Hypofractionated radiation therapy in the treatment of canine thymoma: Retrospective study of eight cases
Abstract
Thymomas are one of the most common tumors of the cranial mediastinum in dogs; however there is limited information available on the use of radiation therapy for treating this neoplasm. Objectives of the current retrospective observational study were to describe outcomes and side effects of a hypofractionated radiation therapy protocol in a group of dogs with confirmed thymoma. A total of eight dogs were included. To generate individualized treatment plans, we designed the planning target volume according to the limits on mean lung dose and the percentage of the total lung volume exceeding 20 Gy (V20). The total administered dose was 48–49 Gy, with one fraction per week for a total of six to seven fractions. After therapy, two dogs achieved complete responses, two achieved partial responses, and the disease remained stable in two. Two dogs died during the radiation therapy protocol and were not classified. The median mean lung dose and V20 were 6.0 Gy (range: 3.1–15.0 Gy) and 12.4% (range: 2.3–27.5%), respectively. The overall response rate was 50.0%, and the median time to response following treatment initiation was 22 days (range: 14–115 days). Acute and late side effects were common in the skin and/or lung and were self-limiting or asymptomatic. The median survival time was not reached (range: 8–1128 days) and the 1 year survival rate was 75.0%. Hypofractionated radiation therapy was well tolerated in this sample of dogs with thymoma and may be considered when owners decline surgical treatment or the tumor is deemed unresectable.
1 INTRODUCTION
Thymomas are neoplasms of thymic epithelial cells.1, 2 Although thymomas are uncommon in dogs, they are one of the most common tumors of the cranial mediastinum.3, 4 Thymomas typically affect older patients, with a median age of 9.4 years.5 Paraneoplastic diseases are common in dogs with thymoma, including myasthenia gravis, hypercalcemia, erythema multiforme, severe lymphocytosis, and myocarditis.1, 4-12 Paraneoplastic myasthenia gravis often exhibits clinical signs of megaesophagus.1, 5-10
Thymomas rarely metastasize and are classified as either noninvasive or invasive. The former displace surrounding organs and the latter wrap around surrounding structures. Computed tomography (CT) scans can be used to evaluate the degree of invasiveness of thymomas.5, 7 Although surgical excision is the treatment of choice for thymoma, complete curative resection is often difficult or impossible due to the extent of invasiveness. In a retrospective study of 11 dogs, the median survival time for dogs treated with surgery alone was 790 days.7 However, three of 11 dogs died in the hospital within 24 h of surgery, and some of which had invasive thymomas. In another previous study, a dog with invasive thymoma died 5 days after surgery.6 These results suggest that surgery could be associated with high morbidity in some dogs with invasive thymomas. Because another study has reported that lack of surgical treatment is a negative prognostic factor, alternative treatment options are needed for dogs with invasive thymomas in which surgery is challenging or whose owners decline surgical treatment.5
Radiation therapy has been reported to be useful as either adjunctive or primary treatment for humans with thymoma.13-16 Although it is believed that thymoma is sensitive to radiation, little has been reported on the use of radiation therapy to treat canine thymoma. In a retrospective study of 16 dogs treated with radiation therapy, two dogs achieved complete responses and nine dogs achieved partial responses, and the median survival time was 248 days.17 However, many dogs concurrently received surgery and/or chemotherapy in that study. There was only one dog that achieved complete response attributable to radiation therapy alone, since the other dog was treated with radiation therapy after partial excision. Additionally, there were large variations in the radiation therapy protocol among the dogs, including total radiation dose (15–54 Gy), schedule (from daily to once weekly), and machines (orthovoltage and megavoltage). To our knowledge, there have been no published studies evaluating the outcomes and side effects of radiation therapy in dogs with thymoma as a primary therapy, using a standardized protocol.
The objective of this study was to describe the outcomes and side effects of hypofractionated palliative radiation therapy as monotherapy in a group of dogs with thymoma.
