Ultrasonographic quantitative evaluation of acute and chronic renal disease using the renal cortical thickness to aorta ratio in dogs
Abstract
The renal cortical thickness (RCT) has been correlated with renal function. Previous studies have also reported that the RCT:Abdominal aorta(Ao) ratio is constant in normal dogs with various physical factors. This multi-center, retrospective, analytical study aimed to determine if there are differences between actual RCT and predicted value of RCT considering physical factors in dogs with acute or chronic renal disease. We also aimed to demonstrate whether the RCT and Ao ratio index would be useful for evaluating renal pathology. A total of 54 dogs with acute or chronic renal disease and 30 normal healthy dogs were included in this study. The RCT was measured at the center of the renal pyramid as the shortest distance perpendicular to the renal capsule from the base of the renal medullary pyramid at three points. The diameter of the Ao was measured just caudal to the branch of the left renal artery in the sagittal plane in systole. The RCT:Ao ratio of chronic kidney disease (CKD) patients was 0.50 ± 0.11 (mean ± standard deviation). The RCT:Ao ratio in normal dogs was 0.67 ± 0.07. The RCT:Ao ratio in patients with acute kidney injury (AKI) was 0.83 ± 0.05. There was a statistically significant difference between normal dogs and dogs with CKD (P < 0.001) and between normal dogs and dogs with AKI (P < 0.001). In conclusion, findings from the current study supported using the RCT:Ao ratio as a non-invasive quantitative method for characterizing kidney pathology in dogs with acute or chronic renal disease.
Abbreviations
-
- ACKD
-
- acute on chronic kidney disease
-
- AKI
-
- acute kidney injury
-
- Ao
-
- abdominal aorta
-
- BCS
-
- body condition score
-
- BW
-
- body weight
-
- CKD
-
- chronic kidney disease
-
- GFR
-
- glomerular filtration rate
-
- IRIS
-
- International Renal Interest Society
-
- RCT
-
- renal cortical thickness
-
- SD
-
- standard deviation
-
- US
-
- ultrasonography
1 INTRODUCTION
Acute kidney damage is a sudden decline in kidney function that leads to azotemia.1 Because the kidneys, especially cortical structures, are prone to ischemic and toxic damage, ischemia induced by hypotension, shock, or infectious disease results in several metabolic and structural changes within the renal tubular cells, resulting in cortical swelling or infiltration.1, 2 These factors induce reversible or irreversible damage to the kidney.3 Chronic kidney disease (CKD) is characterized by the progressive loss of functioning nephrons caused by irreversible damage, leading to atrophy of the renal cortex.4, 5
The degree of renal injury is assessed by evaluating serum creatinine, blood urea nitrogen (BUN), symmetric dimethylarginine (SDMA), and other serum chemistry parameters such as serum phosphate, calcium, and potassium level. However, these biochemical markers are only increased after a certain percentage of damage to the renal functional mass.6 In addition to the above tests, to assess the concomitant structural change, ultrasound (US) can be used as a versatile tool for evaluating the internal architecture or anatomical information of the kidneys as it is quick and non-invasive.7, 8 Abdominal US has been widely used for assessment of renal size, renal cortical echogenicity, renal pelvis dilation, cyst, and mineralization.7, 9, 10 Typical ultrasonographic changes characterizing CKD include irregular contour, decreased renal size, increased cortical echogenicity, abnormal corticomedullary junction, and pyelectasia.9, 11, 12 On the other hand, in AKI, renal size increase can be seen.13, 14 Although most of these findings are significantly correlated with renal disease, except for renal size, they were not quantitative and can be subjective.15, 16 Also, some of them have shown little correlation to kidney function in previous studies.10, 15
