Evaluation of a direct lymphocyte proliferation test for the diagnosis of canine food allergies with delayed reactions after oral food challenge
Abstract
enBackground
In humans, food allergies (FAs) are divided into those with immunoglobulin (Ig) E-mediated (immediate FA), cell-mediated (delayed FA) or both mechanisms (mixed FA). In dogs, lymphocyte stimulation tests have the highest concordance with oral food challenges (OFCs).
Objectives
To report the evaluation of a lymphocyte proliferation test (LPT) in dogs with FA and delayed reactions (≥6 h) after OFC.
Animals
Thirty-five healthy and 28 dogs with delayed FA.
Materials and Methods
Peripheral blood mononuclear cells (PBMCs) were isolated and automatically counted before and after a 5-day culture with food allergens. Stimulation indices (SIs) were then calculated. Food allergen-specific IgE was quantified using the Pet Allergy Xplorer (PAX).
Results
None of the 10 healthy laboratory beagles and 25 healthy pet dogs had an SI greater than the 3.0 cut-off, indicating a specificity of 100%. All 28 dogs with delayed FA had at least one positive stimulation to a food item that induced delayed flares after OFC; the sensitivity of this LPT for the identification of delayed canine FA was 100%. The LPT correctly identified 57 of 68 food items causing delayed flares after OFC (84%). The PAX was negative for food-specific IgE in 18 of the 28 dogs (64%), as expected for delayed FA. In three dogs (11%), PAX results overlapped with those of the LPT, suggesting a mixed FA.
Conclusions and Clinical Relevance
Food allergies with delayed reactions after OFC—those suspected of having a cell-mediated mechanism—seemed to be the most common type of FA in the studied dogs. The LPT was helpful in identifying such cases.
Zusammenfassung
deHintergrund
Beim Menschen werden die Nahrungsmittelallergien (FA) in jene mit Immunglobulin (Ig)E-vermittelte (unmittelbare FA), Zell-vermittelte (verzögere FA) oder jene mit beiden Mechanismen (gemischte FA) aufgeteilt. Bei Hunden zeigt der Lymphozyten Stimulationstest die höchste Übereinstimmung mit Futterprovokationen per os (OFC).
Ziele
Die Publikation der Evaluierung eines Lymphozyten Proliferationstests (LPT) bei Hunden mit FA und verzögerter Reaktionen (≤6 h) nach OFC.
Tiere
35 gesunde Hunde und 28 Hunde mit verzögerter FA.
Materialien und Methoden
Periphere Blut Mononuklearzellen (PBMCs) wurden isoliert und vor und nach einer 5-tägigen Kultur mit Futterallergenen automatisch gezählt. Stimulationsindices (SI) wurden danach kalkuliert. Die Futterallergen-spezifischen IgE wurden mittels Pet Allergy Xplorer (PAX) quantifiziert.
Ergebnisse
Keiner der 10 gesunden Labor-Beagles und der 25 gesunden Haustier-Hunde hatten einen höheren SI als der 3,0 Cut-Off, was auf eine Spezifität von 100% hinweist. Alle 28 Hunde mit verzögerter FA zeigten zumindest eine positive Stimulation auf einen Futterbestandteil, was verzögerte Schübe nach OFC induzierte; die Sensibilität dieses LPT betrug bei der Identifizierung der verzögerten caninen FA 100%. Der LPT identifizierte 57 von 68 Futterbestandteilen, welche verzögerte Schübe nach OFC (84%) verursachten. Der PAX war bei 18 der 28 Hunde (64%) negativ für Futter-spezifisches IgE, was bei der verzögerten FA zu erwarten war. Bei drei Hunden (11%) überlappten die PAX-Ergebnisse mit jenen des LPT, was auf eine gemischte FA hinwies.
Schlussfolgerungen und klinische Bedeutung
Futtermittelallergien mit verzögerten Reaktionen nach OFC – jene bei denen vermutet wird, dass es sich um eine Zell-vermittelten Mechanismus handelte- schienen den häufigsten Typ der FA bei den untersuchten Hunden darzustellen. Der LPT war bei der Identifizierung dieser Fälle hilfreich.
