Effect of N-Acetylcysteine, Ascorbic Acid, and a Vitamin E Analog on Oxidative and Storage Lesions in Canine Packed Red Blood Cells
A scientific abstract presentation of these results was presented at the ACVIM Forum in June 2022, Austin, TX.
Funding: This study had two funding sources: (1) Graduate Student Competitive Research Grant, Veterinary Clinical Sciences, Purdue University, College of Veterinary Medicine and (2) ACVIM-Purina Resident Research Grant.
ABSTRACT
Objective
To describe storage and oxidative lesions in canine packed red blood cells (pRBCs) during routine storage with additives, including saline, N-acetylcysteine (NAC), ascorbic acid (AA), and vitamin E analog (VE).
Design
Prospective, comparative study of canine pRBCs with or without antioxidant additives during routine 42-day storage.
Setting
University teaching hospital.
Animals
Nine leukoreduced units of canine pRBCs were aseptically separated into three aliquots (Groups 1, 2, and 3) on the same day as collection (day 0). All aliquots were shipped overnight and received by the investigators on day 1.
Interventions
Antioxidants (or control solution) were added on day 1, with three treatment groups that included saline (control, Group 1), NAC and AA (Group 2), and AA and a VE (Group 3).
Measurements and Main Results
Blood was collected from each aliquot on day 1, before the addition of antioxidants for baseline measurement of glutathione and intraerythrocytic reactive oxygen species (ROS). Additional samples were collected from each aliquot on days 7, 28, and 42. Type 3 fixed-effects tests were used to compare the impact of group and time on each measurement. All groups showed storage lesions and glutathione depletion by day 42 compared with baseline, regardless of the antioxidant additive. Intraerythrocytic ROS accumulation was lower in Group 3 (AA and a VE) compared with other groups at all time points after baseline (p < 0.0001).
Conclusions
The addition of AA and a VE to canine pRBCs reduced ROS accumulation but did not prevent glutathione depletion during routine storage. Further studies using antioxidants as additives in canine pRBCs are warranted.
Abbreviations
-
- AA
-
- ascorbic acid
-
- ABRI
-
- Animal Blood Resources International
-
- DMSO
-
- dimethyl sulfoxide
-
- GSH
-
- glutathione
-
- NAC
-
- N-acetylcysteine
-
- NACA
-
- N-acetylcysteine amide
-
- PBS
-
- phosphate-buffered solution
-
- PBSA
-
- phosphate-buffered solution with 1% bovine albumin
-
- pRBC
-
- packed red blood cell
-
- ROS
-
- reactive oxygen species
-
- TNB
-
- 5-thio-2-nitrobenzoic acid
-
- VE
-
- vitamin E analog (Trolox)
1 Introduction
The current standard for handling of canine packed red blood cells (pRBCs) includes a maximum storage time of 42 days. Storage of canine pRBCs causes time-dependent modification of RBCs, resulting in osmotic fragility and cell lysis (storage lesions). These storage lesions are theorized to contribute to transfusion reactions and decreased efficacy of blood transfusions [1-3]. Oxidative stress, the cause of which is multifactorial, is considered to be the most significant contributor to the accumulation of lesions in stored RBCs [3, 4]. Because of their oxygen-carrying function, RBCs are in continuous contact with oxygen, which exposes them regularly to reactive oxygen species (ROS). The hematogenous transport of neutrophils exposes RBCs to hydrogen peroxide, produced when neutrophils release myeloperoxidase in an inflammatory response [5]. The impact of WBC in blood transfusion products has been addressed through the process of leukoreduction, in which leukocytes are filtered out during the preparation of the pRBCs. This has been shown to reduce the risk of a transfusion reaction in both people and dogs, although this finding is not consistent across the literature on leukoreduction [6-10].
During storage, the lipid peroxides found in the phosphorus-rich bilayer of the RBC membrane decompose and form reactive carbonyl compounds, which also cause oxidative stress [11]. A risk factor for both febrile hemolytic and nonhemolytic transfusion reactions in dogs is the age of the pRBC unit, which supports that oxidative stress within the blood product is increased by cellular senescence [6, 7, 12].
