorylated by fructokinases and that d-psicose may play a role in the signal sensing resulting in growth inhibition. d-Psicose-induced growth inhibition is one of the useful model systems for studies of the hexokinase-independent sugar-sensing function and for developing new types of weed-control agents.
Acknowledgments
The authors are grateful to Dr Ken Izumori (Rare Sugar Research Center, Kagawa, Japan) for providing the sugars.