摘要
zh背景
在人类中,食物过敏(FA)分为免疫球蛋白(Ig)E介导的(速发型FA)、细胞介导(迟发型FA)或两种机制(混合型FA)。在犬中,淋巴细胞刺激试验与食入食物激发(OFC)的一致性最高。
目的
报告对患有FA和OFC后迟发型反应(≥6小时)的犬进行淋巴细胞增殖试验(LPT)的评估。
动物
35只健康犬和28只迟发型FA的犬。
材料和方法
分离外周血单个核细胞(PBMCs),并在与食物过敏原培养5天前后自动计数。然后计算刺激指数(SI)。使用宠物过敏Xplorer(PAX)定量食物过敏原特异性IgE。
结果
10只健康实验室比格犬和25只健康宠物犬的SI均不大于3.0,表明特异性为100%。所有28只患有迟发型FA的犬在OFC后至少对一种食物产生一次阳性刺激,导致迟发型过敏;该LPT用于鉴定犬迟发型FA的敏感性为100%。LPT正确识别了68种在OFC后引起迟发型发作的食品中的57种(84%)。28只犬中有18只(64%)的PAX对食物特异性IgE呈阴性,与迟发型FA的预期一致。在3只犬(11%)中,PAX结果与LPT结果重叠,表明FA混合。
结论和临床相关性
OFC后出现迟发型反应的食物过敏——疑似具有细胞介导机制——似乎是研究犬中最常见的FA类型。LPT有助于识别此类病例。
Résumé
frContexte
Chez l'homme, les allergies alimentaires (FA) sont divisées en deux catégories: celles à médiation immunoglobuline (Ig)E (FA immédiate), celles à médiation cellulaire (FA retardée) ou les deux mécanismes (FA mixte). Chez le chien, les tests de stimulation lymphocytaire présentent la meilleure concordance avec les tests de provocation alimentaire orale (OFC).
Objectifs
Rapporter l'évaluation d'un test de prolifération lymphocytaire (LPT) chez des chiens souffrant de FA et de réactions retardées (≥6 h) après l'OFC.
Animaux
35 chiens sains et 28 chiens présentant des réactions retardées.
Matériels et méthodes
Les cellules mononuclées du sang périphérique (PBMC) ont été isolées et comptées automatiquement avant et après une culture de 5 jours avec des allergènes alimentaires. Les indices de stimulation (IS) ont ensuite été calculés. Les IgE spécifiques aux allergènes alimentaires ont été quantifiées à l'aide du Pet Allergy Xplorer (PAX).
Résultats
Aucun des 10 beagles de laboratoire sains et des 25 chiens de compagnie sains ne présentait un IS supérieur au seuil de 3,0, ce qui indique une spécificité de 100%. Les 28 chiens atteints de FA retardée présentaient au moins une stimulation positive à un aliment qui induisait des poussées retardées après l'OFC ; la sensibilité de ce LPT pour l'identification de FA canine retardée était de 100%. Le LPT a correctement identifié 57 des 68 aliments provoquant des éruptions retardées après l'OFC (84%). Le PAX était négatif pour les IgE spécifiques aux aliments chez 18 des 28 chiens (64%), comme prévu pour la FA retardée. Chez trois chiens (11%), les résultats du PAX se sont superposés à ceux du LPT, ce qui suggère une FA mixte.
Conclusions et pertinence clinique
Les allergies alimentaires avec des réactions retardées après l'OFC – celles suspectées d'avoir un mécanisme à médiation cellulaire – semblent être le type de FA le plus courant chez les chiens étudiés. Le LPT s'est avéré utile pour identifier ces cas.
