TECHNIQUE FOR ULTRASOUND-GUIDED INTRAARTICULAR CERVICAL ARTICULAR PROCESS INJECTION IN THE DOG
Funding Support provided through a grant by the Merial Veterinary Scholars Program. Abstract presented at the ACVR Annual Meeting in Las Vegas, USA, 2012 and at the International Veterinary Radiology Association Meeting in Bursa, Turkey, August 2012.
Abstract
Ultrasound-guided intraarticular injection of cervical articular process joints is a well-established procedure in both humans and horses for neck pain resulting from osteoarthritis, but the technique has not been described in dogs. Aims of this study were to describe the ultrasonographic anatomy and landmarks for cervical articular process joint injections in the dog, develop a technique for articular process joint injections using these landmarks, and determine the accuracy of injections and factors that may influence it. Eleven canine cadavers were used and bilateral joint spaces from C2–3 to C7-T1 were injected under ultrasound guidance with a blue radiopaque solution. A computed tomographic scan was acquired following each injection, and an injection score was assigned and compared with other patient-specific factors. Of the 132 injections performed, 110 (83.3%) were intraarticular, 20 (15.1%) were periarticular within 5 mm, and 2 (1.5%) were periarticular beyond 5 mm from the joint. There was no significant difference in mean scores between dogs. Only C2–3 had a significantly lower mean score than any other joint. There was no significant correlation between injection score and any other factors measured. The transverse processes of the cervical vertebrae served as excellent ultrasonographic landmarks for identifying the cervical articular process joints in dogs regardless of the size of the dog or location along the vertebrae. Accuracy of ultrasound-guided intraarticular process joint injection was 83% in dogs and similar to published techniques in horses. Further studies are needed to examine the safety and efficacy of this procedure in live animals.
Introduction
Cervical spondylomyelopathy is a common disease in large breed and giant breed dogs due to both disc disease and osseous lesions of the cervical spine, and the compression of the spinal cord and nerve roots associated with cervical spondylomyelopathy is a common source of neck pain.1 Compressions of the spinal cord in dogs with bone-associated cervical spondylomyelopathy are caused by a combination of vertebral malformations and osteoarthritic changes of the articular process.1 Diagnosis of bone-associated cervical spondylomyelopathy requires ruling out other sources of neck pain, whether from osseous, soft tissue, or disc disease. Cervical spondylomyelopathy has traditionally been diagnosed with clinical signs and myelography, but MRI and CT has also been used.1-3 MRI is more accurate than myelography in the diagnosis of site, severity, and nature of spinal cord compression with cervical spondylomyelopathy.3 Bone-associated cervical spondylomyelopathy can be diagnosed on MRI as hypointense bony proliferations associated with the articular processes, lamina, and pedicles in both T1- and T2-weighted images.1
Surgical treatment of cervical spondylomyelopathy depends on factors such as the severity of neurologic signs and pain,1 but medical management has been shown to stabilize or improve clinical signs in 81% of dogs.4 Traditional medical management of pain due to arthrosis of the cervical articular process joints in dogs has typically consisted of systemic corticosteroid or nonsteroidal anti-inflammatory therapy.4 However, due to the potential negative effects of systemic treatments,5 alternatives to systemic therapies are being explored. One study found that fluoroscopically guided intraarticular injection of the cervical articular process joints was feasible and effective for control of pain due to arthrosis.6
Ultrasound-guided intraarticular injection of cervical articular process joints is a well-established procedure in both humans and horses for neck pain resulting from osteoarthritis, but it has not been described in dogs.7, 8 One technique in horses describes locating the characteristic “S-shaped” curvilinear echogenic interface, often referred to as the “chair” sign, formed by the cranial and caudal articular process in a craniocaudal plane parallel to the long axis of the neck.8 A cadaveric study in horses found the reliability of a similar technique to be 98%.9 The course of disease in cervical spondylomyelopathy is similar and dogs and horses,1, 10 and ultrasound-guided injection of the cervical articular process joint is commonly performed in horses.11, 12 Ultrasound-guided injection avoids the potential adverse effects of systemic therapies, and ultrasound is more widely available than fluoroscopy and does not use ionizing radiation.
