Hypofractionated radiotherapy in nine dogs with unresectable solitary lung adenocarcinoma
Abstract
Although lung lobectomy is the most common treatment option for dogs with solitary lung tumors, surgery often cannot be performed at the time of diagnosis. In this retrospective, case series study, we described the effects of hypofractionated radiotherapy for tumor mass reduction in nine dogs with solitary lung adenocarcinoma that were later considered for surgical resection, and we assessed the tolerability of the radiation protocol. Tumors were deemed unresectable by the attending veterinarian. The dose prescription was 7.0-12.0 Gy/fraction in four to seven fractions, administered weekly for a total dose of 40-50 Gy. Treatment planning prioritized normal tissue dose constraints. The median interval between the last radiotherapy session and maximum tumor size reduction was 56 (range: 26-196) days, with six and three dogs exhibiting a partial response and stable disease, respectively. Although acute and late radiation-induced toxicity to the skin and/or lungs developed in all nine dogs, it was self-limiting or improved with short-term anti-inflammatory treatment. Tumor progression after initial size reduction was confirmed in three dogs at 62, 126, and 175 days, respectively, after the last radiotherapy session. Seven of the nine dogs underwent lobectomy a median of 68 days after radiotherapy when tumors were in partial response or stable disease or at the time of progression, and five received systemic chemotherapy concurrent with or after radiotherapy. These findings suggest that hypofractionated radiotherapy for canine solitary lung adenocarcinoma is useful when the tumor is large or when surgery cannot be performed immediately after diagnosis.
1 INTRODUCTION
Primary lung tumors are well recognized but remain relatively uncommon in pet dogs.1 The most common histopathological diagnosis for primary lung tumors in dogs is adenocarcinoma. Other reported tumor types include squamous cell carcinoma, sarcoma, and anaplastic tumors.1-3 The reported median survival time after the clinical detection of spontaneous lung neoplasms of 24 beagles in a research colony was 313 days (range: 5-1266 days) in the absence of treatment.4 The median survival time of pet dogs with advanced tumors may be shorter, because most of the tumors of these beagles were detected incidentally by annual thoracic radiography.
Lobectomy via thoracotomy or thoracoscopy is the most common treatment option for dogs with solitary lung tumors. Mehlhaff et al reported a postoperative survival time of 4-46 months (mean: 13 months) in 15 dogs with primary pulmonary neoplasms treated by lung lobectomy.5 In addition, they found that the median survival time was greater for eight dogs with adenocarcinoma (19 months) than for three dogs with squamous cell carcinoma (8 months). Tumor size and location were also prognostic. The medial survival time of seven dogs with neoplasms <100 cm3 was 20 months, of five dogs with neoplasms >100 cm3 but <1000 cm3 was 6.8 months, and of three dogs with neoplasms >1000 cm3 was 9-10 months. The median survival time of seven dogs with peripheral neoplasms was 15 months, of four dogs with neoplasms at the base of the lung lobe was 17.5 months, and of four dogs with involvement of the entire lobe was 8 months. Ogilvie et al also reported that dogs with lung tumors that achieved surgical remission without residual gross disease exhibited a significantly increased median survival time compared with dogs that did not achieve remission (330 days [n = 55] vs 28 days [n = 21]).6 In addition, several studies have evaluated other prognostic variables in dogs with primary lung tumors. Dogs with clinical signs at the time of diagnosis exhibit significantly shorter disease-free intervals and survival times compared with dogs without clinical signs that were diagnosed with a pulmonary tumor during routine examinations.7 Furthermore, metastatic disease within the lymph nodes is also associated with a significantly shorter disease-free interval and survival time in dogs.3, 6-8
Although early surgical intervention is recommended as a first-line therapy, it may not be possible to perform surgery at the time of diagnosis because of various factors such as owner reluctance or large tumor size. In general, preoperative irradiation may be an option for the size reduction of large tumors. In humans, thoracic radiotherapy plays a well-established and indispensable role in the treatment of advanced small cell lung cancer, aiding in the palliation of locoregional and/or metastatic disease and improving the overall survival of patients when combined with chemotherapy.9, 10 Similarly, with regard to nonsmall cell lung cancer, radiotherapy has been suggested to prolong the survival of medically inoperable patients or patients refusing surgery, including those with stage I/II disease; furthermore, a survival advantage with the addition of surgery to chemoradiotherapy has been reported.11, 12 To our knowledge, however, no published studies have evaluated the efficacy and side effects of radiotherapy in dogs with lung tumors.