要約
ja背景
ヒトでは、食物アレルギー(FA)は免疫グロブリン(Ig)Eを介するもの(即時型FA)、細胞を介するもの(遅延型FA)、あるいは両方の機序を有するもの(混合型FA)に分けられる。犬では、リンパ球刺激試験が経口食物負荷試験(OFC)と最も高い一致率を示す。
目的
本研究の目的は、OFC後FAおよび遅延反応(6時間以上)を示した犬におけるリンパ球増殖試験(LPT)の評価を報告することであった。
対象動物
健常犬35頭、遅延型FA犬28頭。
材料と方法
末梢血単核球(PBMC)を単離し、食物アレルゲンによる5日間の培養の前後に自動的にカウントした。その後、刺激指数(SI)を算出した。食物アレルゲン特異的IgEをPet Allergy Xplorer(PAX)を用いて定量した。
結果
健常な実験用ビーグル10頭および健常なペット犬25頭のうち、SIがカットオフ値3.0を超えた犬はおらず、特異度は100%であった。遅発性FAの犬28頭すべてに、OFC後に遅発性フレアを誘発する食物に対する陽性刺激が少なくとも1回あった。このLPTの遅発性FA同定感度は100%であった。このLPTは、OFC後に遅発性フレアを引き起こす68の食物のうち57を正しく同定した(84%)。PAXは28頭中18頭(64%)で食物特異的IgE陰性であった。3頭(11%)では、PAXの結果がLPTの結果と重なり、混合型FAが示唆された。
結論および臨床的意義
OFC後に遅延型反応を示す食物アレルギー、すなわち細胞介在性機序が疑われる食物アレルギーは、調査対象犬において最も一般的なFAのタイプであった。LPTはこのような症例の同定に有用であった。
Resumo
ptContexto
Em humanos, as alergias alimentares (FA) são divididas nos seguintes subgrupos: mediada por imunoglobulina (Ig)E (FA imediata), mediada por células (FA tardia) ou ambos os mecanismos (FA mista). Em cães, os testes de estimulação de linfócitos têm a maior concordância com os desafios alimentares orais (OFC).
Objetivos
Relatar a avaliação de um teste de proliferação de linfócitos (LPT) em cães com FA e reações tardias (≥6 h) após OFC.
Animais
35 cães saudáveis e 28 cães com FA tardia.
Materiais e métodos
Células mononucleares do sangue periférico (PBMCs) foram isoladas e contadas automaticamente antes e depois de uma cultura de 5 dias com alérgenos alimentares. Os índices de estimulação (SI) foram então calculados. A IgE específica para alérgenos alimentares foi quantificada usando o Pet Allergy Xplorer (PAX).
Resultados
Nenhum dos 10 beagles saudáveis de laboratório e 25 cães de estimação saudáveis apresentaram um SI maior que o limite de 3,0, indicando uma especificidade de 100%. Todos os 28 cães com FA tardia tiveram pelo menos uma estimulação positiva a um item alimentar que induziu reações tardias após OFC; a sensibilidade deste LPT para a identificação de FA canina tardia foi de 100%. O LPT identificou corretamente 57 de 68 itens alimentares que causaram reações tardias após OFC (84%). O PAX foi negativo para IgE específica para alimentos em 18 de 28 cães (64%), conforme esperado para FA tardia. Em três cães (11%), os resultados do PAX se sobrepuseram aos do LPT, sugerindo uma FA mista.
Conclusões e relevância clínica
Alergias alimentares com reações tardias após OFC – aquelas suspeitas de terem um mecanismo mediado por células – parecem ser o tipo mais comum de FA nos cães estudados. O LPT foi útil na identificação de tais casos.
RESUMEN
esIntroducción
En humanos, las alergias alimentarias (FA) se dividen en aquellas con mecanismos mediados por inmunoglobulina (Ig)E (FA inmediata), mediada por células (FA retardada) o ambos (FA mixta). En perros, las pruebas de estimulación de linfocitos tienen la mayor concordancia con las exposiciones alimentarias orales (OFC, por sus siglas en inglés).
Objetivos
Informar sobre la evaluación de una prueba de proliferación de linfocitos (PTL) en perros con FA y reacciones retardadas (≥6 h) después del OFC.
Animales
35 perros sanos y 28 perros con FA retardada.
Materiales y métodos
Se aislaron células mononucleares de sangre periférica (PBMC) y se contaron automáticamente antes y después de un cultivo de 5 días con alérgenos alimentarios. Luego se calcularon los índices de estimulación (IE). La IgE específica de alérgenos alimentarios se cuantificó utilizando Pet Allergy Xplorer (PAX).
Resultados
Ninguno de los 10 beagles de laboratorio sanos y 25 perros domésticos sanos tuvo un SI mayor que el límite de corte de 3.0, lo que indica una especificidad del 100%. Los 28 perros con FA tardía tuvieron al menos una estimulación positiva a un alimento que indujo brotes tardíos después de OFC; la sensibilidad de esta LPT para la identificación de FA canina tardía fue del 100%. La LPT identificó correctamente 57 de 68 alimentos que causaron brotes tardíos después de OFC (84%). La PAX fue negativa para IgE específica de alimentos en 18 de 28 perros (64%), como se esperaba para FA tardía. En tres perros (11%), los resultados de PAX se superpusieron con los de la LPT, lo que sugiere una FA mixta.