This study aims to establish the ultrasonographic anatomy and landmarks for the cervical articular process joint in the dog and, using these landmarks, to develop a technique for cervical articular process joint injections. The accuracy of injections and the factors, which may influence it, will also be assessed.
Materials and Methods
Eleven mixed-breed dog cadavers ranging from 5.3 to 24.2 kg (mean = 18.1, SD = 5.6) body weight that were euthanized for nonorthopedic and nonneurologic reasons were used in this study.
An ultrasound examination of two cadaver spines with the soft tissues dissected away and immersed in a water bath was performed to identify bony landmarks for each cervical articular process joint from C2–3 to C7-T1 and establish their ultrasonographic appearance. It was observed that extending the cervical spine at a 125° angle to the thoracic spine opened the joint spaces from C2-T1 compared with a neutral position and would potentially facilitate needle placement.
Dog cadavers were placed in lateral recumbency for injection. A goniometer was used to extend the neck to form a 125° angle between the cervical and thoracic spine and the forelimbs were pulled caudally to expose the caudal cervical region. In order to localize the joint for injection, the wing of the atlas was palpated, and the transducer was placed on it in a dorsal plane parallel to the long axis of the neck. The transducer was moved caudally along the same plane until the transverse processes of two adjacent cervical vertebrae were visible (Fig. 1). The transducer was then angled approximately 30° dorsally until the articular process joint was visible (Fig. 2, Video 1). For each injection, a 22-gauge, 1.5 inch needle was inserted cranial to the transducer in the plane of the ultrasound beam and angled caudally parallel to the orientation of the articular process joint.
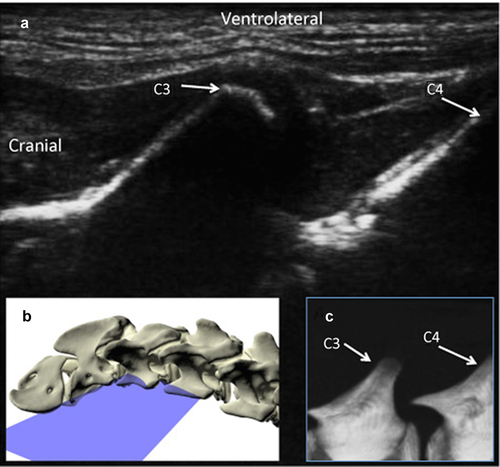
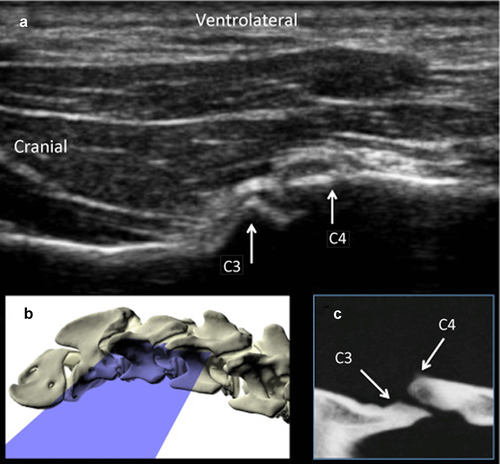
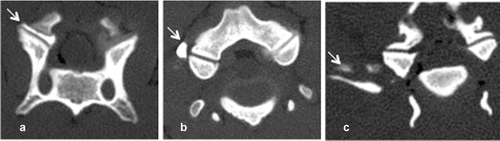
To determine the accuracy of this technique, each joint space from C2–3 to C7-T1 on both the right and left sides was injected individually under ultrasound guidance with 0.1 ml of a solution of 0.67% toluidine blue, 10% gelatin, and 33% iothalamate meglumine* 282 mg/ml organically bound iodine (Conray®, Mallinckrodt Inc., St. Louis, MO).9, 13 A CT examination was acquired following each injection (16-slice Light Speed CT scanner, GE Healthcare, Milwaukee, WI), and an injection score was assigned (Fig. 3). Scores from 1–3 was assigned as follows: 1: intraarticular, 2: peri-articular injections less than 5 mm from the joint, and 3: peri-articular greater than 5 mm from the joint. High accuracy was defined as a score of 1. Less accurate injections were based on the distance from the joint for scores 2 and 3 was determined arbitrarily. In order to assess if a learning curve played a role in the score, injection scores of the first 6 necks were compared with those of the last 5 necks. Three cadavers were frozen after injection and prosected at the level of each joint to confirm the location and extravasation of the injections (indicated by the presence of blue dye).