Objectives of the current study were to describe the effects and tolerability of preoperative hypofractionated radiotherapy for tumor mass reduction in a group of dogs with solitary lung adenocarcinoma.
2 METHODS
2.1 Case selection
In this retrospective, case series study, electronic medical records at the animal medical center of Gifu University were searched to identify dogs with solitary lung adenocarcinoma without evidence of distant metastasis based on radiographs or CT that were treated with hypofractionated radiotherapy, and then later considered for surgical resection, between November 1, 2011 and January 31, 2017. The dogs were diagnosed with lung adenocarcinoma on the basis of histopathological analysis of biopsy specimens obtained at the first visit or tissue samples obtained at the time of surgical excision. All tumor specimens were evaluated by one board-certified veterinary pathologist (H.S.). Radiation therapy was proposed when the attending veterinarian and veterinary surgeon estimated that it would be difficult to perform surgical resection at the time of diagnosis or when the owner was hesitant to accept surgery. For all dogs, surgical resection was considered if tumor size reduction was observed after the radiation protocol. Dogs that received radiotherapy with or without surgery or chemotherapy were included, whereas those who did not complete the radiotherapy protocol for causes other than their condition were excluded. Decisions for subject inclusion and exclusion were made by an experienced clinical veterinarian of oncology (M.K.). All clients received information about the treatment and provided consent for the treatment as well as publication of the findings.
2.2 Medical records review
The following information was retrieved by an experienced clinical veterinarian of oncology (M.K.) from the medical records of the animals: signalment, physical examination findings, clinicopathological findings, diagnostic imaging findings, biopsy results, administered treatments, treatment-related adverse effects, and follow-up findings, including the survival time (defined as the time between the first radiotherapy session and the date of death). Data related to long-term outcomes were obtained from the medical records or contact with the owners and primary-care doctors via telephone calls or fax surveys.
2.3 Efficacy and toxicity assessment
The tumor response was determined by an experienced clinical veterinarian of oncology (M.K.) using CT and classified according to the Veterinary Cooperative Oncology Group RECIST guidelines for dogs.13 A complete response was defined as the disappearance of all targeted lesions. A partial response was defined as a decrease of ≥30% in the sum of the longest diameters of the targeted lesions compared with the baseline sum. Stable disease was defined as a decrease of <30% or an increase of <20% in the sum of the longest diameters of the target lesions, with the smallest longest of the previous measurement designated as the reference value. Progressive disease was defined as the appearance of one or more new lesions or an increase of ≥20% in the sum of the longest diameters of the target lesions, with the smallest longest diameter of the previous measurement designated as the reference value; the sum must also have increased by ≥5 mm. The longest diameter of the tumor was measured on axial CT images.
Radiation-induced tissue toxicity was assessed through medical record reviews and characterized according to the radiation morbidity scoring scheme described by the Veterinary Radiation Therapy Oncology Group.14 Radiation side effects that developed during the first 6 months after the initiation of radiotherapy were classified as acute effects, whereas those that developed more than 6 months after the initiation of radiotherapy were classified as late effects. Acute and late effects on the thoracic organs were evaluated using chest CT and physical examinations.
The radiation dose delivered to the organs at risk contoured on the CT images was determined and evaluated according to the guidelines of the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC).15
2.4 Statistical analysis
A Kaplan-Meier survival curve was generated for all dogs. Dogs that were alive at the end of the investigation period were censored. All statistical analyses were performed by the principal and mentoring investigators (M.K. and T.M.) using statistical analysis software (JMP, version 10.0.0 SAS Institute Inc., Cary, NC).
3 RESULTS
In total, nine dogs were included in this study. The median weight of the dogs was 6.7 kg (range: 2.2-39.1 kg), and the median age at the first visit was 10.7 years (mean: 10.1 years; range: 6 to 13 years). A total of 10 dogs began receiving radiotherapy; however, one dog was excluded from the study because the radiation protocol was discontinued after the second radiation session in order to perform surgery at the request of the owner. Surgery was not performed at the time of diagnosis in nine dogs because the attending clinician and surgeon estimated that the tumors were unresectable due to tumor size or location. Only for the dog that was excluded did owner circumstances preclude surgery at the time of diagnosis.
Five of the nine dogs exhibited clinical signs at diagnosis (Supporting Information 1). The most common clinical sign was cough (n = 4). In the remaining four dogs, lung tumors were incidentally detected on radiographs during a routine examination (n = 2) or detailed examinations for other diseases (n = 2).
