Biocompatibility of allogenic canine fascia lata: In vitro evaluation and small case series
Funding information: Veterinary Transplant Services Inc., Kent, WA; Western University clinical partnership grant
Abstract
Objective
To evaluate the biocompatibility of canine fascia lata (FL) in vitro and after FL allograft implantation in dogs with clinical disease.
Study design
In vitro experiment and small case series.
Sample population
Six dogs treated with allogenic freeze-dried FL.
Methods
Fibroblasts were cultured on disks of FL, polypropylene mesh (PM; negative control), and porcine small intestinal submucosa (SIS; positive control). Constructs were compared at 3, 7, and 14 days for water content, DNA amounts, scanning electron microscopy, and histology.
Records of dogs treated with FL allografts with follow-up examination were reviewed for signalment, indication for surgery, surgical procedure, and outcomes. All owners were invited to complete a standardized questionnaire for long-term follow-up.
Results
Water content was greater in FL and SIS than in PM (P = .03). Fascia lata constructs contained more DNA compared with PM constructs at days 7 and 14 (P < .05), whereas SIS constructs did not differ from FL or PM. Fibroblasts appeared spherical and distributed throughout FL constructs, whereas they appeared stellate and remained on the surface of SIS and PM. Fascia lata allografts were implanted in six dogs with surgical conditions. No incisional complications were noted. All dogs had good to excellent long-term outcomes, except one that experienced recurrence of a perineal hernia 2 years after repair.
Conclusion
In vitro, canine FL allowed attachment and proliferation of fibroblasts throughout layers of the graft. Canine allogenic FL was clinically well tolerated in this small population of dogs.
Clinical significance
Allogenic FL is biocompatible and can be considered an alternative to SIS for soft tissue augmentation in dogs.
Video Abstract
Biocompatibility of allogenic canine fascia lata: In vitro evaluation and small case series
by El‐Taliawi et al.1 INTRODUCTION
Soft tissue substitutes are used to augment repairs or guide tissue regeneration in a variety of soft tissue, orthopedic, oral and neurological procedures.1-4 Although autogenous tissue is preferred to promote healing of tension free closures, especially in contaminated areas, limited supply or altered biomechanical properties of native tissues justify alternative approaches.5 Among these, polypropylene mesh (PM) is the most widely used synthetic material to repair soft tissue defects. However, implantation of PM has been associated with complications such as granulomatous foreign body reaction, inflammation, increased wound contraction and seroma formation, infections due to biofilm formation and intestinal adhesions, and obstruction as well as erosion and formation of fistulas when used in visceral applications.6-12 These limitations have prompted the search for alternatives, such as biological decellularized scaffolds derived from native tissues.
One of the most studied biological scaffolds in small animals consists of porcine small intestinal submucosa (SIS).13 Small intestinal submucosa has been reported to enhance cell adhesion and angiogenesis and to elicit an integrative host response. These properties have been attributed to the collagenous extracellular matrix, hormones and growth factors contained in SIS.10, 14-20 The main limitation of SIS relates to its biomechanical weakness because both single and multilaminate SIS have lower tensile strength and resistance to suture pullout than PM and canine fascia lata (FL).21
In this context, FL seems appealing as an alternative biological scaffold to SIS because of its superior biomechanical properties.21 Autogenous FL was first used in human patients in 1901 to treat inguinal hernias.22 Shortly thereafter, Parys et al23 described its use to correct congenital ptosis. Since then, autogenous FL has been used in various ophthalmologic procedures, tympanoplasty, as a sling in the treatment of facial paralysis, and to reconstruct injured anterior cruciate ligament (ACL).23-27 Today, FL is most commonly used as a suburethral sling to treat urinary incontinence in women.2 Other reported uses of allogenic FL in human patients include sacral colpopexy for pelvic organ prolapse, abdominal wall reconstruction, duraplasty in neurosurgery, and guided tissue and bone regeneration in dental applications.4, 28, 29 Orthopedic applications include ACL reconstruction; ankle, hip, and shoulder suspension; and other ligament and tendon repairs.3
In dogs, autogenous FL harvested from the thigh has been successfully transplanted to treat perineal hernias and urethral defects.30, 31 Fascia lata was reported to integrate adjacent tissue without substantial tissue reaction on histological examination of a dog with perineal hernia.30 Atalan et al31 found no evidence of inflammation or complications on gross examination of dogs with urethral defects, and the FL-lined graft survived in all dogs. However, lameness of the donor limb was the most frequent complication related to autogenous FL transplantation.30 Allogenic canine FL has recently become commercially available (Fascia-VTS; Veterinary Transplant Services, Kent, Washington) to reduce surgical time and eliminate donor site morbidity. The product however, has limited experimental data in canines. One study involving implantation of allogenic canine FL into the stifle joint reported no biocompatibility issue, but more research is warranted.32
The objective of this study was to assess the biocompatibility of canine FL in vitro and after implantation in dogs with clinical disease. We hypothesized that cell attachment and proliferation would not differ between FL and SIS in vitro and would be decreased on PM. Our second hypothesis was that dogs treated with allogenic FL would not exhibit clinical evidence of a foreign body reaction to the implanted FL allograft.