Glutathione (GSH) is a tripeptide made up of cysteine, glutamine, and glycine. It is the primary antioxidant defense for RBCs and is depleted when exposed to excessive oxidative stress [3, 13]. GSH will neutralize ROS, becoming oxidized in the process. Reducing compounds (e.g., ascorbic acid [AA]) and enzyme systems (e.g., glutathione peroxidase, glutathione reductase) are vital for restoring glutathione and other free radical scavengers (e.g., α-tocopherol) to a reduced state, thus recycling them for further use [14, 15]. During storage of pRBCs, endogenous antioxidants are used until depleted, which increases ROS and lipid peroxides and, ultimately, leads to RBC damage [2, 3, 13].
The use of antioxidants as additives to human pRBCs is an area of ongoing research. This novel storage practice has been evaluated using AA, α-tocopherol, water-soluble vitamin E analogs (VEs), N-acetylcysteine (NAC), and a combination of these [1, 16, 17]. In people, water-soluble VEs have been shown to have similar or, in some cases, increased antioxidant activity compared with the use of α-tocopherol in experimental conditions [16, 18]. The addition of antioxidants to human pRBCs, especially in combination, resulted in less overall depletion of GSH, decreased lipid peroxidation, and decreased hemolysis throughout storage [1, 16, 17]. Although a clinical benefit has not yet been investigated in people and therefore antioxidant supplementation is not routinely used in blood bank protocols, the research is encouraging and supports further study, including evaluating the effect of adding antioxidants to canine pRBCs.
This study has two primary objectives, the first of which is to determine the degree to which oxidative stress and storage lesions occur in canine pRBCs during standard collection and 42 days of storage. Further, the study aims to determine the effect of adding antioxidants to canine pRBCs on the development of oxidative and storage lesions during routine collection and storage. We hypothesized that oxidative stress and storage lesions are time dependent and would increase throughout storage. Furthermore, we hypothesized that adding antioxidants to canine pRBCs would reduce oxidative stress and storage lesions compared with the control group.
2 Materials and Methods
2.1 Study Design
In this prospective comparative study, nine units of canine leukoreduced pRBCs were obtained from Animal Blood Resources International (ABRI)a. ABRI is a Class R Research Facility and Class B Dealer under the Animal Welfare Act, licensed by the United States Department of Agriculture. ABRI donors were screened according to the most current consensus on canine and feline blood donor screening for bloodborne pathogens [19]. The method of collection and storage of blood from donors followed the standard protocol established by ABRI. Blood was collected into a commercially available leukoreduction bagb containing citrate, phosphate, dextrose, and adenine anticoagulant. The blood was then drained through a leukoreduction filter, with the remaining contents in the original collection bag discarded. The filtered blood was then centrifuged, with plasma separated, and the final RBC product was suspended in saline, adenine, glucose, and mannitol, allowing for a storage time of 42 days. For this study, ABRI further aseptically separated each standard unit into three 150-mL transfer bags, making three aliquots per one standard unit. After collection, processing, and separation into aliquots (day 0), the blood product was shipped overnight to the authors’ institution for baseline diagnostics and antioxidant supplementation (day 1).
The 3 aliquots from each donor unit were used to establish three treatment groups so that each donor served as its own control. Upon receipt of the blood, each aliquot was weighed to determine the exact volume of blood. Each aliquot was spiked using a sterile vented dispensing pinc. NACd and AAe were diluted to 10-mmol stock solutions using deionized water and sterilized through a 0.2-µmol filter. A water-soluble VEf was diluted to a 10-mmol stock solution using a phosphate-buffered solution (PBS 1×: pH 7.4, 137 mmol NaCl, 10 mmol Na2HPO4, 2.7 mmol KCl, and 1.8 mmol KH2PO4) and filtered similar to the NAC and AA.