All dogs underwent CT at the first visit using a 4-detector row (AsteionTM Super 4; Toshiba Medical Systems, Tochigi, Japan) or 16-detector row CT device (AlexionTM Advance Edition; Toshiba Medical Systems, Tochigi, Japan). General anesthesia was induced according to protocols determined by the clinician responsible for each dog. The dogs were positioned in sternal recumbency. Thoracic images were acquired under the following settings: slice thickness, 1.0 or 2.0 mm; beam pitch, 0.9 or 1.4; tube rotation time, 0.75 s/rot; tube voltage, 120 kV; and tube current, 30-40 mA. Imaging at follow-up visits was also performed under similar conditions.
Computed tomographic measurements of the mass were available, and the mean longest diameter was 66 mm (range: 25-88 mm). Anatomical locations of the mass included the cranial lobe of the left lung (n = 3: cranial part [n = 1], caudal part [n = 1], and cranial and caudal parts [n = 1]), caudal lobe of the left lung (n = 2), cranial lobe of the right lung (n = 1), middle lung lobe (n = 1), caudal lobe of the right lung (n = 1), and accessory lung lobe (n = 1). In one dog, the affected lobe was identified at the time of lung lobectomy, because the tumor was so large that the affected lobe could not be identified on initial CT images (Dog 4; Figure 1A). Six dogs also underwent contrast-enhanced CT. The CT images were acquired after intravenous injection of iohexol (300 mg/mL, 1.5 mL/kg, 1.0-2.0 mL/s; Omnipaque 300 injection syringe; Daiichi Sankyo, Tokyo, Japan) by a pressure injector (Smart Shot; Nemoto Kyorindo, Tokyo, Japan). Four of these showed poor enhancing effects. The remaining two dogs exhibited distinct enhancing effects, and the unenhanced area was located near the center of the tumor in these dogs. This unenhanced area was noted to enlarge after radiotherapy.
Evidence of pleural effusion was observed in two dogs at the time of diagnosis. In one dog, pleural effusion was not collected for examination because it was present in a small amount (Dog 6). In the other dog, the hematocrit of the pleural effusion was 14% and the total protein was 1.8 g/dL, and no tumor cells could be observed in the pleural effusion (Dog 4). The effusion resolved after two radiotherapy sessions in both dogs without any additional treatment.
Mild enlargement of the intrathoracic lymph nodes was detected on CT images, but sampling of these lymph nodes was not performed (Supporting Information 1). In cases in which mild abdominal lymphadenopathy was observed, neither relevant clinical symptoms nor other abnormal findings on the abdominal images were noted (Dogs 1, 3, 5, 6, and 8).
All dogs underwent hypofractionated radiotherapy. Treatments were delivered with a linear accelerator that had an X-ray energy output of 4 MV (Primus Mid-Energy 4 MV linear accelerator; Siemens Healthcare, Malvern, PA). Planning CT scans were obtained for each dog placed in the treatment position (sternal recumbency) on a vacuum-mattress immobilization device (Vac-Loc; MED-TEC, Orange City, IA). Individualized treatment plans were constructed using a three-dimensional CT-based computer-generated treatment planning system (XiO Version 4.60; Elekta Japan, Tokyo, Japan). The gross tumor volume was defined as gross disease on CT images. Intrathoracic lymph nodes were not included in the radiation target. The planning target volume margins were contoured to include regions at risk for respiratory motion and ranged from 0.3 to 1.0 cm around the gross tumor volume limits. On the basis of the assumption that lobectomy would be performed after tumor size reduction, the risk of microscopic lesions was not considered and clinical target volume was not contoured. Adjacent critical tissues or organs that were deemed healthy and at risk for radiation toxicity, including the lung, heart, spinal cord, and liver were also contoured on the CT images. The treatment planning priority in these cases was to maintain the radiation dose to normal tissues within the chosen dose constraints, not to optimize the dose in the targets. For cases where the limit of irradiation for at-risk organs was exceeded (this will be described later), the planning target volume was set with no margin or minus the margin in order to ensure protection of the at-risk organs and reduction of only the tumor mass. Therefore, at times the planning target volume was smaller than the gross tumor volume. A multileaf collimator with 10-mm leaves was used on the linear accelerator to shape the fields exposing the target volume and block the surrounding normal tissues. The isocenter and beam arrangements for each plan were determined by the location of the tumor and adjacent critical normal structures. The radiation dose prescription point was set at the isocenter, not covering 95% of the planning target volume. Organs at risk included the lungs, heart (pericardium), spinal cord, and liver, and the dose information for these organs, which was based on the QUANTEC guidelines, is presented in Supporting Information 2.16 Radiation was delivered in six to nine unequally weighted fields using multiple oblique beam arrangements. Portal imaging was performed prior to each session to ensure accurate patient positioning.