2 MATERIALS AND METHODS
2.1 Patient selection
The study was a retrospective, observational design. The medical database at the Animal Medical Center of Gifu University was searched for dogs with histopathologically or cytologically confirmed thymoma that received hypofractionated radiation therapy from December 2011 to April 2015.2, 18, 19 Decisions for subject inclusion and exclusion were made by an experienced veterinarian (S.G.). Dogs were diagnosed as thymoma on the basis of combined findings of a solitary mediastinal mass, and cytologic evidence of a mixed population of epithelial cells and small lymphocytes with mast cells. These findings help distinguish lymphocyte-predominant thymoma from other disease including lymphoma.5 Data from the medical database were recorded by an experienced veterinarian (S.G.) and included signalment, physical examination results, clinicopathologic findings, CT findings, treatments, and outcomes. Dogs that had concurrently received surgery and/or chemotherapy, except for prednisolone, or that had inadequate baseline or follow-up information were excluded from the study
2.2 Radiation therapy
As part of the inclusion criteria for the study, all dogs received the same radiation therapy protocol using external beam megavoltage three-dimensional conformal radiation therapy. Radiation was delivered by 4 MV photons from a linear accelerator (Primus Mid-Energy 4 MV linear accelerator; Siemens Healthcare, Malvern, PA). Radiation therapy planning was performed based on CT (Asteion Super 4; Toshiba Medical Systems, Tochigi, Japan) scans of each patient in the treatment position (sternal recumbency) in a vacuum-mattress immobilization device (Vac-Lok; MED-TEC, Orange City, IA). Individualized treatment plans were generated with a three-dimensional CT-based computer-generated treatment planning system (XiO Version 4.60; Elekta Japan, Tokyo, Japan) utilizing heterogeneity correction. Gross tumor volume (GTV) was defined as gross disease on CT images. No expansion for subclinical disease (gross tumor volume to clinical target volume margin) was used. The dose-volume histogram constraints for the organs at risk were as follows: mean lung dose of <20 Gy and V20 < 30% for the lungs, and dose delivered to 33% of the heart (D33%) < 60 Gy. The isocenter and beam arrangements were determined by the planning target volume locations and adjacent critical normal structures. The linear accelerator was equipped with a multileaf collimator (41, 10-mm leaves), which was used to shape the fields to expose the target volume and block adjacent normal tissue. A standard single isocenter technique with nonopposing coplanar fields was used. Treatments were delivered in six to nine unequally weighted fields in multiple oblique (0°, 40°, 80°, 120°, 160°, 200°, 240°, 280°, and 320°) beam arrangements. Dogs were administered 7 to 8 Gy per fraction, delivered to a total dose of 48 to 49 Gy with one fraction per week for a total of six to seven fractions. The radiation dose prescription point was set at the isocenter, rather than covering 95% of the planning target volume. For planning target volume, the parameters D2% and D98% were used for plan evaluation, which are, respectively, defined as the dose received by 2% and 98% of the planning target volume volume. These two values represent the maximum and minimum dose in the planning target volume. The source-axis distance was 100 cm, and source-to-surface distances were based on both dog size and positioning, and automatically determined by the treatment planning system. Megavoltage portal imaging was performed prior to treatment to verify the appropriate positioning before each treatment.
2.3 Efficacy assessment
Follow-up information was obtained by an experienced veterinarian (S.G.) using a review of the medical records. Tumor response was determined using CT scans and was classified according to the Veterinary Cooperative Oncology Group RECIST guidelines for dogs.20 A complete response was defined as the disappearance of all targeted lesions. A partial response was defined as a ≥30% reduction in the sum of the longest diameters of the targeted lesions, compared with the baseline sum. Stable disease was defined as a <30% reduction or <20% increase in the sum of the longest diameters of the targeted lesions, with the smallest sum of the diameters during the study designated as the reference value for at least 10 weeks. Progressive disease was defined as the appearance of ≥1 new lesion or a ≥20% increase in the sum of diameters of the target lesions, with the smallest sum during the study designated as the reference. Overall survival was defined as the time (days) from the date of the initiation of radiation therapy until death.
2.4 Toxicity assessment
Radiation side effects were assessed by a single observer (S.G.) through review of the medical records. Acute and late effects were characterized according to the radiation morbidity scoring scheme described by the Veterinary Radiation Therapy Oncology Group.21 Radiation side effects that developed during the first 6 months after the initiation of radiation therapy were classified as acute, and those that developed more than 6 months after the initiation of radiation therapy were classified as late effects. Acute and late effects on the thoracic organs were evaluated using CT scans of the chest.