Three treatment groups were then established based on additive. For Group 1 (control), 5 mL of blood was removed from the bag, with 5 mL of 0.9% saline added. For Group 2 (0.5 mmol NAC + 0.23 mmol AA) and Group 3 (125 µmol VE + 0.23 mmol AA), the volume of each stock solution necessary to achieve the desired concentration of antioxidant was calculated, and an equivalent amount of blood was removed from the bag before adding the antioxidant [1, 16].
Each bag was then rocked for 10 min before being stored at 4°C–6°C for 42 days. The blood collected before the addition of antioxidants was used for baseline measurement of all tests of oxidative stress (day 1). Additional sampling of each aliquot was performed on days 7, 28, and 42 of storage. Five milliliters of blood was collected from each bag at each of these time points, separated, and prepared for the various assessments of oxidative and storage lesions as described as follows. A PCV was measured at each time point, and a blood smear was prepared for evaluation by light microscopy. Plasma was collected at each time point for measurement of thiobarbituric acid reactive substances, but concerns about the assay protocol arose after results were obtained. A description of this assay, the results, and its limitations are available as Supporting Information (File S1 and Figure S1).
2.2 Flow Cytometry
Flow cytometry was used to measure the accumulation of intraerythrocytic ROS based on a method previously validated in dogs [20]. One milliliter of pRBCs was collected from each treatment group and centrifugedg at 3000 × g for 5 min at room temperature. A pipette tip was then passed through the plasma into the RBC pellet, removing 10 µL of RBCs. The pipette tip was then cleaned with a KimWipeh to minimize cellular contamination, and the RBCs were diluted in 5 mL of PBS with 1% bovine albumini (PBSA). Each sample was exposed to four treatment conditions based on the presence or absence of a fluorochrome used to identify intraerythrocytic ROS and the presence or absence of an oxidative agent. The four treatment conditions included fluorochrome/oxidant (stimulated), fluorochrome/control (unstimulated), vehicle/oxidant, and vehicle/control. The fluorochrome used was 2ʹ,7ʹ-dichlorodihydrofluorescein diacetatej, which was diluted to 25 mmol with dimethyl sulfoxide (DMSO)k. DMSO was used as the vehicle in samples treated without fluorochrome. Hydrogen peroxide (H2O2, 30%) was used as the stimulant of oxidative stress and was diluted to 0.5 mmol using deionized water. A PBS solution was used as a negative control in the samples treated without stimulation.
One hundred microliters of the prepared RBC/PBSA solution was used for each sample, and each sample was run in triplicate. Ten microliters of 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate or DMSO was added to 100 µL of the prepared RBC solution. Cells were incubated for 20 min at 37°C. Ten microliters of H2O2 or PBS was then added to each tube, and cells were incubated for 20 min at room temperature in the dark. Samples were then diluted with 200 µL of 1% PBSA and immediately analyzed by flow cytometry. Flow cytometry was performed using a commercially available machinel. ROS-dependent fluorescence intensity was detected by green fluorescence with an excitation wavelength of 488 nm (FL1 channel) with gating around the isolated RBCs. Fluorescence was measured using log amplification, and median fluorescence intensity was analyzed with commercially available softwarem.
2.3 GSH
GSH concentrations were measured on RBC hemolysate solutions and evaluated via an enzymatic recycling method using a commercially available kitn. RBC hemolysates were prepared by diluting 1 mL of canine pRBCs with 4 mL of ice-cold HPLC-grade water and centrifuging at 10,000 × g for 15 min at 4°C. The supernatant/hemolysate was collected with a Pasteur pipette and deproteinated before assaying. The samples were stored at −80°C. The glutathione assay was performed within 1 month of collection. At the time of assay, 50-µL aliquot samples were brought to room temperature. This assay uses a carefully optimized enzymatic recycling method, glutathione reductase, to quantify GSH. The sulfhydryl group of GSH reacts with 5,5ʹ-dithio-bis-2-(nitrobenzoic acid), Ellman's reagent, and produces a yellow 5-thio-2-nitrobenzoic acid (TNB). The mixed disulfide of GSH and TNB is concomitantly produced and is reduced by glutathione reductase to recycle the GSH and produce more TNB. The rate of TNB production is directly proportional to this recycling reaction, which is, in turn, directly proportional to the concentration of GSH in the sample. The absorbance of TNB was measured at 405–414 nm on a multiplate reader, and the concentration was determined based on a standard curve. The interassay coefficient of variation was 3.6% (N = 5), and the intraassay coefficient of variation was 1.6%. Samples are stable when stored at −80°C for up to 6 months.