Renal function is often evaluated using the estimated glomerular filtration rate (eGFR).17 Recent studies have shown that renal length and volume are significantly correlated with eGFR.18 However, renal lengths are thought to have a low specificity in predicting renal impairment because they include sinus fat and do not reflect the true sizes of functional tissue.18, 19 Other studies in human medicine have revealed that renal cortical thickness (RCT) better correlates with eGFR than renal length or renal volume.20-23 Evaluation of the renal cortex would be useful because the glomeruli, which act as the actual filtration barrier, are distributed in the cortex as a functional unit whereas, the primary function of the medulla is to maintain urine concentration.24 One study in human medicine described that when the cortical volume decreases, the volume of the medulla increases to some extent as a compensatory mechanism.25 In addition, previous studies in human medicine have shown that renal medullary change is not significantly different between normal and CKD because of altered hemodynamics of the kidney.19, 24 Therefore, RCT measurements are considered important imaging techniques in human patients with renal disease. In veterinary medicine, although there is a normal reference range (3−8 mm) of RCT of the dog or renal length-to-aorta (Ao) ratio (5.5–9.1), these normal reference ranges are too broad to be applied to patients for evaluating renal pathology.26, 27 For dogs, the body type and size between breeds vary28; therefore, an accurate evaluation cannot be made within the broad normal range. This is because renal size is correlated with physical factors, such as body weight (BW), obesity, and height.29, 30 A previous study established a normal reference range for RCTs in dogs considering BW and body condition score (BCS). It revealed that this reference range was a more accurate assessment of normal RCTs than the conventional normal range considering the various physical factors of dogs. In addition, this study demonstrated that the RCT:Ao ratio is constant, regardless of the physical factors of normal dogs.31
Based on these facts, we hypothesized that RCTs of CKD or AKI patients considering BW and BCS would be outside the normal ranges. We also hypothesized that the RCT:Ao ratio would be smaller in CKD patients and larger in AKI patients than in normal dogs. The purpose of our study was to determine whether there is a significant difference in actual RCT and predicted value of RCT considering the BW and BCS of dogs with acute or chronic renal disease. In addition, we aimed to assess the usefulness of the RCT:Ao ratio as a quantitative index reflecting the pathology of the kidney in dogs with acute and chronic renal disease.
2 MATERIALS AND METHODS
2.1 Selection and description of subjects
The study was a multicenter, retrospective, analytical design. The study protocol was approved by the Institutional Animal Care and Use Committee of Jeonbuk National University, Iksan-si, Jeollabuk-do, Republic of Korea (Approval no. JBNU 2021-0103). The electronic medical records and ultrasound image data from four hospitals (Jeonbuk National University Animal Medical Center, Bundang Leaders Animal Medical Center, VIP Animal Medical Center, and Pyeongchon Nell Animal Medical Center) from April 2019 to June 2021 were retrieved. Dogs were classified into normal, acute renal disease, or chronic renal disease groups. Inclusion criteria for the chronic renal disease group required dogs with a clinical diagnosis of CKD. They were classified according to the International Renal Interest Society (IRIS) guideline of BUN, serum creatinine or SDMA, proteinuria or low urine specific gravity, and ultrasonographic abnormalities. Inclusion criteria for acute kidney injury group required dogs based on relevant medical history with acute onset of azotemia with known prerenal, renal, or postrenal cause of AKI (e.g., hypotension, pyelonephritis, urethral obstruction). Exclusion criteria were dogs without laboratory tests or abdominal US and those with a period of more than 1 week between laboratory tests and abdominal US. The final decisions regarding inclusion or exclusion of subjects were based on a consensus of two graduate-trained veterinary medical imaging specialists (H.Y.Y., K.C.L.) with 11 years and 20 years of clinical experience, respectively.