2.5 Statistics
The Kaplan-Meier curve for overall survival was generated using the Kaplan-Meier product-limit method. Data were censored if dogs were alive at the end of the study. None of the dogs were lost to follow-up. The analysis was performed by principal and mentoring investigators (S.G. and T.M) with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for the R programming language (The R Foundation for Statistical Computing, Vienna, Austria).22
3 RESULTS
3.1 Patient data
Eighteen dogs with thymoma were identified by the retrospective search of the medical database during the investigation period. Three dogs were excluded because their owners declined any treatment. Tumor excision was performed for four dogs and three of them received hypofractionated preoperative radiation therapy. Two dogs were treated with hypofractionated radiation therapy and concurrent chemotherapy. Nine dogs received hypofractionated radiation therapy alone and one dog was excluded from the analysis because the information of this dog was inadequate. Overall, eight dogs met all of the study inclusion criteria. In four dogs, the planning target volume was defined by a margin of 5 mm around the gross tumor volume to accommodate positioning errors and motion. In the remaining four dogs, the planning target volume margin was 0 mm beyond the gross tumor volume to minimize radiation dose to the surrounding organs, especially the lungs. To prevent any increase in lung exposure with tumor regression, the tumor size was reevaluated with CT scans just before the third or fourth radiation therapy session in all but one dog (dog 8), which died before receiving the third radiation therapy fraction, and the radiation therapy planning was modified if a ≥30% reduction was observed in the longest diameter of the tumor.
The results of the individual patients are summarized in Appendices 1 and 2. The median age was 11 years (range: 10–14 years). The median weight was 7.18 kg (range: 1.98–27.7 kg). Two dogs were Shih Tzus; two were Yorkshire terriers, and there were one each of the following breeds: mixed breed dog, Papillon, Chihuahua, Golden retriever. Clinical signs at the time of initial examination included: lethargy (4/8), anorexia (3/8), exercise intolerance (3/8), vomiting or regurgitation (3/8), dyspnea (2/8), cough (2/8), and weight loss (1/8). Seven of eight dogs presented with clinical signs related to thymoma. In one dog (dog 6), thymoma was incidentally detected with no clinical signs. For all dogs, complete blood count and serum biochemical analysis, including the calcium concentration, were performed at the time of initial examination. None of the dogs had a high total calcium concentration. Two dogs were determined to have myasthenia gravis on the basis of detection of serum acetylcholine receptor antibody (AchR Ab). Of these dogs, one dog (dog 1) had megaesophagus and the other dog (dog 8) had no clinical signs or CT findings associated with myasthenia gravis. The remaining six dogs were not tested for AchR Ab.
Ultrasound-guided or CT-guided fine-needle aspiration of masses was performed in all dogs. Four of eight dogs were diagnosed as thymoma based on both histologic and cytologic evaluation. Cytologic and histopathologic findings were in agreement in these dogs. The remaining four dogs were diagnosed based on cytological evaluation alone.
3.2 Diagnostic imaging
Total body CT was performed on a 4-detector row CT scanner (Asteion™ Super 4; Toshiba Medical Systems) and a cranial mediastinum mass was found in all dogs. Computed tomographic technical parameters were as follows: rotation time, 0.75 s; slice thickness, 1–2 mm; field of view, 160–340 mm; matrix dimensions, 512 × 512; reconstruction interval, 0.5–1 mm; detector pitch, 3.5; collimator pitch, 0.875; X-ray tube potential, 120 kV; and X-ray tube current, 30–100 mA. Computed tomographic measurements of the mass were available and the mean longest diameter was 6.1 cm (range: 3.8–17.4 cm). Evidence of pleural effusion was observed in one dog (dog 7). One dog (dog 8) had regional lymph node (sternal lymph node) enlargement. Evidence of pulmonary metastasis was not detected in any dogs. Megaesophagus was observed by CT in one dog with myasthenia gravis and there was no evidence of aspiration pneumonia.