The hypofractionated radiation protocol included one fractionated dose of 7-12 Gy (median: 9 Gy) that was administered weekly for a total dose of 40-50 Gy (median: 48 Gy). The median values of the dose delivered to 98% (D98%), mean, and the dose delivered to 2% (D2%) for the planning target volume were 29.6 (range: 13.1-40.8), 45.4 (range: 30.7-47.3), and 48.5 (range: 38.8-50.9) Gy, respectively. The median of the mean lung dose and V20 to the lungs were 10.1 Gy (range: 4.2-14.0 Gy) and 16.3% (range: 2.7-28.1%), respectively. Because of tumor size reduction during radiotherapy, a new plan with modified gross tumor volume and planning target volume was generated and used to complete the course of radiotherapy in two dogs (Dogs 3 and 6).
Three of four dogs that initially presented with cough (Dogs 6, 8, and 9) and one dog that initially presented with tachypnea (Dog 4) experienced symptom amelioration during or after radiotherapy.
Seven of the nine dogs received one or more adjunctive treatments (Supporting Information File 1), including lung lobectomy (n = 2), or lung lobectomy and chemotherapy (n = 5).
Although seven of the nine dogs underwent tumor removal surgery after radiotherapy, two dogs did not undergo surgery. The reasons included no tumor size reduction (Dog 9) and owner refusal despite mass reduction (Dog 8). In the seven dogs that underwent lung lobectomy, one affected lobe was resected using median sternotomy (n = 3) or lateral thoracotomy (n = 4). Microscopically, one of seven dogs exhibited tumor cell infiltration across the lung pleura (Dog 2), while another exhibited tumor cells on the excised stump (Dog 7). Tumor-free margins were achieved for the other five dogs. Surgery was tolerated after radiotherapy by all dogs. Although moderate pleural adhesion was observed in one dog (Dog 4), it was impossible to judge whether it was derived from the tumor itself or was a consequence of radiation, because the tumor was originally very large. All surgeries were performed after radiotherapy, and the median time to surgery from the last radiotherapy session was 68 days (range: 26-203 days). In five of the seven dogs, surgery was performed after radiotherapy while the tumors were in partial remission or stable disease, whereas in two dogs (Dogs 2 and 3), surgery was performed at the time of progressive disease after an initial tumor response.
Five of the nine dogs underwent systemic chemotherapy with a variety of agents (Supporting Information 3). Two dogs underwent chemotherapy as first-line therapy to prevent metastasis, which was administered concurrently with radiotherapy in one dog (Dog 2) and after completion of radiotherapy in another dog (Dog 5). Systemic chemotherapy was initiated for two dogs when metastasis was detected (Dogs 4 7). In these cases, reduction of metastatic lesions was observed in response to treatment. For another dog, chlorambucil was initiated during radiotherapy for the management of an inflammatory colonic polyp (Dog 1).
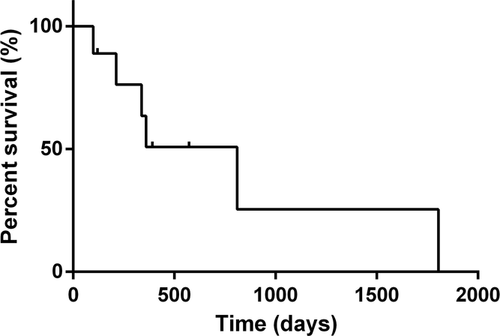
The median survival time for the nine dogs was 810 days (95% confidence interval, 99-1805 days; Figure 2). Three dogs that were alive at study completion were censored from the survival analysis. Five dogs died because of the lung adenocarcinoma and one died because of an unrelated disease. There were no deaths caused by radiation-related side effects. The 1-year survival rate was 44% (4/9 dogs).
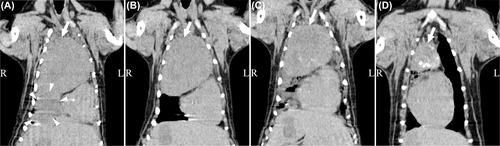
Computed tomography was routinely performed for all nine dogs 1 month after the last radiotherapy session; three (33%) dogs exhibited a partial response and six (67%) exhibited stable disease. Subsequently, the seven dogs that underwent surgery received CT examinations every month until surgery. Two dogs that did not undergo surgery received CT examinations every month until death (Dog 9) or until its return to the referral animal hospital seven months later (Dog 8). The median interval between the last radiotherapy session and maximum tumor size reduction was 56 days (range: 26-196 days). At this time, six dogs (67%) exhibited a partial response and three (33%) exhibited stable disease.