Conclusiones y relevancia clínica
Las alergias alimentarias con reacciones tardías después de OFC (aquellas sospechosas de tener un mecanismo mediado por células) parecían ser el tipo más común de FA en los perros estudiados. La LPT fue útil para identificar dichos casos.
INTRODUCTION
In humans, food allergies (FAs) are defined as adverse reactions to foods mediated by an immunological mechanism involving either immunoglobulin (Ig) E (i.e. IgE-mediated FA), cell-mediated mechanisms (i.e. non-IgE-mediated) or both (mixed IgE- and non-IgE-mediated).1, 2 The clinical signs of IgE-mediated (immediate) FA are typical of those associated with mast cell and basophil degranulation. They can affect multiple organs and organ systems, such as the skin (e.g. urticaria, angioedema, pruritus, flushing and erythema), gastrointestinal tract (e.g. vomiting, diarrhoea, oro-pharyngeal pruritus and oedema), eyes (e.g. conjunctivitis), respiratory tract (e.g. rhinitis, cough and dyspnoea) and cardiovascular system (from pallor to shock).3 Examples of non-IgE-mediated (i.e. cell-mediated or delayed) FA include contact dermatitis, food protein-induced enterocolitis syndrome (FPIES), food protein-induced enteropathy, dermatitis herpetiformis and coeliac disease; the pathogenesis of the latter two diseases also involves autoimmune mechanisms. Finally, food-induced exacerbations of atopic dermatitis (AD) or asthma, as well as eosinophilic oesophagitis or gastritis/enteritis, are categorised as ‘mixed’3 or ‘predominantly non-IgE-mediated’ FA.1
The latest European guidelines for IgE-mediated FA recommend that in human patients suspected of IgE-mediated FA, food allergen sensitisations should be determined either by skin prick tests or by allergen-specific IgE serological testing to help select food items for ensuing oral food challenges (OFCs).3 Conversely, these IgE sensitisation tests are of little value whenever a non-IgE-mediated mechanism is suspected based on the history and clinical signs exhibited by the patient.
In order to determine allergen sensitisations in cell-mediated delayed FA, cellular assays to analyse lymphocyte proliferation or cytokine production after the short-term cultures of peripheral blood mononuclear cells (PBMCs) with food extracts are preferred. One can also use the ‘atopy patch tests’, which are in vivo challenges aiming to reproduce allergic reactions after the application of the suspected food allergens to the skin.4
Food allergies have been recognised in animals for decades. Nevertheless, within the realm of veterinary allergology, their separation between IgE-mediated, cell-mediated or mixed FA has been mentioned only rarely, and the consequences of these pathogenetic differences for the identification of sensitisations have not been incorporated into routine clinical practice.5 The introduction of such a classification system would allow one to discern between manifestations of FA based on their pathogenesis and facilitate the selection of the most appropriate tests to identify culprit food allergens optimally.
In dogs and cats, detection tests for IgE sensitisations (intradermal or serological) to food allergens have had too variable a diagnostic accuracy to warrant their wide acceptance in practice. Conversely, lymphocyte stimulation tests (LSTs) and patch tests with food allergens appear to have higher accuracy for such a diagnosis (reviewed previously6). Unfortunately, in none of the studies assessing the proliferation of lymphocytes7 or their expression of activation markers8-11 after incubation with food allergens were the dogs separated based on their types of clinical presentations, time-to-flare (TTF) after OFC, or suspected FA mechanisms. As a result, the value of these LSTs to help diagnose dogs suspected of cell-mediated, delayed FA, or to determine offending food allergens in such patients is unknown; their negativity in normal client-owned pets (i.e. their specificity) also needs to be clarified.
In this paper, we describe the suitability of a direct lymphocyte proliferation test (LPT) in food-allergic dogs with delayed flares after OFC.