2 MATERIALS AND METHODS
2.1 In vitro evaluation
2.1.1 Scaffolds
Three commercially available scaffolds were evaluated: PM (Ethicon, Somerville, New Jersey), canine lyophilized FL (Veterinary Transplant Services), and multilayer porcine SIS (Vetrix, Cumming, Georgia). All scaffolds were cut into 1-cm-diameter discs with a biopsy punch (Acuderm, Fort Lauderdale, Florida) and sterilized by gamma irradiation.
2.1.2 Cell culture
Mouse skin fibroblasts derived from Mus dunni (Clone III8C) were obtained from American Type Culture Collection (ATCC; Manassas, Virginia) and were maintained in ATCC-formulated McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS) and 0.1% antimicrobials (10 000 IU/mL penicillin, 10 000 μg/mL streptomycin, and 25 μg/mL amphotericin B) at 37°C, 95% humidity, and 5% CO2.
Cells were seeded onto scaffolds by using the drop-seeding method.33 Briefly, the scaffolds were placed in a non–tissue culture treated 48-well plate (Corning, Corning, New York), soaked in FBS, and incubated overnight at 37°C. This type of plate was selected to prevent cell attachment to the bottom of the wells. On the next day, FBS was removed, and cells were seeded at 5 × 105 cells/cm2 in 20 μL of medium. After 1 hour of incubation, 500 μL of medium was added into each well, and cells were maintained at 37°C, 95% humidity, and 5% CO2. Constructs were collected at days 3, 7 and 14 postseeding.
2.1.3 Water content and DNA quantitation
Constructs were lyophilized with FreeZone 2.5 liter benchtop freeze dry system (LABCONCO, Kansas City, Missouri) by using a freeze-drying technique.34 Samples were weighed before and after lyophilization to determine water content by using a previously reported technique.34 The DNA content of constructs was then measured after digestion in papain (Sigma, St Louis, Missouri) for 4 h at 65°C with the Hoechst 33258 assay, as previously reported.35
2.1.4 Scanning electron microscopy
Constructs were fixed with 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, Pennsylvania) in 0.1 M sodium cacodylate (Sigma) at 4°C for 2 h, postfixed in 1% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M sodium cacodylate for 90 minutes, and dehydrated in gradient ethanol (37%–100%). The samples were placed in hexamethyldisilazane (Electron Microscopy Sciences) for 45 minutes for critical point drying prior to drying under a fume hood. The fixed samples were sputter coated with iridium in a vacuum coater and imaged under a scanning electron microscope (SEM; FEI Magellan3 400 L, Hillsboro, Oregon) at 5.0 kV accelerating voltage and 50 pA probe current. Cell morphology and distribution across the scaffolds were examined at the superficial surface of the scaffold in contact with medium.34
2.1.5 Histopathology
Constructs cultured for 14 days were collected and fixed in 4% paraformaldehyde. Samples of SIS and FL were embedded in paraffin and stained with hematoxylin and eosin stain, while samples of PM were embedded in plastic and stained with Stevenel's blue stain, which is optimal for this type of material.36 Slides were examined to evaluate cell morphology, distribution within constructs, and integrity of the scaffold.