Local tumor progression after initial size reduction was confirmed in three dogs at 62, 126, and 175 days (Dogs 3, 9, and 2, respectively) after the last radiotherapy session. Dogs 2 and 3 underwent surgery immediately after tumor progression was detected, whereas Dog 9 did not undergo surgery and died 32 days after tumor progression was detected.
Three of the seven dogs that underwent lobectomy developed metastasis after surgery. The metastatic lesions were confirmed by abdominal ultrasound, CT, or MRI of the intra-abdominal or intrathoracic lymph nodes, lungs, and brain at 84, 287, and 314 days (Dogs 7, 2, and 4, respectively) after the last radiotherapy session. Sampling of these lesions was not performed. Moderate enlargement of the mesenteric lymph nodes with ascites fluid was detected in one dog and metastasis was suspected, but detailed examinations were not performed (Dog 2).
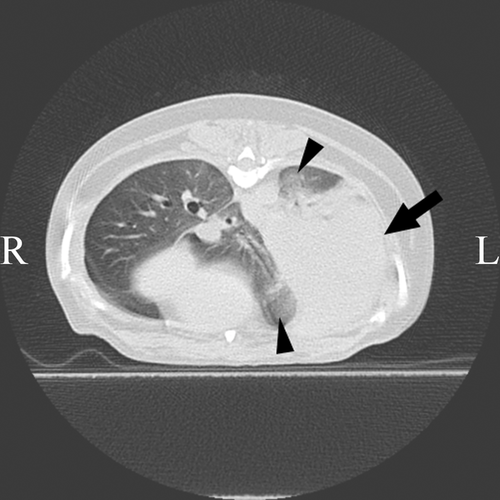
Acute and late radiation toxicity to the thoracic organs, including the lungs and heart, was evaluated using chest CT and electrocardiography in all nine dogs. Toxicity to the skin was assessed by visual inspection. With regard to acute toxicity, eight dogs exhibited grade 1 (n = 7 dogs) or 2 (n = 1) toxicity of the lung and three exhibited grade 1 toxicity of the skin (Supporting Information 2). One dog presented with grade 2 alveolar infiltrate (Dog 9) on CT images, which was resolved by a single dose of prednisolone (1.0 mg/kg given subcutaneously). Mild cough seen in five of eight dogs that had acute lung injury improved with a single dose of prednisolone (Dog 9) or without treatment (Dogs 1, 3, 4, and 7). Late radiation-induced toxicity was determined in two dogs; this included grade 1 toxicity of the lung in both dogs and grade 1 toxicity of the skin in one dog. Acute and late lung toxicity was represented by heterogeneous ground glass opacities on CT (Figure 3). These findings were consistent with the findings for radiation pneumonitis reported in a previous study.16
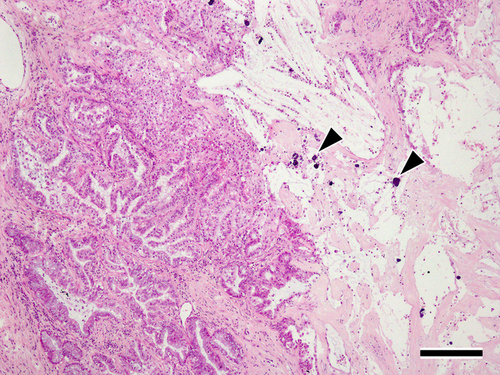
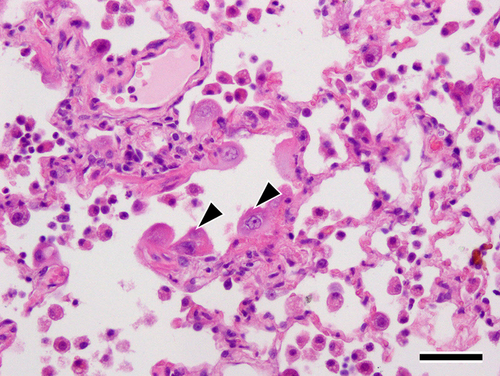
The excised tumors and surrounding lung tissue for the seven dogs that underwent lobectomy were pathologically evaluated. Tumor specimens and adjunctive lung tissue obtained from dogs that underwent lobectomy were fixed in 10% buffered neutral formalin, embedded in paraffin, and stained with hematoxylin and eosin for routine histopathological examination. All specimens exhibited similar findings of massive necrosis with moderate fibrosis and calcification was observed in the peripheral area of the tumor (Figure 4). In addition, focal coagulative necrosis was observed in the center of the tumor, and hyperplasia of type II pneumocytes secondary to the injury of type I pneumocytes, detachment of ciliated bronchiolar epithelial cells, and a small amount of fibrin deposition along with alveolar wall thickening were observed in the surrounding lung tissues (Figure 5). Macrophages and neutrophils infiltrated the alveolar spaces. No fibrotic lesions were observed in the surrounding lung tissues. Three dogs were alive at the time of the survey. For the remaining six, necropsy was not performed because owner consent could not be obtained.