2.2 Data collection and analysis
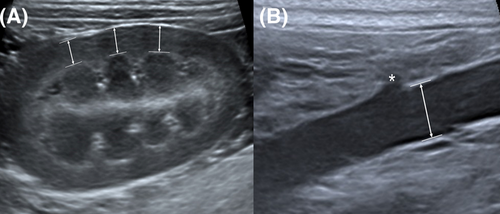
All ultrasound images were evaluated using the same image analysis workstation (INFINITT PACS, INFINITT Healthcare Co., Ltd., Seoul, Korea). The measurements of RCT and Ao diameter were determined by consensus of a second-year resident (D.H.C.) and two graduate-trained veterinary medical imaging specialists (H.Y.Y., K.C.L.) with 11 years and 20 years of ultrasound experience, respectively. All readers were blinded to the subjects’ clinical information, such as disease status, BW, and BCS at the time of image analysis. Cineloop images including the mid-sagittal plane were examined, and a still image in which the renal medullary pyramid and renal cortex were clearly visible was selected. In previous studies, RCT was measured from the base of the renal medullary pyramid to the renal capsule.20, 21, 26, 31 In the current study, RCT was measured on the mid-sagittal plane at the center of the renal pyramid as the shortest distance perpendicular to the renal capsule from the leading edge of the base of the renal medullary pyramid to the trailing edge of the renal capsule at three points in one kidney (Figure 1A). The averages of the measurements were then calculated. It was measured in both left and right kidney in each dog in this way. The diameter of the abdominal aorta (Ao) was measured just caudal to the branch of the left renal artery in the sagittal plane, and the diameter was measured from a still image in systole while examining cineloop images for maximal luminal diameter (Figure 1B).27
2.3 Statistics
The statistical tests were selected and conducted based on a consensus of the second-year resident (D.H.C.) and a veterinary medical imaging specialist (H.Y.Y.) with PhD training in statistics. Statistical analysis software (IBM SPSS Statistics, version 26.0; Chicago, IL, USA) was used for all analyses, and a P-value < 0.05 was considered significant. The correlation between BW, BCS, and RCT in normal dogs was determined using multiple linear regression analysis. In addition, to determine whether renal disease affected RCT considering BW and BCS, a moderated regression analysis was conducted. The difference in the RCT:Ao between patients with a diagnosis of CKD or AKI and normal dogs was determined through one-way analysis of variance. In addition, the difference in the RCT:Ao ratio between normal dogs and those in each of the IRIS stages and AKI patients was also assessed by one-way analysis of variance. A receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff for each RCT:Ao ratio for discriminating between normal dogs and dogs with CKD, and between normal dogs and dogs with AKI. The sensitivity and specificity of the RCT to Ao ratio were calculated.
3 RESULTS
Records for 578 clinically affected dogs were reviewed and 524 of these were excluded due to insufficient data (e.g., lack of laboratory tests, lack of abdominal US, or period exceeding 1 week elapsing between laboratory tests and abdominal US). The remaining 54 patients (45 with CKD and nine with AKI) were included in the current study. Thirty normal dogs were also included based on random selection of dogs that had been used in a previous study.31. Dogs had been classified as normal based on history, clinical examination, laboratory tests, and abdominal US. The most common laboratory findings for the affected dogs were elevated BUN (n = 39, 72%), and creatinine (n = 33, 61%), hyperphosphatemia (n = 26, 48%), and decreased urine specific gravity (n = 31 in 34, 91%). The most common clinical signs for the affected dogs were anorexia (n = 34, 63%), lethargy (n = 19, 35%), and vomiting (n = 9, 17%). Further details for clinical data, symptoms, age (years), BW (kg), BCS (1-9), and sex are provided in Table 1.