Of the seven dogs that underwent contrast-enhanced CT, three had a tumor that appeared noninvasive and displaced the surrounding structures, and the remaining four dogs had tumors that appeared invasive and were wrapping around the surrounding structures. These four tumors were deemed to be likely unresectable because of the extent of invasiveness.
3.3 Treatment
The prescribed hypofractionated radiation protocol consisted of one fractionated dose of 7 or 8 Gy given weekly to a total dose of 48 or 49 Gy. Two of the eight dogs (dog 7, dog 8) died due to respiratory failure before completing the course of radiation therapy and received a total dose of 28 and 14 Gy, respectively. The mean values of the minimum (D98%), mean, and maximum (D2%) dose for the planning target volume were 31.2 Gy (range: 20.5–41.5 Gy), 44.8 Gy (range: 40.8–48.4 Gy), and 49.8 Gy (range: 47.9–52.5 Gy), respectively. Due to tumor shrinkage during the radiation therapy, a new plan with modified gross tumor volumes and planning target volumes was generated and used to complete the course of radiation therapy in three dogs (dog 2, dog 3, dog 4). In dog 8, the enlarged regional lymph node was not included as part of the gross tumor volume because the degree of enlargement in the regional lymph node was mild while the tumor at the primary site was very large. Therefore, the gross tumor volume was confined to the tumor at the primary site as palliative treatment.
The median mean lung dose and V20 to the lungs were 6.0 Gy (range: 3.1–15.0 Gy) and 12.4% (range: 2.3–27.5%), respectively. Three of the eight dogs (37.5%) concurrently received prednisolone from the date of the initiation of radiation therapy at a median dose of 0.7 mg/kg (range: 0.25–1.0 mg/kg) once a day. The median duration of administration was 17 days (range: 8–29 days).
3.4 Response and survival assessment
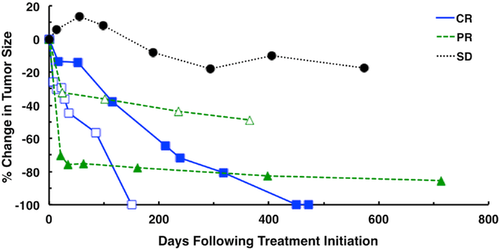
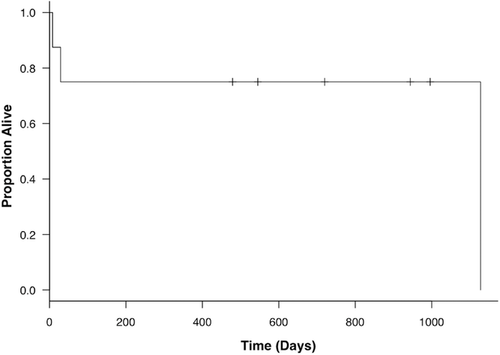
All eight dogs were followed until they died from thymoma or unrelated diseases; the dogs still alive at study completion were censored from the survival analysis. The median follow-up period was 632 days (range: 8–1128 days). According to the CT scans, the responses were as follows: two (25.0%) dogs had complete response, two (25.0%) had partial response, two (25.0%) had stable disease, and two (25.0%) had died during the radiation therapy protocol for an overall response rate of 50.0% (four dogs). Radiation therapy successfully controlled tumor growth in both the short term and long term. The median time to response that we observed following treatment initiation was 22 days (range: 14–115 days). Furthermore, five dogs (two complete response, two partial response, and one stable disease) were followed by CT scans to evaluate the tumor-inhibitory effects of radiation therapy over a long time period. In these dogs, tumor progression was not observed for more than 150 days (range: 150–713 days). Of the two dogs that achieved complete response, the time to achieve complete response was 150 and 450 days (Fig. 1). Five of eight dogs (62.5%) were still alive at study completion, and median survival time was not reached (range: 8–1128 days) (Fig. 2). Out of the three dogs that died before study completion, two (dog 7, dog 8) died as a result of respiratory failure attributable to large tumor or myasthenia gravis. The remaining one (dog 5) died from unknown causes. We obtained information from the referring veterinarian, and it was assumed that this dog (dog 5) died due to old age (17 years), though the cause of death had potential to be associated with thymoma. Of the two dogs that died of respiratory failure, one dog (dog 7) had a large tumor with pleural effusion, and demonstrated dyspnea and anorexia at the time of initial examination. These clinical symptoms and pleural effusion continued after the radiation therapy and the dog died as result. The other dog (dog 8) with myasthenia gravis demonstrated severe respiratory failure after the first radiation therapy session that progressed to respiratory arrest. In four of seven dogs (57.1%) with clinical signs (dog 7 did not show any clinical signs), the clinical signs noted at the time of diagnosis achieved improvement over a median time of 15 days (range: 5–17 days). In one dog with myasthenia gravis (dog 1), clinical signs consistent with megaesophagus, including regurgitation and lethargy, partially resolved after the radiation therapy, although megaesophagus was still observed. The dog was still alive at 996 days following treatment initiation.