4 DISCUSSION
In the present study, we investigated the effects and tolerability of hypofractionated radiotherapy for tumor mass reduction in a group of dogs with solitary lung adenocarcinoma. In our radiotherapy protocol, of which treatment planning goal was to prioritize normal tissue dose constraints instead of target coverage, suppression of tumor growth or tumor size reduction was achieved within months (median 56 days) from the onset of irradiation. In addition, radiation toxicity to the intrathoracic tissue was mild according to CT and histopathology results. Although the effectiveness of hypofractionated radiotherapy was suggested, there were cases where tumor progression after initial size reduction or metastatic lesions in the lungs were confirmed within the observation period after radiotherapy.
In humans, surgery remains the cornerstone of treatment for early-stage nonsmall cell lung cancer.17-19 Similarly, timely surgical intervention for solitary lung tumors is recommended in the field of veterinary medicine. However, surgery may not always be possible because of various factors. The nine dogs with unresectable solitary lung adenocarcinoma in the present study could not undergo immediate surgery; therefore, we performed hypofractionated radiotherapy to shrink the tumors or slow down the rate of tumor enlargement. The findings of this study suggested that this was a useful and effective management option.
The breed, sex, and lesion location varied among the nine dogs included in the present study. The mean age was 10.1 years, which is consistent with the mean ages of 9.5-11.5 years reported in previous studies on primary lung tumors in dogs.1, 5, 7 The most common clinical sign in the present study was cough (4/5 dogs); this finding was also consistent with that in a previous study.7
The median survival time for the nine dogs in the present study was 810 days. Despite a small planning target volume given the goal to prioritize tissue constraints, we recorded partial remission for six dogs and stable disease for three dogs in our study, with no record of a complete response. Tumor regression was observed slowly after the initiation of treatment, with the median interval between the last radiotherapy session and maximum tumor size reduction being 56 days. In three of the six dogs that exhibited a partial response, there was minimal variation in the tumor size at 1 month after the last radiotherapy session. Furthermore, tumor progression after initial size reduction was confirmed in three dogs at 62, 126, and 175 days, respectively, after the last radiotherapy session. Dog 2 underwent a follow-up examination without surgery at 28 days after the last radiation session because of a remarkable decrease in the tumor size (reduction ratio: 42%). However, tumor progression was confirmed at 175 days after the last radiation session, and lobectomy was performed 28 days later. Unfortunately, intrapulmonary metastasis was detected on chest CT at 84 days after surgery (287 days after the last radiation session). Pulmonary metastasis was also detected in two other dogs that underwent lobectomy at 245 and 58 days, respectively, after surgery (314 and 84 days, respectively, after the last radiation session). Although no metastasis was detected during the observation period for four dogs that underwent lobectomy and two dogs that did not undergo surgery, the risk of metastasis during long-term follow-up after radiotherapy should be adequately investigated.
Although three of four dogs that initially presented with cough experienced symptom amelioration after radiotherapy (Dogs 6, 8, and 9), they all exhibited stable disease 1 month after the last radiotherapy session. In addition, two dogs with pleural effusion before treatment exhibited complete resolution of the effusion after two sessions of radiotherapy, even though the tumors themselves did not decrease in size. These results suggest that hypofractionated radiotherapy appears to improve clinical symptoms, even if tumor size reduction is not achieved.