MATERIALS AND METHODS
Animals
Between March and November 2023, we received samples from 55 dogs diagnosed with FA by veterinary specialists. In these dogs, the diagnosis was based on compatible cutaneous or digestive signs, a full improvement after a restrictive diet, and a subsequent flare after OFC, per current practice standards. The symptomatology, TTF of signs after OFC and food ingredients causing flares were noted in a standardised questionnaire (Data S1). Of these 55 dogs with FA, 44 (80%) had a flare of cutaneous signs that occurred ≥6 h after OFC, the longest duration expected for an IgE-mediated immediate FA in humans.12 In 28 of the 44 (64%) dogs with delayed FA, and after verbal consent was obtained from their owners, 10 mL of peripheral blood was collected by a single venipuncture in a dry tube and either used for serum isolation for routine allergen-specific IgE determination (Pet Allergy Xplorer; Nextmune) or placed in an ethylenediaminetetraacetic acid (EDTA)-containing tube for the performance of the LPT. After securing approval from the local institutions' animal care and use committee, we also obtained 5 mL of blood in EDTA from 10 laboratory beagles that had eaten the same chicken-and-rice diet (Nutribest Adult7+ Chicken and Rice; Picart Petcare) in the preceding 3 years. Finally, a 5 mL blood sample in EDTA was collected after informed verbal consent from the owners of 25 healthy dogs from Belgium and Lithuania presented to their veterinary surgeons for vaccination or neutering. All samples were kept refrigerated until shipment to the laboratory. All results were provided back to the veterinary surgeon for sharing with pet owners.
Allergen source
Most food extracts used to stimulate canine PBMCs were produced at Nextmune (Madrid) either with phosphate-buffered saline (PBS) or 0.02% sodium hydroxide (NaOH) (for flour extracts). All were prepared from raw ingredients and, in the case of meats, with the lean parts of muscles. For plant foods, we used different parts and varieties of vegetables and fruits. The peanut extract was a 50% glycerol, 0.4% phenol and saline commercial solution for human prick testing (Inmunotek). Bovine IgG (Bos d 7; Merck KGaA) was the only molecular allergen tested.
Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells were isolated by density gradient separation using Histopaque 1077 Hybri-Max (Sigma-Aldrich/Merck Life Sciences). First, 5 mL of blood in EDTA was diluted and mixed 1:1 in 0.9% saline before the addition of 3 mL of Histopaque 1077. This mixture was centrifuged for 30 min at 440 g without braking. The PBMC layer was aspirated and subjected to three successive cycles of saline wash. After the last centrifugation, the supernatant was removed, and PBMCs were resuspended in 5 mL of Gibco AIM-V serum-free medium (Fisher Scientific, Spain). After mixing 20 μL of the PBMC suspension with 20 μL of trypan blue (Fisher Scientific), we counted live cells on a TC20 automated cell counter (Bio-Rad); we only kept samples with >70% of viable cells for food allergen stimulation. The PBMC suspension was adjusted to 1 million cells/mL in the AIM-V medium.
Lymphocyte proliferation after food allergen stimulation
In dogs, PBMCs contain >90% of T and B cells.13, 14 These are the only cells with the ability to proliferate after being cultured with antigens. Therefore, the culture of PBMCs with food allergens is referred to as an LPT, just as it is for humans.15 We placed 100,000 cells in 100 μL of medium in each well of a tissue culture plate (Corning 96; Fisher Scientific). Then, 100 μL of a 10 μg/mL solution of each food allergen extract diluted in the medium was added to the PBMCs for a final allergen concentration in each well of 5 μg/mL. Food allergen stimulations were done in duplicates in 22 of 28 dogs. The three negative controls were similar volumes of saline, NaOH and 50% glycerol/0.4% phenol (all from AppliChem); these last two controls being added as some food allergen extracts contained such chemicals (see above). The positive control consisted of 100 μL of the lymphocyte mitogen concanavalin A at 10 μg/mL (Sigma-Aldrich/Merck). Food allergen-PBMC co-culture plates were then kept in an incubator at 37°C in 5% CO2 for 5 days. On Day (D)5, 20 μL of trypan blue was added to 20 μL of cell culture to evaluate cellular viability after stimulation, and cells were counted again using the TC20 automated cell counter. Subsequently, the food allergen-induced proliferation was expressed as a stimulation index (SI), denoting the ratio of cell numbers after 5 days of allergen co-culture relative to those cultured with saline at the same time point.