3.5 Side effects
The individual patient data regarding the total radiation dose and side effects are summarized in Appendix 2. Acute and late effects on the thoracic organs, including the lungs and heart, were evaluated using CT scans of the chest in all dogs. Acute effects could be retrospectively evaluated in six dogs and were observed in five (83.3%) of these dogs, consisting of grade 1 lung (n = 4), and grade 1 skin (n = 2) effects. The median time to occurrence of acute effects was 63 days (range: 56–85 days). Of the four dogs with acute effects in the lung, three showed radiographic changes for which they were asymptomatic. These were followed up with CT scans. These effects were resolved or improved by the next session. The remaining dog showed clinical symptoms, including cough, that were resolved after 3 weeks. Late effects could be retrospectively evaluated in four dogs and were observed in three (75.0%) of these dogs, consisting of grade 1 lung (n = 3) effects. Late effects on the heart were not observed in these dogs. The median time to occurrence of late effects was 211 days (range; 173–294 days). All three dogs with late effects in the lung were followed up by CT scans over multiple time points. The radiographic changes did not progress but continued to be observed in these dogs. All side effects were self-limiting or asymptomatic, which did not warrant any specific treatment.
4 DISCUSSION
The goal of this retrospective study was to describe the outcomes and side effects of hypofractionated palliative radiation therapy as monotherapy in dogs with thymoma. In this study, we made two important observations. First, dogs with thymoma demonstrated good responses to hypofractionated radiation therapy and the tumor-inhibitory effects of the treatment lasted for a long time period. Second, the degree of side effects with hypofractionated radiation therapy was self-limiting or asymptomatic, which suggests that this protocol was a tolerable treatment approach.
Hypofractionated radiation therapy was effective for the induction of complete response and partial response as well as stable disease in the dogs with thymoma that were sampled in the current study. Of the eight dogs that were evaluated by CT scans according to the RECIST guidelines in this study, two (25.0%) dogs achieved complete response and two (25.0%) achieved partial response, for an overall response rate of 50.0%. In a previous study, a complete response rate of 2 of 16 (12.5%) and partial response rate of 9 of 16 (56.3%) were reported, for an overall response rate of 68.8%.17 However, radiography was only used to evaluate tumor responses and the RECIST criteria were not available at that time. Additionally, some dogs also received other therapy, including partial excision and chemotherapy. Therefore, the results of our study cannot be compared with this earlier study. Tumor regression was observed quickly after initiation of the treatment and most of the dogs in this study achieved complete response or partial response, and the median time to response was 22 days. However, the tumors gradually regressed for several months in most dogs, and only one dog had experienced a >50% reduction in the longest diameter of tumor at 1 month after treatment initiation. Five of eight dogs (62.5%) were still alive at the end of follow-up, and the median survival time was not reached. The 1-year survival rate in this study was 75.0%, which is comparable to that reported in a previously published study of canine thymoma patients treated by surgery alone (64%), although another study reported that lack of surgery was a negative prognostic factor in the treatment of canine thymoma.5, 7 Interestingly, two dogs that were classified as stable disease achieved long-term survival (>2 years), with a survival time of more than 996 days (still alive) and 1128 days, respectively. At the time of initial examination, bronchial compression and paraneoplastic diseases were not seen in either dog and their physical condition was good, although mild atelectasis in part of the cranial lung lobe was detected in one of them. In contrast, two dogs that had died during the radiation therapy protocol presented with severe clinical symptoms due to a large tumor size at the time of diagnosis, including lethargy, coughing, and dyspnea. Additionally, myasthenia gravis was also observed in one of them. These results demonstrate the possibility of long-term survival after radiation therapy even in patients with stable disease, if the patients have no significant complications attributable to the space-occupying mass, to adhesions into neighboring organs, or to paraneoplastic diseases.