Age, gender, body weight, body condition score from 1 to 9,14 transverse and dorsal angle of each joint,15 neck diameter at each joint, vertebral canal diameter for each vertebra, and vertebral body/height and length for each vertebra16 were recorded for each dog. All measurements were performed using an image analysis workstation (EFilm version 3.1.0.21, Merge Healthcare, Milwaukee, WI). Transverse angles of the articular process joints were defined as the mean of three measurements of the angle made between the line connecting the dorsal and ventral edge of the cranial articular process and the mid-sagittal line in transverse CT scans. Dorsal angles of the articular process joints were defined as the mean of three measurements of the angle between the line connecting the cranial and caudal edge of the cranial articular process and the mid-sagittal line in dorsal CT scans.15 Neck diameter was defined as the mean length of three evenly spaced lines bisecting the neck connecting the outermost layer of skin in a transverse CT image. Vertebral canal diameter was defined as the narrowest portion of the vertebral canal in a mid-sagittal plane at the cranial 25 % of the vertebra, perpendicular to the vertebral canal and on the inner surface of the vertebral canal from the cranioventral aspect of the lamina to the craniodorsal aspect of the vertebral body.16 Vertebral body height (H) was defined as the maximum height of the cranial 25% of the vertebral body made parallel to the cranial vertebral endplate in a mid-sagittal CT scan.16 Vertebral body length (L) was defined as the length of the line parallel to the vertebral canal starting at the center point of the cranial vertebral endplate and ending at the most caudal part of the caudal vertebral endplate in a mid-sagittal CT scan.16 Vertebral ratios were calculated as vertebral canal diameter/H and vertebral canal diameter/L, and neck diameter to vertebral canal diameter ratios were calculated as vertebral canal diameter/neck diameter.
Data were analyzed using commercially available statistics software (SAS®, version 9.2 Cary, NC). The mean injection score between each dog and between each site were compared using two-sample t-tests. The injection scores of the first 6 dogs were also compared with the last 5 injections in order to determine if experience improved injection score. Spearman correlations and analysis of variance with post-hoc Tukey's test were used to determine the effect of patient-specific factors on injection score. Variability of joint angles and neck diameters and ratios was examined with Levene's test. All comparisons were considered significant at P ≤ 0.05.
Results
Of the 132 injections performed on 11 cadavers, 110 (83.3%) had a score of 1 (intraarticular), 20 (15.1%) had a score of 2 (periarticular within 5 mm), and 2 (1.5%) had a score of 3 (periarticular beyond 5 mm) (Table 1). The mean injection score for all the injected joints for each dog was not significantly different from the mean score of any other dog. However, with respect to joint location, C2–3 had a significantly greater (P < 0.001) mean score than any other joint. The first six necks injected had a significantly greater mean score than the last five necks injected (P < 0.001), though total number of necks injected did not have a significant correlation to average injection score (Table 2). The three dogs that were prosected following articular process joint injection showed that scores of 1 did not have blue dye within the periarticular soft tissues while all other scores did. This reflected the same appearance of the location of the contrast in the CT images.