2.4 Light Microscopy
A standard blood smear was prepared and stained using Wright's staino on the day of collection. Slides were evaluated by the same author (J.R.T., small animal internal medicine resident) using a commercially available microscopep. This author was not blinded to the source of blood for each smear. Two high-power fields in the monolayer, which have been found to encompass more than 500 RBCs each, were evaluated. When applicable, RBC morphologic changes were described with the percentage noted.
2.5 Statistical Methods
The sample size for this experimental study was determined using a resource equation approach [21, 22], in which 10–20 degrees of freedom was used as an acceptable range for the equation. For the current study, using group comparison and repeated measures, we used the equation:where n is the number of subjects per group, DF represents the degrees of freedom, k is the number of groups, and r is the number of repeated measures.
Using a minimum and maximum DF of 10 and 20, respectively, and with three treatment groups and four repeated measurements (days 1, 7, 28, and 42), the following was calculated:
Minimum n = 10/3 × 4 + 1 = 1.8 (rounds up to 2), minimum two animals per group,
Maximum n = 20/3 × 4 + 1 = 2.6 (rounds up to 3), maximum three animals per group.
Once the minimum and maximum number of subjects per group was determined (n), the total number of subjects required (N) was calculated by multiplying n by the number of groups (3), giving us a final required sample size of between six and nine dogs [21, 22].
Statistics were performed using commercially available softwareq. Numerical data for outcome variables were compared by group, time, and their interaction using a linear mixed model with a unit of pRBCs as a random effect. Within- and between-group comparisons at each time point and versus baseline were performed in a pairwise fashion, with P-values adjusted for multiple comparisons using the Scheffé method. A P-value of <0.05 was considered significant. Summary statistics are reported as mean ± SD.
3 Results
A total of 27 samples were used for this study (nine units of canine pRBCs each separated into three aliquots), with nine samples in each treatment group (Group 1 [control], Group 2 [NAC + AA], and Group 3 [VE + AA]). There was no statistically significant difference in any measured parameter when groups were compared on day 1.
The mean PCVs for Groups 1, 2, and 3 were 59.83% (±0.29), 61.33% (±1.53), and 61.33% (±1.53), respectively, on day 1. The mean PCVs for Groups 1, 2, and 3 were 60.06% (±2.36), 60.61% (±6.78), and 63.17% (±5.24), respectively, on day 42. The mean PCV did not differ by group (P = 0.087) or time (P = 0.11). On blood smear analysis, changes in erythrocyte shape were common, with too many echinocytes to count noted on all blood smears at all time points. No Heinz bodies or eccentrocytes were noted.
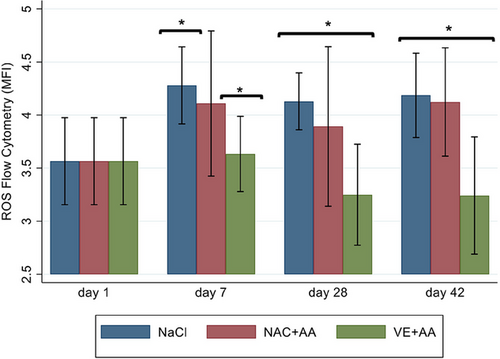
The mean hemolysate GSH concentrations differed by time (P = 0.0022) but not by group (P = 0.55; Figure 1). In all groups, glutathione concentrations were significantly lower on days 28 and day 42 compared with day 1. Flow cytometry for the detection of intraerythrocytic ROS differed by group (P < 0.0001) but not by time (P = 0.35). Intraerythrocytic ROS accumulation was significantly lower in Group 3 (VE and AA) on days 7, 28, and 42 when compared with the other groups (Figure 2).