The surgical specimens and surrounding lung tissue obtained from the seven dogs that underwent lobectomy were pathologically evaluated. Both the tumor center and margins exhibited necrosis, although the pathological features differed (Figure 4). Although necrotic cells in the marginal region were aggregated around fibroconnective tissues, those in the center were scattered among active tumor cells. These findings suggest that the necrosis in the marginal region of the tumors was radiation induced, whereas that in the central region was due to other factors such as ischemic necrosis.16 Recently, the molecular pathways for tumor necrosis induced by hypoxia have been elucidated. Rapid tumor growth outstripping the vascular supply creates a hypoxic environment that subsequently results in hypoxia and, finally, necrosis.20 In two of the six dogs that underwent contrast-enhanced CT, an unenhanced area was observed near the center of the tumor at the first visit and enlarged after radiotherapy. However, the histopathology findings from the surgical specimens suggest that the unenhanced area on contrast-enhanced CT after radiotherapy may not necessarily reflect necrosis due to radiation.
Lung toxicity is one of the most common concerns in thoracic radiotherapy. In human medicine, radiation pneumonitis usually occurs within the first 6 months after treatment completion, whereas radiation fibrosis typically occurs at 6-12 months after treatment completion.21 Some studies demonstrated a correlation of V20 (percentage of lung volume that receives a radiation dose of 20 Gy or more) or V30 (percentage of lung volume that receives a radiation dose of 30 Gy or more) and the mean lung dose with the incidence of radiation pneumonitis.22-28 Graham et al reported that fatal pneumonitis occurred in human patients with a V20 of ≥35%, while high-grade pneumonitis occurred in patients with a V20 of ≥32%.28 In addition, Kong et al stated that, when the cutoff values for V20, mean lung dose, and the normal tissue complication probability are 30%, 20 Gy, and 10%, respectively, the positive and negative predictive values for these factors are 50-71% and 85-89%, respectively.22 However, the QUANTEC guidelines, which are a summary of the available three-dimensional dose-volume/outcome data in people, have been used as a more concrete quantitative evaluation of normal tissue toxicity risk because of concern in recent years that these normal tissue complication probability models are not ideal.15 In the QUANTEC guidelines, the risk of symptomatic pneumonitis is considered to be less than 20% with lung V20 <30% and mean lung dose <20 Gy. However, these reports were based on a standard fractionation scheme (ie, 1.8-2.0 Gy per daily fraction) used in people, so the conditions were different from those for hypofractionated radiotherapy involving weekly radiation sessions, as used in the present study. Nonetheless, our radiotherapy protocol was a tolerable treatment when dose constraints described for people treated with standard fractionation were used. In addition, published data indicate that dogs with lung cancer may have a greater tolerance for radiation than do humans.29 Furthermore, in the QUANTEC guidelines, the dose and volume parameters associated with a 15% risk of pericarditis are a mean dose >26 Gy and V30 >46% for irradiation of pericardium.15 Because these limitations were also based on a standard fractionation, further careful investigation of the chest irradiation dose for dogs is necessary.
Acute radiation toxicity was detected in all our dogs, although it was mild (grade 1 or 2) according to the radiation morbidity scoring scheme described by the Veterinary Radiation Therapy Oncology Group.14 Pericarditis was not observed on CT images and electrocardiograms. Lung toxicity, grade 1 (n = 7) or 2 (n = 1), was detected in eight dogs, who demonstrated heterogeneous ground glass opacities in the surrounding normal lung fields on CT, as reported in a previous study.16 These opacities disappeared spontaneously (n = 7) or by administration of a single dose of prednisolone (n = 1, grade 2). This was consistent with the findings in a previous human study where mild to moderate symptomatic radiation pneumonitis resolved either spontaneously or with symptomatic treatment such as corticosteroid therapy.30
As observed in any other condition causing diffuse alveolar damage, radiation toxicity in human lung results in a limited and stereotypical response of the lung, which is characterized by an acute exudative phase, an organizing or proliferative phase, and a chronic fibrotic phase. These phases appear at approximately 0-2 months, 2-9 months, and >9 months after treatment, respectively.31, 32 The first two phases correspond to the clinical and radiological stages of radiation fibrosis, and all resected lung samples from the seven dogs in our study exhibited these phases.32 The changes observed in the surrounding lung tissues in the present study, such as thickening of the alveolar walls, swelling or peeling of type II pneumocytes, deposition of fibrin, and inflammatory cell infiltration (Figure 5), were similar to findings reported in dog models with radiation-induced lung injury.16 Hyaline membranes, which are seen in humans, were not observed.32 The proliferation of type II pneumocytes occurs during the early phase of acute lung injury as a reparative phenomenon.33 The pathological findings in our dogs, such as hyperplastic changes in type II pneumocytes, were considered to be subacute changes occurring as part of the repair process after injury, and this suggests that the radiation toxicity was reversible.