| Articular process joint | Injection score = 1 | Injection score = 2 | Injection score = 3 | Mean score | Mean transverse joint angle ± SD (range) | Mean dorsal joint angle ± SD (range) | Mean VCD/H ± SD (range) | Mean VCD/L ± SD (range) | Mean crVCD/neck diameter ± SD (range) |
|---|---|---|---|---|---|---|---|---|---|
| C2–3 | 12 | 8 | 2 | 1.5* | 62.1 ± 7.3 (48.3–78.7)** | 23.5 ± 7.7 (9.0–39.7) | 0.8 ± 0.2 (0.5–1.0) | 0.2 ± 0.0 (0.2–0.3) | 0.1 ± 0.003 (0.09–1.0)*** |
| C3–4 | 18 | 4 | 0 | 1.2 | 67.1 ± 7.7 (55.3–75.0) | 20.7 ± 7.8 (4.3–35.0) | 0.7 ± 0.1 (0.0.5–0.9) | 0.3 ± 0.0 (0.3–0.4) | 0.08 ± 0.003 (0.07–0.08) |
| C4–5 | 20 | 2 | 0 | 1.1 | 67.8 ± 4.9 (59.7–80.3) | 22.7 ± 6.8 (11.0–36.7) | 0.7 ± 0.1 (0.5–0.1) | 0.3 ± 0.0 (0.3–0.4) | 0.08 ± 0.003 (0.07–0.08) |
| C5–6 | 20 | 2 | 0 | 1.1 | 68.0 ± 4.6 (60.0–76.3) | 34.3 ± 11.3 (17.0–54.3)¶¶ | 0.7 ± 0.1 (0.6–1.0) | 0.4 ± 0.1 (0.3–0.5) | 0.08 ± 0.003 (0.07–0.08) |
| C6–7 | 20 | 2 | 0 | 1.1 | 61.9 ± 9.0 (35.0–73.3)** | 45.7 ± 9.3 (22.3–58.7)¶ | 0.8 ± 0.1 (0.6–1.0) | 0.5 ± 0.0 (0.5–0.6) | 0.08 ± 0.003 (0.07–0.08) |
| C7-T1 | 20 | 2 | 0 | 1.1 | 52.1 ± 5.5 (41.3–52.1)§ | 46.0 ± 9.8 (24.0–46.0)¶ | 0.7 ± 0.1 (0.6–0.9) | 0.6 ± 0.2 (0.5–0.7) | 0.06 ± 0.003 (0.05–0.07)*** |
- *C2–3 injection score was statistically significantly greater (P < 0.001) than the other joints.
- **C2–3 and C6–7 were statistically significantly different joint angle morphology (P < 0.001) than the other joints but not from each other.
- §C7-T1 was statistically significantly different (P < 0.001) than the other joints.
- ***C2–3 and C7-T1 were statistically significantly different (P < 0.001) from other joints and from one another.
- ¶¶C5–6 was statistically significantly different (P < 0.001) than all other joints.
- ¶C6–7 and C7-T1 were statistically significantly different (P < 0.001) than the other joints but not from each other.
| Dog | Injection score = 1 | Injection score = 2 | Injection score = 3 |
|---|---|---|---|
| 1 | 10 | 2 | 0 |
| 2 | 10 | 1 | 1 |
| 3 | 11 | 1 | 0 |
| 4 | 10 | 2 | 0 |
| 5 | 7 | 4 | 1 |
| 6 | 9 | 3 | 0 |
| 7 | 10 | 2 | 0 |
| 8 | 8 | 4 | 0 |
| 9 | 11 | 1 | 0 |
| 10 | 12 | 0 | 0 |
| 11 | 12 | 0 | 0 |
- VCD, vertebral canal diameter; H, vertebral body height; L, vertebral body length.
No joint had significant interindividual variability with respect to process joint angle in the transverse or dorsal plane. C2–3 and C6–7 were statistically significantly different (P < 0.001) for transverse joint angle compared to the other sites but were not different from one another. C7-T1 was statistically significantly different (P < 0.001) for transverse joint angle compared to all other joints. For the dorsal joint angle, C6–7 and C7-T1 were statistically significantly different (P < 0.001) for transverse joint angle compared to the other sites but were not different from one another and C5–6 was statistically significantly different (P < 0.001) compared to all other joints. With respect to the ratio of cranial vertebral canal diameter to total neck diameter, C2–3 was statistically different (P < 0.001) than any other site, as was C7-T1.
There was no significant correlation between injection score and age, gender, body weight, body condition score, transverse and dorsal angles of each joint, neck diameter at each joint, or vertebral canal diameter, vertebral canal diameter/H, vertebral canal diameter/L, or vertebral canal diameter/neck diameter.