2.1.6 Statistical analysis
All experiments were performed in triplicate (n = 3/scaffold type/culture period: total N = 27 samples), and data are presented as mean ± SD. The water content of FL, SIS, and PM constructs at days 3, 7, 14 was averaged and compared between groups with analysis of variance (ANOVA), followed by Tukey's test for multiple comparisons. DNA amounts were compared between FL, SIS, PM constructs at each time point with ANOVA followed by Tukey's test. DNA amounts of each construct across time points were compared with repeated-measure ANOVA, followed by a Sidak test for multiple comparisons. Statistical significance was set as P < .05, and trends were considered as 0.05 ≤ P < .1.
2.2 Case series
Medical records from a multidisciplinary specialty practice (VCA Veterinary Specialty Center of Seattle, Lynnwood, Washington) and a veterinary dental practice (Animal Dental Clinic, Lake Oswego, Oregon) were searched to identify dogs that had been treated with FL allografts between May 1, 2014 and May 1, 2017. Dogs were included in the study when follow-up by a board-certified specialist (American College of Veterinary Surgeons diplomate or American Veterinary Dental College diplomate) was available. Signalment, indication for surgery, surgical procedure, and clinical outcomes were extracted from the records. A questionnaire was sent to all owners for long-term follow-up (approximately 2 years postoperatively). The questionnaire (Appendix S1) included the Canine Brief Pain Inventory (CBPI) as a validated measure of function, chronic pain, and quality of life.37 Questions targeting client-specific outcome measures were added to assess the outcomes of patients treated for nonorthopedic conditions.38
3 RESULTS
3.1 In Vitro evaluation
3.1.1 Water and DNA content
Fascia lata (78.65 ± 4%, P < .01) and SIS (78.25 ± 6.02%, P < .01) constructs contained more water compared with PM (56.17 ± 14.81%). At day 3, FL and SIS contained more DNA compared with PM (P = .02, Figure 1). At day 7, FL contained more DNA compared with PM (P = .03), while the DNA content of SIS did not differ from that of FL (P = .28) and PM (P = .38) constructs. At day 14, FL contained more DNA compared with PM (P = .03), while DNA content of SIS did not differ from that of FL (P = .20) and PM (P = .58) constructs.
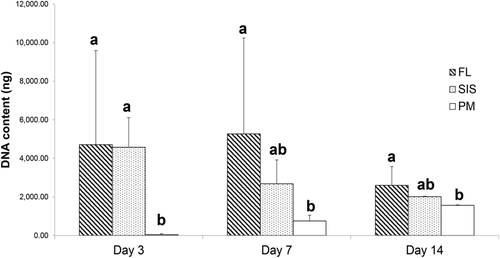
The DNA content of FL did not differ between time points. The DNA content of SIS constructs decreased between days 3 and 7 (P = .02) and remained unchanged from day 7 to day 14. On the other hand, DNA contained in PM constructs increased from day 3 to day 7 (P < .01) and from day 7 to day 14 (P < .01; Figure 1).
3.1.2 Scanning electron microscopic appearance
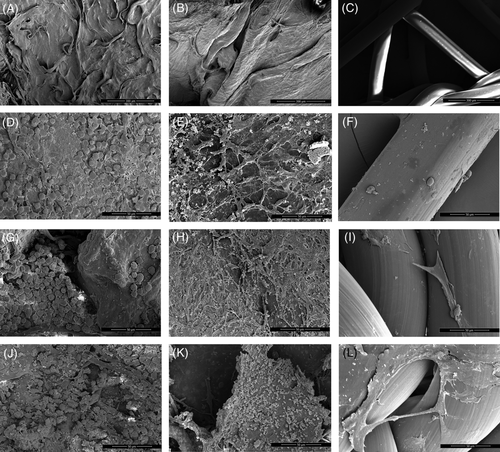
Cells on SIS appeared stellate with prominent cytoplasmic extensions, whereas cells on FL were spherical. Cells on PM were initially spherical but became spindled by day 7, with fewer cytoplasmic extensions than cells on SIS. Cells on SIS and PM also tended to coalesce into sheets, while cells on FL tended to remain more isolated (Figure 2).