In the five dogs that were followed by CT scans for >100 days, the tumor-inhibitory effects of radiation therapy were found to last for at least 150 days after the initiation of therapy. There was no evidence of local recurrence or distant metastasis in these dogs, thus progression-free time was not determined. Generally, the frequency of metastasis is low. Most patients, dog or human, that experience progressive disease experience it locally.5, 7, 23, 24 A retrospective study reported that recurrence was identified in 17% of dogs after surgery and the mean interval to tumor recurrence was 518 days.5 In a case series of 11 human patients with thymoma treated with radiation therapy, 27% of patients who achieved complete responses experienced recurrence within the thorax but outside the radiation field after treatment with radiation therapy.14
The second noteworthy finding was that side effects with hypofractionated radiation therapy were self-limiting or asymptomatic. No dogs died of radiation-related side effects. In a previous study, dogs treated with orthovoltage X-ray radiation developed significant dermatitis and pneumonitis that was life-threatening.17 Generally, lung toxicity is one of the most common concerns and a dose-limiting toxicity with thoracic irradiation. In human medicine, a recent review found that a >20% risk of radiation pneumonitis is associated with a mean lung dose of >20 Gy.25 It has been also reported that V20 to the lungs is a useful parameter for predicting the risk of radiation pneumonitis; when V20 was 22–31%, the incidence was less than 10% in human patients with lung cancer who received radiation therapy.26 On the basis of these data, mean lung dose of <20 Gy and V20 of <30% are recommended to minimize lung toxicity when using radiation therapy to treat thymic neoplasm in humans.27 In our study, mean lung dose and lung V20 were kept below the same limits. Acute and late side effects frequently occurred, but all of them were self-limiting or asymptomatic. It may be useful to evaluate those dosimetric factors, such as V20 and mean lung dose, which are obtained from the three-dimensional treatment planning system to avoid severe pneumonitis in dogs with thymoma. However, a causal relationship between lung toxicity and these factors remains unclear, and further investigation is required. In humans, the dose levels associated with a 5% risk of pericarditis within 5 years of treatment (TD5/5) are 60 Gy for irradiation of one-third of the heart (D33%).28 More recently published studies proposed to keep the mean pericardial dose <26 Gy with V30 < 46% to keep the risk of pericarditis <15%.29, 30 For Hodgkin's lymphoma and breast cancer patients, it has been suggested that V25 for the whole heart should be <10% to avoid long-term cardiac mortality31, 32. In the present study, some dogs exceeded the limits of those dose volume parameters (data not shown), although D33% of the heart was kept below the limit for all dogs (<60 Gy). Dogs with thymomas that have large tumors tend to receive high radiation doses to the heart. In the present study, although radiation-induced cardiotoxicity was not observed, careful monitoring should be required. Generally, a hypofractionated protocol tends to increase the risk of late effects, compared to a multifractionated protocol, due to the larger dose per fraction. We modified the planning target volume according to the normal surrounding tissue constraints for the lungs in the present study. Expansion for the gross tumor volume to clinical target volume margin and the gross tumor volume-planning target volume margin was not used in four dogs. This may have resulted in inadequate dosing of parts of the tumor due to positioning errors or breathing motion. Multifractionated protocols that use lower doses per fraction are more tolerable by normal tissues and allow for tumor dose homogeneity, which may improve clinical outcomes. Multifractionated radiation therapy is common in the treatment of human thymoma and is recommended in doses of 40–60 Gy given in 20–30 fractions.13, 14, 33 However, dogs with thymoma often have clinical signs associated with the respiratory tract or paraneoplastic myasthenia gravis. In such patients, a hypofractionated protocol may be more suitable, as it requires fewer anesthetic episodes compared to a multifractionated protocol. Three of four dogs (75%) in the current study developed late effects but all of them were asymptomatic.