Discussion
Ultrasound-guided cervical articular process joint injection can be performed accurately in the canine cervical spine from C2-T1. The transverse processes of the cervical vertebrae serve as excellent ultrasonographic landmarks for identifying the cervical vertebral joints. A limitation in this study may be the size of the cadaver dogs used. The size of dogs in this study was smaller than the population of dogs that are affected by cervical vertebral myelopathy clinically as affected breeds tend to be large breed dogs. However, given that a similar technique can be used in horses, it is likely that this technique can also be performed in larger dogs. Larger dogs will have larger articular process joints, which may facilitate needle placement. The reason for the higher mean injection score of C2–3 is not clear but may be explained by unique morphology of the joint since joint angles measured in this study were significantly different for that joint in the transverse and dorsal planes compared to most other joints. However, the same is true for a number of other joints such as C6–7, which was not statistically different from C2–3 in the transverse angle measurement. The authors also did not feel that during the procedures that there was more difficulty to perform the injections at C2–3 compared to the other joints. Concerning the accuracy of the injections, although the percentage of both intra- and extraarticular injections within 5 mm of the joint was 98.5% in this study, the therapeutic significance for intra- vs. peri-articular injection is not known.8, 9
Cervical spondylomyelopathy occurs primarily in large breed dogs and mainly affects the C4-C7 sites,1 which can be injected with accuracy using ultrasound guidance based on our study. Therapeutic cervical articular process joint injection has been used in Deerhounds with C2–3 arthrosis and severe cervical pain. Myelography was performed in those dogs prior to joint injection in order to rule out spinal cord compression as the cause of pain. Those dogs underwent fluoroscopically guided joint injections with triamcinolone acetonide (10 mg/joint) and lidocaine hydrochloride (20 mg/joint), which resolved their clinical signs within 1–2 days post injection. Relief lasted up to four months.
The statistically significant difference in mean injection scores between the first six and last five necks injected likely indicates that a learning curve plays a role in the accuracy of injections. In Great Dane dogs with cervical spondylomyelopathy, it has been shown that 86.2% of dogs have degenerative changes of the articular process joints.17 This could potentially create difficulty in identifying the process joint sonographically due to periarticular new bone formation and could result in a peri-articular injection.
While this study did not investigate volumes of the injection, injecta was observed throughout the entire joint without extravasation on prosections with an injected volume of 0.1 ml. Therapeutic injection volumes in horses are typically 2–3 ml,18 and volumes of 2 ml have previously been used in the dog.5 Injection volumes for therapeutic treatment may need to be explored in dogs to determine if leakage or extravasation occurs and what its significance is in clinical disease.
Since only cadavers were used in this study, further studies are necessary to examine the safety and efficacy of this procedure on live animals. Complications have been reported as self-limiting in the horse since being established as a technique for treating articular process joint arthrosis.8 Fluoroscopically guided injections of the cervical articular process joints in dogs to relieve pain have already been shown to be effective without complication.7 With any interventional procedure, there is potential for pain or infection. Articular process joint injections are performed commonly in horses and thought to be safe and efficacious, even if the injections are performed peri-articularly.19 Due to the proximity of the articular process joint to the vertebral foramen, the potential exists for inadvertent intraspinal canal injection. The effect of medications entering the spinal canal or subarachnoidal space has been reported in people.20 It is known that epidural corticosteroid injections can enter the subarachnoidal space and intrathecal formulations can be neurotoxic in people and is thought to be due to the additives.20
The use of ultrasound for joint injection would make the technique more widely available in dogs compared with fluoroscopy. Furthermore, ultrasound does not use ionizing radiation. Ultrasound-guided intraarticular cervical articular process joint injection is a feasible technique in the dog, and use of this technique to inject with anti-inflammatory drugs warrants future clinical trials to determine safety and efficacy in treating arthrosis in dogs with cervical spondylomyelopathy.
ACKNOWLEDGMENTS
The authors would like to recognize the Merial Veterinary Scholars Program for their funding as well as Michael Kearney, Dr. Rhett Stout, Mark Hunter, and Emily Levy for assistance with this project.