| Dogs with acute or chronic renal disease (N = 54) | Normal dogs (N = 30) | |
|---|---|---|
| Age (y) | Range 2–19 (mean 12.5) | Range 0.9-9 (mean 4.36) |
| BW (kg) | Range 1.4-29 (mean 4.36) | Range 1.69-29.7 (mean 11.16) |
| BCS (1-9) | Range 1–7 (mean 4) | Range 3–8 (mean 5) |
| Sex (n) | F (7), NF (23), M (3), NM (21) | F (9), NF (9), NM (12) |
| Breed (n) | Maltese (17), Yorkshire terrier (7), Shih tzu (5), Miniature poodle (5), Cocker spaniel (4), Mixed (5), Miniature pincher (2), Pekingese (2), Chihuahua, Dachshund, Schnauzer, Siberian husky, Samoyed, Spitz, Pomeranian | Mixed (7), Pomeranian (4), Bichon fries (3), Jindo (3), Border collie (2), Miniature poodle (2), Alaskan malamute, Beagle, Chihuahua, Cocker spaniel, Jack Russell terrier, Poongsan, Shetland sheep dog, Standard poodle, Shih tzu |
| Abnormal laboratory findings (n, frequency) |
Elevated BUN (39, 72%), Elevated Creatinine (33, 61%), Hyperphosphatemia (26, 48%), Decreased hematocrit (17, 31%), Decreased urine specific gravity (31/34, 91%), Albuminuria (17/31, 50%) |
N/A |
| Clinical signs (n, frequency) | Anorexia (34, 63%), lethargy (19, 35%), vomiting (9, 17%), diarrhea (8, 15%), polydipsia/polyuria (8, 15%), weight loss (3, 6%), hematuria (2, 4%) | N/A |
- BW, body weight; BCS, body condition score; BUN, blood urea nitrogen; F, female; n, number; NF, neutered female; NM, neutered male; M, male.
Abdominal US was performed by three experienced veterinary imaging specialists and a second-year resident (D.H.C.) at four hospitals, using a 12−13 MHz linear array transducer and an 8 MHz convex array transducer on one of three US machines (Apolio 300, Canon Medical system, Europe B.V., Zoetermeer, Netherlands; Apolio 500, Canon Medical system, Europe B.V., Zoetermeer, Netherlands; Philips EPIQ Elite, Philips Healthcare, Bothell, WA). All dogs were positioned in dorsal recumbency. Additional technical details of the parameters used for ultrasound examinations are provided in Supporting Information 1.
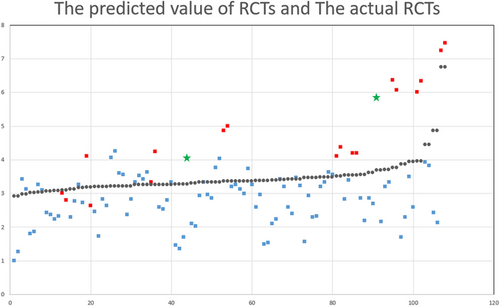
In normal dogs, RCT showed a positive correlation with BW and a negative correlation with BCS. This relationship was expressed similar to a previous study as RCT (mm) = 0.135 × BW − 0.113 × BCS + 3.171 (R2 = 0.85).31 A difference was noted between the predicted value of the RCT obtained by substituting the BW and BCS of the dogs with acute or chronic renal disease into this equation and the actual RCT value (Figure 2). The RCT of CKD and AKI patients was mostly smaller and larger, respectively, than the predicted value. Moderated regression analysis revealed that the pathology of the kidney (normal or renal disease) had a moderating effect in RCT considering the BW and BCS (P < 0.05) (Table 2).
| Change Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression Model | R | R Square | Adjusted R Square | Std. Error of the Estimate | R Square Change | F Change | df1 | df2 | Sig. F change |
| 1 | 0.640a | 0.409 | 0.404 | 0.722 | 0.409 | 73.756 | 2 | 213 | 0.000* |
| 2 | 0.660b | 0.436 | 0.428 | 0.707 | 0.027 | 9.976 | 1 | 212 | 0.002* |
| 3 | 0.673c | 0.452 | 0.439 | 0.700 | 0.017 | 3.187 | 2 | 210 | 0.043* |
- a Predictors : (Constant), BW, BCS.
- b Predictors : (Constant), BW, BCS, Pathology of the kidney.
- c Predictors : (Constant), BW, BCS, Pathology of the kidney, BW x Pathology of the kidney, BCS x Pathology of the kidney.