4 Discussion
The current study compared storage and oxidative lesions in canine pRBCs during routine storage with NAC and AA, VE and AA, or saline placebo. Although hemolysis was expected during storage, the PCV did not differ significantly on day 42 compared with day 1 in any group. Rather than the PCV decreasing, as would be expected with hemolysis, a slight increase was noted in Groups 1 and 3 during storage. This increase in PCV has been described in stored canine and human blood in which the RBCs become engorged due to storage-related osmotic and deformability alterations [23, 25]. In addition to cellular engorgement, spicule formation on the RBC surface has been described in human and canine blood during storage, appreciated as echinocytes and spheroechinocytes on light or electron microscopic examination. Echinocytes were commonly seen in the current study at all time points in all groups. Although echinocytosis can be a crenation artifact, in stored human RBCs these changes in shape are theorized to be due to lipid peroxidation and metabolic alterations, including depletion of ATP and 2,3-diphosphoglycerate, leading to changes in the sodium–potassium balance in the cells [23, 24]. Additionally, a steady decrease in pH has been noted during storage of canine pRBCs collected in citrate, phosphate, dextrose, and adenine anticoagulant, attributed to the citric acid in the anticoagulant and the accumulation of lactic acid during storage. This decrease in pH may further influence cellular electrolyte homeostasis and therefore size and deformability [25]. In one study in people, approximately 90% of pRBCs demonstrated some morphologic alteration (echinocyte or stomatocyte) by day 7 of storage [23]. These alterations in cellular size and morphology are described in vitro and differ from the expected decrease in cellular size that occurs during aging and senescence of RBCs in vivo [23, 25].
In the current study, numerous echinocytes were noted on day 1, and these findings persisted at all time points in all groups. This early identification of morphologic changes may result from inherent differences between canine and human RBCs. Unlike human RBCs, which are high in potassium and low in sodium, most canine RBCs are low in potassium intracellularly and rely more heavily on calcium pumps and sodium–calcium exchange to prevent cellular swelling [25]. These differences in electrolyte concentrations may lead to earlier changes in RBC shape in stored canine RBCs compared with stored human RBCs. Additionally, while the current study only measured glutathione as an antioxidant, canine RBCs contain only one-thirtieth the amount of catalase, a common antioxidant enzyme, compared with human RBCs [26]. In comparison, this may make canine RBCs more susceptible to oxidant-related storage lesions. Future studies could measure catalase activity in canine pRBCs over time. In the current study, hemolysis would have been more easily assessed if hemoglobin had been measured, because this value combined with the PCV would have allowed calculation of the percentage of hemolysis in the samples [25]. Additionally, electron microscopy may have allowed for more accurate description of cellular morphologic changes.
The addition of VE and AA resulted in less accumulation of ROS in canine pRBCs when compared with controls and other antioxidant additives. This is similar to human pRBCs, in which the combination of VE and AA resulted in significantly decreased markers of oxidative stress [16]. Both of these vitamins exert their antioxidant effect through free radical scavenging. During RBC storage, ROS accumulate due to the breakdown of cellular components, including lipid peroxidation of the cell membrane and auto-oxidation of hemoglobin, which is a significant source of ROS, namely, hydroxyl radicals [12, 14, 27, 28]. VEs, like all tocopherols, are especially capable of neutralizing hydroxyl radical via hydrogen ion donation. AA has some free radical scavenging properties but is also capable of reducing oxidized radicals (e.g., oxidized tocopherols and oxidized glutathione) and returning them to a stable state [15, 16, 18]. VE and AA have been shown to act synergistically to protect against oxidative stress in human pRBCs, and the current study demonstrates a similar effect in canine pRBCs [18]. Our study used concentrations of VE and AA extrapolated from studies in people, so optimization of both of these antioxidants would be necessary to determine the overall safety and utility in canine pRBCs. Once optimized, antioxidant additives to pRBCs have the potential to decrease transfusion reactions, therefore representing an area of study that warrants further research.