At day 3, cells covered most of the top surface of FL and SIS but were sparse on PM. At day 7, cells appeared slightly less abundant on FL and SIS, whereas the cellularity of PM constructs was marginally improved but remained lower than that of SIS and FL constructs. At day 14, cells on FL had a rough surface. Sheets of cells were partially detached from SIS in some areas. Cells expanded on PM, covering a greater surface of the scaffolds (Figure 3).
3.1.3 Histopathology
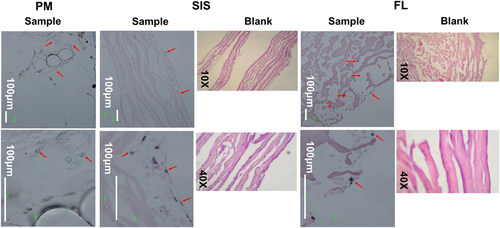
Cells on FL were spherical and remained individually distributed over the surface and cross section of FL constructs. By contrast, cells on SIS and PM were spindle or oval shaped. Cells formed coalescent sheets limited to the surface of SIS in contact with the medium. Cells on PM tended to form aggregates along intersections of woven PM threads (Figure 4).
3.2 Case series
Six dogs met the criteria for inclusion in the study. They weighed an average of 12.4 ± 18.8 kg and were 7.16 ± 3.5 years old. Commercially available canine FL (Fascia-VTS; Veterinary Transplant Services) was implanted in one dog to treat a tendon rupture, implanted in two dogs for herniorrhaphy, and implanted in three dogs undergoing oral surgery for dental/periodontal diseases (Table 1).
| Dog ID | Breed | Sex | Age, y | Weight, kg | Diagnosis |
|---|---|---|---|---|---|
| 1 | Golden retriever mix | MN | 3 | 13 | 2-week-old complete rupture of the right common calcaneal tendon |
| 2 | Welsh corgi | MN | 9 | 13.4 | Left perineal hernia (2-year duration) |
| 3 | Hound mix | MN | 9 | 36.4 | Recurrence after excision of an infiltrative lipoma of the right pelvic diaphragm |
| 4 | Yorkshire terrier | F | 10 | 1.2 | Five-millimeter periodontal pocket on the palatal aspect of maxillary left canine tooth, periodontitis, moderate gingivitis; presumptive hepatic shunt |
| 5 | Yorkshire terrier | MN | 4 | 3 | Seven-millimeter periodontal pocket at the palatal aspect of the maxillary left canine tooth; generalized enamel dysplasia, severe gingivitis with mobile lower incisors |
| 6 | Chihuahua mix | FS | 8 | 7.6 | Six-millimeter periodontal pocket at the palatal aspect of the maxillary left canine tooth; moderate gingivitis |
- Abbreviations: F, female; FL, fascia lata; FS, female spayed; MN, male neutered.