- BW, body weight; BCS, body condition score; df, degrees of freedom; Sig, significance; R, coefficient of determination; RCT, renal cortical thickness; Std, standard; * P < 0.05.
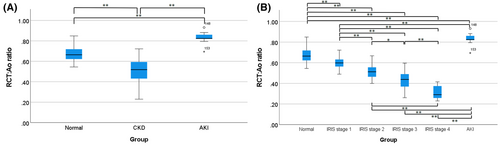
To evaluate whether the RCT:Ao ratio can be used as a quantitative evaluation index, it was calculated and compared between dogs with acute or chronic renal disease and normal dogs. The CKD patients were classified into each IRIS stage. The IRIS stage 1 included 18 dogs, IRIS stage 2 included 14 dogs, IRIS stage 3 included eight dogs, and IRIS stage 4 included five dogs. One dog in IRIS stage 1 and another dog in IRIS stage 2, which had a large difference in cortical thickness of bilateral kidney, were excluded from the statistical analysis. The RCT:Ao ratio of CKD patients was 0.50 ± 0.11 (mean ± standard deviation). The RCT:Ao ratio in normal dogs was 0.67 ± 0.07. The RCT:Ao ratio in patients with AKI patients was 0.83 ± 0.05. The RCT:Ao ratio showed a statistically significant difference between normal dogs and dogs with CKD and AKI (P < 0.01) (Figure 3A). In addition, when CKD patients were classified into IRIS stages, the RCT:Ao ratio showed a statistically significant difference between the normal group and each of the IRIS stages and AKI patients (Figure 3B). The mean RCT:Ao ratio decreased as the IRIS stages increased (summarized in Table 3).
| Group (n) | Mean | SD | 95% confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Normal (30) | 0.67 | 0.07 | 0.65 | 0.69 |
| AKI (9) | 0.83 | 0.05 | 0.80 | 0.86 |
| CKD all stages (43) | 0.50 | 0.11 | 0.48 | 0.53 |
| CKD IRIS stage 1 (17) | 0.59 | 0.05 | 0.58 | 0.62 |
| CKD IRIS stage 2 (13) | 0.51 | 0.06 | 0.49 | 0.54 |
| CKD IRIS stage 3 (8) | 0.43 | 0.08 | 0.39 | 0.47 |
| CKD IRIS stage 4 (5) | 0.31 | 0.07 | 0.26 | 0.36 |
- Ao, abdominal aorta; AKI, acute kidney injury; CKD, chronic kidney disease; IRIS, international renal interest society; n, number; RCT, renal cortical thickness; SD, standard deviation.
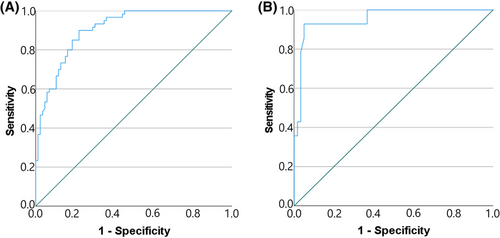
To investigate the usefulness of the RCT:Ao ratio as an index for differentiating normal dogs from dogs with acute or chronic renal disease, ROC curve analysis was performed. ROC curve analysis for normal dogs and CKD patients identified a cut-off value of ≤0.60 for the RCT:Ao ratio to discriminate patients with CKD. The sensitivity and specificity value at this cut-off were 90% and 78%, respectively, with an area under curve (AUC) of 0.90 (95% confidence interval, 0.86–0.95) (Figure 4A). ROC curve analysis for normal dogs and AKI patients identified a cut-off value of ≥0.79 for the RCT:Ao ratio to discriminate patients with AKI. The sensitivity and specificity values at this cut-off were 92% and 95%, respectively, with an AUC of 0.95 (95% confidence interval, 0.90–1.00) (Figure 4B).