GSH decreased significantly in all groups over time regardless of the additive. Its depletion may play a significant role in storage lesions due to impairment in the neutralization of ROS, which leads to increased damage to cellular membranes and nuclear material [3, 13, 14]. GSH depletion is described in human pRBCs with antioxidant additives, although in some studies the rate of depletion was slower in units supplemented with antioxidants [1, 16-18]. In the current study, depletion was seen in all groups, and the sample size was too small to meaningfully investigate subtleties in the trends of depletion between groups. Although NAC is a glutathione precursor, its addition did not prevent glutathione depletion in our study compared with the groups without the addition of NAC. In people, studies evaluating NAC as an antioxidant in various disease states have shown that it is hydrophobic and has poor bioavailability, requiring high doses to counteract these limitations [17, 29]. At physiologic pH, NAC loses a proton from its carboxyl group, giving it a negative charge and reducing its ability to cross the RBC membrane [29-32]. In one study investigating NAC as an additive in human pRBCs, high concentrations were required to reduce the percentage of hemolysis (20–25 mmol), whereas lower concentrations were able to reduce the depletion of GSH (2.5–5 mmol) [17]. In the current study, 0.5 mmol NAC was used as the additive in canine pRBCs. This low concentration, coupled with the limitations of NAC due to bioavailability, likely contributed to its inability to reduce GSH depletion. Another thiol compound, N-acetylcysteine amide (NACA), has improved bioavailability compared with NAC in human RBCs in vitro [29-32]. This improved bioavailability is a result of the addition of an amide group to the carboxyl, which increases lipid solubility, allowing better membrane permeability [31]. NACA has shown promise in human in vitro studies and murine models, demonstrating superior antioxidant properties at lower doses compared with NAC in RBC disorders of oxidative stress, such as sickle cell anemia and acetaminophen intoxication [29, 30]. NACA has not been investigated in dogs, but an improved effect on markers of oxidative stress may be achievable if NACA were added to canine pRBCs, rather than NAC. Additionally, future studies could investigate supplementation of all peptide components of glutathione (cysteine, glutamine, glycine) because this may allow restoration of glutathione in addition to reducing its depletion.
This study had several limitations. As an initial investigation into antioxidant additives to canine pRBCs, nine units were used. A more robust sample size would be necessary in the optimization of antioxidants and their concentrations in canine blood bank practice to determine the safety and feasibility for use in a clinical setting. In addition, there is no consensus on the best method or methods to evaluate oxidative stress and the efficacy of antioxidant additives. A comprehensive approach to evaluation of oxidative stress in these conditions would require numerous assays for enzymes contributing to the balance of oxidative stress, small molecules and minerals, and byproducts of oxidative damage, among others. An approach such as this would be cost- and time-prohibitive without preliminary data to justify further research. The measured variables chosen for the current study identified several intriguing results. We showed that, during routine storage of canine pRBCs, changes in RBC shape are common, glutathione is depleted, and ROS accumulate. The addition of AA, VE, or NAC did not reduce these alterations in cellular shape, nor did they prevent the depletion of glutathione. However, the accumulation of intraerythrocytic ROS during storage is significantly decreased when a combination of AA and VE is added to canine pRBCs. Future studies are warranted.
Conflicts of Interest
Dr. George E. Moore serves as the Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in the review of this manuscript. The other authors declare no conflicts of interest.
Offprints
Andrew Woolcock, College of Veterinary Medicine, Purdue University, 625 Harrison Street, West Lafayette, IN 47907, USA. Email: [email protected]