3.2.1 Tendon reconstruction
A 3-year-old male neutered golden retriever mixed breed (Dog 1) presented with an acute non–weight bearing lameness of the right pelvic limb of 2-week duration, after playing with a large dog (Table 1). At examination, hyperflexion of the right tarsus, palpation of a defect between the gastrocnemius complex and tarsus and soft tissue swelling and pain led to a tentative diagnosis of Achilles tendinopathy. A complete rupture of the right common calcaneal tendon was confirmed on ultrasonography. The tendon was repaired through a paramedian lateral surgical approach. The ruptured ends of the gastrocnemius tendon complex were exposed, debrided, and approximated with No. 1 Prolene (Ethicon) in a locking loop pattern. An FL allograft was applied around the ruptured tendon and secured with 2-0 PDS (Ethicon). The lateral retinaculum was apposed with 2-0 PDS. After routine closure of the subcutaneous tissues and skin, the limb was placed in a bivalved fiberglass splint. Meloxicam (1.5 mg orally every 24 h) was administered for 30 days, and codeine sulfate (15 mg orally every 8-12 h) was administered for 10 days. The splint was changed once weekly for 3 weeks and then every 2 weeks thereafter. The splint was replaced by a soft padded bandage at 8 weeks posttreatment, for 4 weeks. Exercise was restricted to short walks on a leash for 8 weeks, and physical therapy was initiated for the following 4 weeks. Dog 1 recovered uneventfully, with no record of fever or incisional complications. The skin incision was healed by 2 weeks posttreatment, at which point sutures were removed. At 12 weeks posttreatment, the dog was weight bearing, and the tendon was intact on palpation, with no swelling or pain around the repair. At 2 years posttreatment, answers of the CBPI indicated absence of pain and return to normal function. Improvement of preoperative signs and satisfaction were ranked highest by the owner.
3.2.2 Herniorrhaphy
Two dogs received FL for soft tissue augmentation during perineal herniorrhaphy (Table 1). Dog 2 had a history of recurrent, intermittent constipation for 2 years. A left perineal hernia was repaired with an internal obturator muscle flap, sutured with one PDS to the sacrotuberous ligament and coccygeus muscle.39 This repair was augmented with a 3 × 5-cm graft of FL secured with 0 PDS. Dog 3 presented with recurrence of an invasive lipoma in the right perineal region. Resection of the recurrent tumor, which on a single lateral projection radiograph measured 10 × 9 cm (height × length), required excision of a large portion of the right side of the pelvic diaphragm. A 3 × 5-cm FL allograft was sutured with 2-0 PDS to the sacrotuberous ligament, remnants of the coccygeus muscle and periosteum of the ischium, to reconstruct the pelvic diaphragm. After perineal herniorrhaphy, both dogs received analgesia (opiates for 10 days, and nonsteroidal anti-inflammation drugs [NSAID] for up to 30 days) and antibiotics (a cephalosporin and metronidazole) for 5 to 8 days. Cold compresses were applied locally for 2 days after surgery, followed by warm compresses for 5 days. Exercise was restricted to short leash walks for 4 weeks.
The incisions healed without complication by 2 weeks posttreatment in both dogs. Both were defecating normally, and their perineal repair was intact on rectal palpation. At 2 years posttreatment, the owner of dog 2 was reported to have had a recent recurrence of the perineal hernia, but the owner was unwilling to return for physical examination. The owner of dog 3 reported an uncomplicated recovery after surgery and normal function at 2 years posttreatment.
3.2.3 Oral procedures
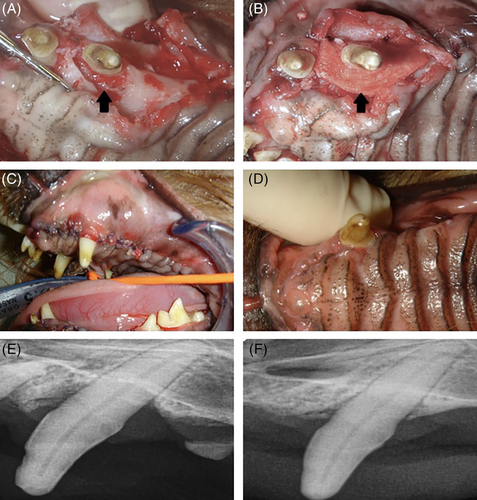
Deep periodontal bone loss on the palatal aspect of the left maxillary canine tooth (204) combined with dental pathology affecting multiple teeth was diagnosed in three middle age to older small breed dogs (Table 1) at physical examination. Dogs were prepared for pocket debridement and guided tissue regeneration with an osseous allograft and FL. Buccal and palatal envelope flaps were created. The 204 palatal pocket was treated with root scaling/planning, pocket curettage, and mild bone smoothing. After the pocket had been cleaned, a bone graft (Periomix; Veterinary Transplant Services) saturated with autogenous blood was placed in the pocket in successive 3-mm layers until the bone graft was even with the palatal bone. An FL allograft was trimmed to closely adapt the graft to the tooth and was sutured over the palatal defect with 4-0 Monocryl (Ethicon; Figure 5). The mucogingival flap was cleaned of granulation tissue and thinned as required. The gingiva was replaced over the graft and sutured around the canine tooth with 4-0 Monocryl. Scaling, subgingival debridement, and polishing of other teeth was completed.