In two patients who developed acute-on-chronic renal disease due to urethral obstruction or pyelonephritis while being managed according to the higher IRIS stage, the RCT:Ao ratio was observed to be within the normal range or slightly greater than the normal range. In two dogs, it was observed that one kidney had a very small RCT:Ao ratio, and the other kidney had a large RCT:Ao ratio. Laboratory assays of the first dog revealed elevated BUN levels and normal serum creatinine levels. Laboratory assays of the second dog revealed elevated BUN and serum creatinine levels, and this dog was considered IRIS stage 2 because of the increased laboratory results. The RCT:Ao ratio in the small kidney of the first dog was 0.33, which was lower than that of higher stage CKD patients, whereas the RCT:Ao ratio in the other kidney was 0.79, which was higher than that in normal dogs. In addition, the RCT:Ao ratio in small kidney of the second dog was 0.32 whereas the RCT:Ao ratio of the contralateral kidney was 0.88.
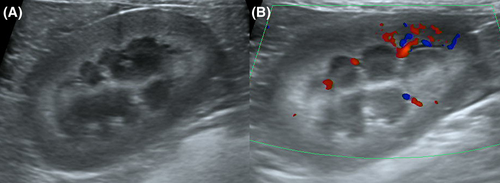
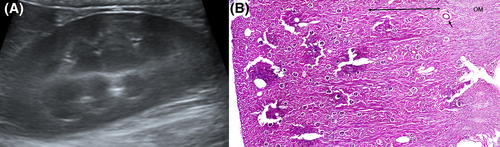
Additionally, when measuring RCT, a hyperechoic layer was observed under a relatively hypoechoic cortex in some individuals (Figure 5A), causing confusion over where the measurements should be made. When color Doppler was used, the hyperechoic renal parenchyma was located toward the cortical side of the arcuate arteries (Figure 5B). To further evaluate this finding, we imaged dogs from a cadaver lab and selected one that also had a hyperechoic layer deep to the hypoechoic renal cortex (Figure 6A). The procedures were conducted by a second-year resident (D.H.C.), a veterinary medical imaging specialist (H.Y.Y.), and a veterinary anatomy specialist (E.Y.L.). Dogs were positioned with dorsal recumbency for acquiring the B-mode mid-sagittal plane images (Apolio 300, 13 MHz linear array transducer, Canon Medical system, Europe B.V., Zoetermeer, Netherlands). The left kidney of selected canine cadaver was removed, and sagittal paraffin sectioning and hematoxylin and eosin staining were performed. In the histologic section of kidney, the lower third cortical parenchyma toward the cortical side of the arcuate artery corresponded to juxtamedullary cortex (Figure 6B). Comparing the ultrasound image with the histologic section, the hyperechoic layer of the renal parenchyma was interpreted to be the renal cortex.
4 DISCUSSION
Our study was conducted to assess whether the difference in RCT considering BW and BCS between normal and dogs with acute or chronic renal disease is indicated by the renal pathology and to determine a quantitative method using RCT for assessing renal pathology that can differentiate disease renal pathology from normal. Results supported the hypothesis that RCTs of CKD or AKI patients considering BW and BCS would be outside the normal ranges and RCT:Ao ratio would be smaller in CKD patients and larger in AKI patients than in normal dogs.
In our study, in normal dogs, RCT showed a positive correlation with BW and a negative correlation with BCS, as demonstrated in a previous study.31 A moderated regression analysis showed that the significance of F change was <0.05 in model 3. This means that the relationship between actual RCT and the predicted value of RCT considering BW, and BCS depends on the renal pathology; RCT can deviate from the normal range according to renal pathology. When comparing the predicted value of the RCT and actual RCT, the RCT of patients with CKD was observed to be smaller. However, the RCT of patients with AKI was larger. The results of the current study were in agreement with the findings of previous reports; in CKD, the RCT decreased due to renal cortical atrophy, glomerulosclerosis, and fibrosis caused by chronic inflammation.5, 32, 33 In contrast, in AKI, ischemia caused by decreased intrarenal blood flow leads to cellular damage and structural changes within renal tubular cells. The inflammatory response induced by these reactions is believed to play a major role in AKI, resulting in changes in vascular permeability and endothelial cell integrity, causing cortical edema.2, 5, 14 Therefore, the RCT in AKI patients is found to be thickened.