Thymoma is associated with autoimmune disorders, and myasthenia gravis is the most common paraneoplastic disease in both humans and dogs.1, 5-10, 23 Only two dogs (dog 1, dog 8) in the present study were determined to have myasthenia gravis on the basis of detection of serum AchR Ab. One dog (dog 1) had myasthenia gravis as well as megaesophagus, which was associated with clinical signs, including regurgitation, and lethargy. This dog achieved complete response and clinical signs consistent with partial resolution of myasthenia gravis, but megaesophagus was observed. It has been reported that after thymectomy, both dogs and human patients with myasthenia gravis experience attenuation of the clinical signs but not necessarily complete resolution.5, 6, 8, 10, 34, 35 In one report describing human thymoma, a patient with uncontrollable myasthenia gravis experienced marked improvement after radiation therapy.16 Future studies are needed to determine the degree to which radiation therapy for canine thymoma contributes to the improvement of myasthenia gravis. The other dog (dog 8) developed severe respiratory failure after the first radiation therapy session and died as a result. There was no overt clinical evidence of myasthenia gravis in this dog and no radiographic evidence of megaesophagus at the time of treatment. The acetylcholine receptor antibody titer was measured after the initiation of radiation therapy, and the result was only obtained after the death of this dog. In human medicine, it is thought that myasthenia gravis can increase the perioperative mortality rates and the leading cause of deaths in thymectomy is myasthenic crisis.36 In a previous report, a dog with thymoma had subclinical signs at the time of surgery, but clinical signs of myasthenia gravis were observed soon after removal of the thymoma.6 A small proportion of human patients without initial signs of myasthenia gravis have been reported to develop the disease after removal of the thymoma.37 Therefore, we suggest that an assessment of the AChR Ab titer before the initiation of radiation therapy may help reduce the risk associated with anesthesia.
Our study has several limitations, including a small sample size, and a lack of evaluation of histopathologic subtypes and pathological stages. The identification of prognostic factors was not an objective of the present study; it would have been difficult to find significant factors because of the small sample size. A previous study reported that the pathological stage was a prognostic factor.5 However, this staging system could not be applied to dogs in the present study, because the pathological stage could not be fully determined without surgery. Thymomas are composed of thymic epitheliums with variable lymphocytic infiltration.1, 3, 4 Lymphocytes are typically radiosensitive and it may be possible that the lymphocyte-predominant type is more radiosensitive than other forms. The World Health Organization's (WHO) classification scheme for thymoma is the most widely used in humans, and this classification scheme can also apply to canine thymoma.2 It was impossible to differentiate these histopathologic subtypes by tumor biopsy sample, and the lymphoid component of each tumor was not evaluated. Furthermore, it is difficult to diagnose canine thymoma based only on biopsy samples, except for the lymphocyte-predominant type that corresponds to type AB, B1, and B2 thymoma under the WHO classification scheme. This implies that the majority of thymomas in the present study were likely to be the lymphocyte-predominant type, potentially biasing our results.
In conclusion, results of the present study indicated that the hypofractionated radiation therapy was well tolerated in a sample of dogs with thymoma when the lung dose constraints were followed and that durable tumor responses were observed. Although further studies are necessary to evaluate the prognostic factors and time to recurrence when administering radiation therapy as monotherapy in dogs with thymoma, our findings suggest that local control may be achieved by hypofractionated radiation therapy and that evaluation of radiation therapy as an alternative to surgery for canine thymoma may be warranted. Further investigation is also needed to determine the optimal radiation therapy protocol.