Computed tomography revealed late radiation-induced grade 1 lung toxicity in two of our dogs. The findings were consistent with radiation-induced pneumonitis, and there was no evidence of lung fibrosis such as volume loss, pleural thickening, bronchiectasis, or cicatricial changes.32 Although fibrosing lesions in the surrounding lung tissue were not observed on histopathological examination, this finding may be related to the timing of surgery, considering that the dogs underwent lobectomy within 6 months after the last radiotherapy session. The possibility of fibrosis increases after 6 months; however, no findings of pulmonary fibrosis were confirmed in the remaining normal lung field of the seven dogs that underwent lobectomy and the normal lung of the two dogs that did not undergo surgery after 6 months or later within the observation period. It should be noted that the normal lung field that was most exposed to radiation was probably resected in the seven dogs that underwent surgery. In general, fraction size is the dominant factor in determining late effects, and late reactions are more severe if large doses per fraction are given. Nevertheless, our findings suggest that the hypofractionated protocol described here was well tolerated, although further investigations with a larger number of cases are required to determine the optimal radiotherapy protocol. Because the priority of our radiotherapy protocol was to maintain the radiation dose to normal tissues within the chosen dose constraints, target coverage was not optimized. More conformal therapies (eg, intensity-modulated radiation therapy) may enable delivery of higher doses to the tumor while maintaining normal tissue tolerance.
Although hypofractionated radiotherapy showed good effects in dogs with solitary lung adenocarcinoma in our study, the findings are limited by several factors. First, statistical examinations related to prognostic factors were not conducted because of the small sample size. Although all dogs were considered to have unresectable lung tumors in the present study, many cases were favorable in parameters considered to affect prognosis: four of nine dogs were incidentally diagnosed without clinical signs, some had small tumors (4/9 dogs had tumors smaller than 4.5 cm), and some did not have intrathoracic lymphadenopathy (3/9 dogs). Second, the influence of radiation alone could not be determined in cases that received concurrent surgery or chemotherapy. Seven of nine dogs underwent surgery, five of which had a complete excision. It is notable that resection became possible after radiotherapy in most dogs. Surgical resection was considered complete in 5 dogs, which may have contributed to the overall favorable outcome of this study. Whether the favorable outcome was due to radiation or surgery was not clear in the present study. In addition, the effects of chemotherapy could not be sufficiently verified, because the type, dose, time of initiation, and duration of use of combined drugs were not standard. Further studies evaluating the influence of chemotherapy are necessary, considering the potential risk of radiation toxicity, specifically pulmonary fibrosis, because some chemotherapy drugs may cause pulmonary fibrosis or may increase the incidence of radiation pneumonitis.31, 34 Late radiation-induced toxicity was not properly assessed, because most of the cases underwent lobectomy within 3 months after the last radiotherapy session and necropsy was not performed. Lymph nodes status including presence of metastasis was not thoroughly known as no sampling of lymph nodes was performed. Finally, the delivered radiation dose to tumors, as well as normal tissues, may be different from the calculated dose as there was no control for respiratory motion during radiotherapy.
In conclusion, the results of the present study suggest that preoperative hypofractionated radiotherapy may be useful for solitary lung adenocarcinoma in dogs, particularly when the tumor is too large or immediate surgery is not possible. The tumor response to radiation, the resolution of clinical signs in most dogs following radiation treatment, and the tolerability of radiation therapy when treatment planning prioritized normal tissue tolerance over the dose to the tumor were demonstrated. Although tumor size reduction occurs gradually with time, the possibility of progression or metastasis remains. Long-term monitoring, including monitoring for late radiation effects, should be performed.
LIST OF AUTHOR CONTRIBUTIONS
Category 1
- (a) Conception and Design: Kawabe, Kitajima, Murakami, Iwasaki, Goto, Mori
- (b) Acquisition of Data: Kawabe, Kitajima, Sakai
- (c) Analysis and Interpretation of Data: Kawabe, Kitajima, Murakami, Iwasaki, Goto, Sakai, Mori
Category 2
- (a) Drafting the Article: Kawabe, Kitajima
- (b) Revising Article for Intellectual Content: Murakami, Iwasaki, Goto, Sakai, Mori
Category 3
- (a) Final Approval of the Completed Article: Kawabe, Kitajima, Murakami, Iwasaki, Goto, Sakai, Mori