In this study, the RCT:Ao ratio was significantly different between dogs with CKD and AKI and normal dogs. As shown in Table 3, there is little overlap between the RCT:Ao ratio of normal dogs versus dogs with CKD versus dogs with AKI. In addition, the RCT:Ao ratio showed a statistically significant difference as the IRIS stages increased. This is because that the RCT changes according to the pathology; the RCT:Ao ratio, which was observed constantly regardless of BW and BCS in previous studies,31 was also changed according to the pathology. Since the optimal cut-off value for CKD and normal dogs is 0.60 with a reliable sensitivity of 90% and specificity of 78%, it is considered that the possibility of CKD is high when the RCT:Ao ratio is less than or equal to 0.60. Likewise, since the optimal cut-off value for AKI and normal dogs is 0.79, with a reliable sensitivity of 92% and specificity of 95%, it is considered that the possibility of AKI is high when the RCT:Ao ratio is greater than or equal to 0.79. According to these results, the RCT:Ao ratio could be a useful quantitative index to evaluate renal pathology with other typical US findings. Even if there are no abnormalities in the blood test, the RCT:Ao ratio could be used as an index for early recognition, suggesting that the kidney is pathological.
Some patients with CKD may experience a sharp decrease in GFR due to sudden parenchymal damage caused by ischemia, infection, or nephrotoxicity.2, 34 This is called acute-on-chronic kidney disease (ACKD). Occasionally, the pathogenesis, clinical signs, and laboratory tests of ACKD may resemble those of AKI, which makes differentiation between AKI and ACKD challenging.35, 36 In our case, the RCT:Ao ratio of two patients considered to have ACKD due to urethral calculi obstruction or pyelonephritis was observed within the normal range or slightly greater than normal. Before the acute insult, the patients were managed according to the high IRIS stage. At that time, abdominal ultrasound showed that the RCT:Ao ratio was similar to the value of the corresponding IRIS stage. When comparing the values before and after the acute insult, we confirmed that the values increased after the acute insult. This is considered to be due to renal cortical edema caused by acute inflammation.5 Furthermore, these cases suggest that when there is a rapid increase in serum creatinine, BUN, and SDMA, the possibility of ACKD cannot be ruled out when the RCT:Ao ratio is not as high as in the case of AKI. Mild or early AKI should be considered in the differential diagnosis.
In two dogs, one kidney had a similar RCT:Ao ratio to IRIS stage 3–4, despite being at a normal or low level of IRIS stage, while the other kidney had a slightly greater RCT:Ao ratio than that of normal dogs. Previous studies reported that children with a small kidney due to unilateral renal disease showed varying degrees of compensatory hypertrophy in the contralateral kidney, which was proportional to parenchymal reduction.37, 38 This is thought to be associated with both an increase in the size of the kidney tubules and glomeruli and an increase in the single nephron glomerular filtration rate. Compensatory hypertrophy and an increase in single nephron glomerular filtration rate result in normalization of the total GFR.38 Therefore, these two patients are regarded to have suffered compensatory hypertrophy due to poor functioning of one side due to previous unilateral ischemic or other kidney injuries, and are considered to have maintained or slightly decreased GFR due to compensatory hypertrophy. However, these compensatory mechanisms could contribute to glomerular or tubular injury and hypertension later in life.38 Thus, a patient with a large difference in cortical thickness between the two kidneys would require continuous monitoring and follow-up, even if the BUN, serum creatinine, or SDMA levels were within the normal range.