After surgery, analgesia was achieved with buprenorphine (0.036 mg orally every 12 h for 3 days) or tramadol (12.5 mg orally every 8-12 h for 3 days) and meloxicam (0.12-0.75 mg orally every 24 h for 7 days). Antimicrobials were prescribed for 7 to 10 days after surgery, consisting of Clavamox (43.75 mg orally every 12 h for 7 days) or clindamycin (75 mg orally every 12 h for 10 days). Tromethamine ethylenediaminetetraacetic acid chlorhexidine oral solution was provided to rinse the mouth twice daily.
All three dogs treated with an FL graft for periodontal bone loss recovered uneventfully from surgery. No evidence of graft rejection (swelling, pain, inflammation, dehiscence, discharge, fever) was observed at reexamination 2 to 3 weeks after surgery. The flaps remained in place, and oral mucosa seemed to be healing normally. At reevaluation 3 to 10 months after surgery, treated pockets had normal depths, determined by probing. Bone ingrowth was identified on radiographs with bone filling the periodontal pockets (Figure 5).
4 DISCUSSION
Our in vitro and in vivo results provide evidence of biocompatibility of a commercially available, lyophilized canine FL product. in vitro canine FL allowed maintenance and distribution of fibroblasts throughout all layers of the graft. The lack of obvious foreign body reaction after implantation in six dogs regardless of the site supports biocompatibility and justifies additional clinical trials of FL allografts in the dog.
The DNA content and SEM of FL and SIS constructs at day 3 were consistent with robust cell attachment on these scaffolds compared with PM. The limited attachment of cells to PM may reflect its physical structure, offering a small surface area. In addition, the higher water content of FL and SIS may have facilitated cellular attachment; hydrophilic scaffolds have been found to enhance attachment of other cell types.40, 41 Longitudinal changes in DNA content and SEM of constructs were used to assess cell proliferation and distribution after initial attachment. Cell proliferation balanced cell death on FL constructs, as evidenced by a stable DNA content. By contrast, the DNA content on SIS constructs dropped between days 3 and 7, although the surface area of cell-covered scaffold was increased according to SEM. These findings were consistent with a previous study42 and likely reflect cells reaching confluence soon after seeding, with subsequent detachment of cellular sheets from the scaffold. This mechanism was less pronounced on FL because this scaffold likely offered a larger surface area with sieved multilayer structure for cell attachment. Indeed, cells migrated inside this thicker scaffold, as evidenced by results of histologic examinations (Figure 4).
Distribution of cells throughout scaffolds is a prerequisite to successful integration of grafts into surrounding tissues.43 Cells were more broadly distributed in FL than in SIS, where cells formed cellular sheets on surface, or in PM, where cells formed aggregates where woven threads intersected. Cellular distributions on SIS and PM were consistent with those described in previous reports.44-46 The difference in cell distribution between scaffolds may stem from difference in cellular attachment to scaffolds. The spindle or stellate morphology of fibroblasts on SIS and PM was consistent with a stronger attachment to scaffolds, as previously described.44, 47 On the other hand, the spherical morphology of cells on FL would be consistent with a weaker attachment, thereby promoting mobility within scaffolds. Cell distribution throughout scaffolds is desirable, serving as a basis for uniform tissue regeneration in vitro or after in vivo implantation.48 Although they were not tested in our study, fibroblasts adjacent to a surgical site in a live dog would be expected to migrate through the implanted FL, allowing its integration into native tissues.