LIST OF AUTHOR CONTRIBUTIONS
Category 1
- (a)
Conception and Design: Goto S, Murakami M, Kawabe M, Iwasaki R, Heishima K, Mori T
- (b)
Acquisition of Data: Goto S, Mori T, Sakai H
- (c)
Analysis and Interpretation of Data: Goto S, Murakami M, Kawabe M, Iwasaki R, Heishima K, Sakai H, Mori T
Category 2
- (a)
Drafting the Article: Goto S
- (b)
Revising Article for Intellectual Content: Murakami M, Kawabe M, Iwasaki R, Heishima K, Sakai H, Mori T
Category 3
- (a)
Final Approval of the Completed Article: Goto S, Murakami M, Kawabe M, Iwasaki R, Heishima K, Sakai H, Mori T
APPENDIX 1: SUMMARY OF CLINICAL CHARACTERISTICS AND OUTCOMES FOR EACH OF THE EIGHT SAMPLED DOGS
| PT | Signalment | Dose | LN enlargement | RECIST response | Time to response (days) | Paraneoplastic syndrome | Survival (days) | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 years, 2.8 kg, FS | 48 Gy/6 fr | − | CR | 115 | Megaesophagus Myasthenia gravis | >720 | Alive |
| 2 | 12 years, 27.7 kg, FI | 48 Gy/6 fr | − | CR | 14 | None | >479 | Alive |
| 3 | 10 years, 7.1 kg, FS | 48 Gy/6 fr | − | PR | 22 | None | >944 | Alive |
| 4 | 13 years, 9.6 kg, MI | 49 Gy/7 fr | − | PR | 25 | None | >545 | Alive |
| 5 | 14 years, 10.6 kg, FS | 48 Gy/6 fr | − | SD | − | None | 1128 | Unknown |
| 6 | 10 years, 1.98 kg, MI | 49 Gy/7 fr | − | SD | − | None | >996 | Alive |
| 7 | 11 years, 7.3 kg, MC | 28 Gy/4 fra | − | N/A | − | None | 29 | Respiratory failure |
| 8 | 12 years, 6.5 kg, FI | 14 Gy/2 fra | + | N/A | − | Myasthenia gravis | 8 | Respiratory failure |
- FS, female spayed; FI, female intact; MI, male intact; MC, male castrate; Gy, gray; fr, fraction; LN, regional lymph node; CR, complete response; PR, partial response; SD, stable disease; N/A, not available.
- aDied before completing the course of radiation therapy.
APPENDIX 2: SUMMARY OF DELIVERED DOSE TO SURROUNDING ORGANS AT RISK AND SIDE EFFECTS FOR EACH OF THE EIGHT SAMPLED DOGS
| Lung dose | Heart dose | Side effects | |||||
|---|---|---|---|---|---|---|---|
| PT | Dose/fraction | RECIST response | Mean (Gy)b | V20 (%)c | D33% (Gy)d | Acute effects | Late effects |
| 1 | 48 Gy/6 fr | CR | 4.2 | 9.0 | 2.4 | None | Lung (grade 1) |
| 2 | 48 Gy/6 fr | CR | 3.7 | 6.7 | 0.9 | Lung (grade 1)e | N/Af |
| [5.1] | |||||||
| 3 | 48 Gy/6 fr | PR | 15.0 | 27.5 | 29.1 | Lung (grade 1)e | Lung (grade 1) |
| [7.1] | |||||||
| 4 | 49 Gy/7 fr | PR | 6.9 | 14.5 | 11.0 | Skin (grade 1) | None |
| [4.7] | |||||||
| 5 | 48 Gy/6 fr | SD | 5.1 | 10.3 | 6.4 | Skin (grade 1), Lung (grade 1)e | Lung (grade 1) |
| 6 | 49 Gy/7 fr | SD | 7.9 | 17.4 | 21.8 | Lung (grade 1) | N/Af |
| 7 | 28 Gy/4 fra | N/A | 3.1 | 2.3 | 12.9 | N/Af | N/Af |
| 8 | 14 Gy/2 fra | N/A | 10.2 | 18.4 | 41.0 | N/Af | N/Af |
- CR, complete response; PR, partial response; SD, stable disease; N/A, not available.
- aDied during the radiation therapy.
- bThe numbers in square brackets indicate the mean lung dose in the new treatment plan.
- cThe percentage of the total lung volume exceeding 20 Gy.
- dDose received to 33% of the volume of heart.
- eRadiographic changes without clinical signs of cough.
- fNot available.