A previous study described evidence that hyperechoic renal parenchyma deep to the hypoechoic renal cortex corresponded to the outer medulla, and this hyperechoic outer medulla has been observed in dogs without any evidence of renal disease.39 In the photograph of the cadaver kidney in the current study, the region marked as the outer medulla was located deep to the arcuate artery. In contrast, in the ultrasound image, the region marked as the outer medulla was located toward the cortical side of the anechoic arcuate artery.39 Histologically, the outer medulla was located deep to the cortex, and the arcuate vessels marked the border between the renal cortex and the outer medulla.40 Although the exact reason for the double echogenicity of the renal cortex in some patients is still unknown, considering the structure of the renal cortex, it is thought to be due to the difference in the degree of vascular distribution between the cortical cortex and the juxtamedullary cortex.40, 41 One textbook of pediatric US described that the renal cortex was subdivided into two zones and juxtamedullary cortex was hyperechoic to the rest of the cortex with acute rejection after kidney transplant.42 Since there may be histological differences between humans and dogs,40 it may be difficult to apply this finding equally, but it is a finding worth consideration. A previous study on the correlation between renal histopathology and renal echogenicity showed that renal cortical echogenicity is mainly influenced by glomerulosclerosis and fibrosis. Renal echogenicity rapidly increases in relation to mild degenerative lesions but decreases as the severity of the lesions progresses.33 Therefore, it is possible to suggest that the difference in perfusion between the cortical cortex and juxtamedullary cortex after a mild injury may be the cause of double echogenicity. Further studies using histopathological diagnosis are necessary to determine the exact cause. A hyperechoic layer should be included when measuring RCT, and the measurement should be started from the leading edge of the base of the renal medullary pyramid based on the arcuate artery.
The limitations of this study include the small sample size and number of patients. This could have prevented an accurate statistical evaluation. Another limitation is that although IRIS staging was classified according to creatinine levels and the data mainly included patients who underwent SDMA, accurate staging may not have been possible because some patients did not undergo SDMA. Also, no actual GFR measurements have been made. A more accurate assessment would have been possible if the patient's RCT:Ao ratio and actual GFR measurements were compared. However, the comparison could not be made because it would have been costly and time consuming. It would be good if further study is conducted on this comparison. Lastly, future studies, including renal biopsy, are needed for a more accurate evaluation of the RCT:Ao ratio index.
In conclusion, the current study introduced new evidence that RCT values were affected by BW and BCS and that values differed for normal dogs and dogs with acute or chronic renal disease in spite of these variations. Findings supported using the RCT:Ao ratio as a non-invasive quantitative method for minimizing these outside sources of variation and evaluating the pathology of kidneys in dogs with acute or chronic renal disease.
ACKNOWLEDGMENTS
The research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1A6A1A03033084). The authors would like to thank Dr. Minji Kwon, Dr. Jeongmin Lee, and Dr. Yunsir Choi for managing the medical records of the dogs, and Dr. Euiyong Lee who assisted with histopathology process
LIST OF AUTHOR CONTRIBUTION
- (a) Conception and Design: Choo, Yoon
- (b) Acquisition of Data: Choo, Kwon, Yoon
- (c) Analysis and Interpretation of Data: Choo, Kim, Lee, Yoon
- (a) Drafting the Article: Choo, Yoon
- (b) Revising Article for Intellectual Content: Choo, Kim, Kwon, Lee, Yoon
- (a) Final Approval of the Completed Article: Choo, Kim, Kwon, Lee, Yoon
- (a) Agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Choo, Kim, Kwon, Lee, Yoon
CONFLICT OF INTEREST
The authors have declares no conflict of interest.
PREVIOUS PRESENTATION OR PUBLICATION DISCLOSURE
None
EQUATOR NETWORK DISCLOSURE
No reporting guideline checklist was used
DATA ACCESSIBILITY STATEMENT
Data used in this study can be accessed by contacting the Corresponding Author