Freeze-dried allogenic FL was used in our short case series for augmentation of soft tissue repairs in three dogs and for guided bone regeneration in three dogs with deep periodontal pockets. The product used in this study is available in 2.0 × 3.0 cm and 3.0 × 5.0 cm, which was sufficient in all dogs described here. However, these dimensions may limit its application to smaller defects. Allogenic FL has previously been reported to lead to normal function and intact repairs 60 days after treatment of perineal hernias in dogs.49 Successful treatment of a chronic complete traumatic rupture of the common calcaneal tendon in a dog had previously been described with a free autogenous FL graft.50 However, this report is the first to describe the use of allogenic canine FL to treat periodontal pockets. The FL graft was implanted to prevent invasion of the defects by rapidly growing tissue (such as connective and epithelial tissues) and instead, guide regeneration with slower growing tissue (bone).4 In man, membranes commercialized for guided tissue regeneration include resorbable membranes such as biological/natural tissues (usually of collagen type; eg, serosa, tendon, fascia, bone, skin) and synthetic (eg, polyester based and liquid polymers). The most common nonresorbable membranes include polytetrafluoroethylene membranes with or without titanium reinforcing. Resorbable membranes are advantageous because they are biodegradable and do not require removal.4 In dogs, only two biological membranes have been labeled for guided tissue regeneration in dogs, the FL used in our study and a flexible bone membrane (Ossiflex-VTS; Veterinary Transplant Services) reported to restore probing depth in 11 dogs with intrabony periodontal pockets.51, 52
The outcomes after implantation of allogenous FL in dogs add to the in vitro evidence of biocompatibility of this material. The most accepted definition of biocompatibility is provided by Williams53 as “the ability of a material to perform with an appropriate host response in a specific application.” Although all animals presented in this study were treated with NSAID postoperatively, which may have decreased inflammation, the lack of recorded fever, obvious inflammation, swelling, dehiscence, or discharge and consistent suture removal at first postoperative reexamination provide evidence to support the host's acceptance of this material. Allogenic FL is most commonly used in human patients as a suburethral sling, first reported by Handa et al54 to treat female urinary incontinence. In 1999, Fitzgerald et al55 reported the failure of the procedure in 12 patients, raising concern over the biocompatibility of FL. Indeed, most grafts retrieved from these patients had histological evidence of disorganized remodeling and degeneration. A year later, the same authors confirmed the presence of residual donor antigens after both freeze drying and solvent treatments of allogenic FL grafts.56 However, all donor antigens were replaced by host antigens within 1 year after successful treatment of a human patient, prompting the authors to conclude that the antigenicity of FL was not an obstacle to their successful clinical application. This finding is consistent with animal experiments in which FL allografts implanted in rabbit corneas lacked donor fibroblasts after 1 week and were completely populated with host fibroblasts after 3 weeks.56
Our in vitro assessment of biocompatibility is limited to the evaluation of cytocompatibility by direct contact and does not include hemocompatibility or genotoxicity testing. The use of a mouse cell line rather than primary canine fibroblasts could also be considered as a limitation in the in vitro study. The FL is commercialized for use in dogs, and canine fibroblasts may have been more prone to attach and infiltrate allogenic FL. Indeed, human fibroblasts have been found to infiltrate allogenic better than xenogenic dermal matrices.57 The model selected here was therefore probably more stringent for FL compared with the use of canine cells, which would have been allogenic for FL but xenogenic for SIS. Because SIS currently represents the standard of care, we sought to eliminate potential confounding factors between SIS and FL. The main limitations of our case series are inherent to its small sample, lack of standardized follow-up, and, because of its clinical nature, inability to evaluate grafted sites with histology. These limitations prevent conclusions regarding the role of allogenic FL in the success or failure of the surgical procedures reported here.
In conclusion, our results provide details of in vitro cellular characteristics supporting the biocompatibility of allogenic FL and describe some possible clinical applications. Additional studies are required to further define its clinical indications and outcomes in a larger population.
ACKNOWLEDGMENTS
We thank Helen Newman PhD CTBS for providing the fascia lata used in this study; Shanti Jha BVSc MS DACVS; and VCA Veterinary Specialty Center of Seattle for providing access to the medical records of dogs with surgical diseases.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to this report.