Determination of interleukin-2 concentrations in cell culture supernatants
As the stimulation of sensitised lymphocytes leads to both their proliferation and the release of interleukin (IL)-2, we aimed to validate the results of the LPT by comparing the proliferation of PBMCs with the concentrations of IL-2 in the supernatant of the cells after culture with food allergens. Concentrations of this cytokine were determined using the Canine IL-2 DuoSet ELISA (DY1815; Bio-Techne R&D Systems) following the manufacturer's instructions. The IL-2 concentrations were measured every day for 5 days in the culture supernatant of eight food-allergic dogs stimulated with between five and seven food allergens, including both positive and negative stimulations. Concentrations of IL-2 were likewise measured in the supernatant of PBMCs of three healthy client-owned dogs after co-culture with nine food allergens. As for the LPT, we expressed results as SIs of IL-2 concentrations after stimulation with food allergens over those cultured with saline on the same day.
Statistical analysis
The IL-2 supernatant concentrations were compared over time by the nonparametric, repeated-measures Friedman ANOVA with Dunn's multiple comparison test. The correlation between SIs of cell proliferation and IL-2 concentrations was calculated using the Spearman test. Stimulation indices obtained with the positive control concanavalin A were compared between healthy and food-allergic dogs using the Wilcoxon–Mann–Whitney U-test. All calculations were done using Prism 10 (GraphPad Software) with a significance level set at 0.05.
RESULTS
Patient characteristics
Table S1 includes details on the signalment, clinical manifestations, suspected allergens and TTF of the 28 dogs with delayed FA.
These dogs belonged to different breeds, and six of them (golden retriever, Labrador retriever, Labrador retriever poodle crossbred, Border collie, French bulldog, American Staffordshire terrier) had two patients included in this cohort. Their age varied from 6 months to 12 years (average 3.5, median 3.0 years), and the female-to-male ratio was 0.75 (Table S1).
Food-induced atopic dermatitis (FIAD) and signs of gastro-enteropathy (e.g. vomiting and diarrhoea) were the main clinical manifestations of delayed FA, as they were seen in 18 of the 28 dogs (64%) each (Table S1). Less commonly, dogs exhibited pruritus without visible lesions (five dogs; 18%), urticaria (two; 7%), angioedema (two; 7%), conjunctivitis or interdigital fistulae (one dog each; 4%). Seventeen dogs (61%) presented with two or more of these clinical manifestations (Table S1).
After performing OFC, veterinary surgeons identified 66 single food ingredients and two commercial diets inducing flares ≥6 h after ingestion in their patients (i.e. 68 different food sources). The number of culprit food sources ranged from one to seven (average 2.0, median 2.4). Nine dogs (32%) only exhibited a delayed flare after eating one food item, while four others had a recurrence of signs after eating five or more ingredients. In descending order of frequencies, the main culprit food sources during OFC were chicken (16 dogs; 57%), beef (10; 36%), turkey, pork and horse (4 each; 14%), and lamb, rabbit, rice and corn (3 each; 11%). Of interest is that one dog exhibited signs after eating peanuts, a rarely reported event.
According to our selection criteria, all dogs with delayed FA exhibited signs ≥6 h after OFC. In four dogs (numbers 6, 11, 12 and 14), some food ingredients caused a delayed flare of signs (≥6 h), while others also induced a relapse before that timeframe (Table S1). Altogether, delayed reactions after OFC were observed within 6–12 h in 3 dogs (11%), between 12 and 24 h in 8 dogs (29%) and beyond 24 h in the remaining 17 (61%) (Table S1).
LPT controls
The co-culture of the cells for 5 days with the three negative controls did not lead to SIs >1.9 in any dog. By contrast, the positive control concanavalin A led to SIs ranging from 7.9 to 18.9 in the 35 healthy laboratory and client-owned dogs (average 12.3, median 11.0) and between 6.7 and 19.7 (average 11.3, median 10.5) in the 28 dogs with FA. SIs after co-culture with concanavalin A were not significantly different between these two groups of dogs (Wilcoxon–Mann–Whitney U-test; p = 0.1541).
Performance of the LPT in healthy dogs
The LPT was first performed using a set of 23 food extracts plus one milk component (Bos d 7, bovine IgG) in the 10 healthy laboratory beagles, all females aged from 4.5 to 7.5 years (average 6.5, median 6.7 years). These dogs all had eaten the same chicken-and-rice diet for the preceding 3 years. In these dogs, the lowest of the 240 possible SIs was 1.0, and the highest was 2.7 (average 1.9, median 2.0). In fact, most SIs were tightly grouped with a 95% confidence interval between 1.9 and 2.0. Importantly, the LPT had SI values <2.7 for all ingredients reported on the beagle's diet label (chicken, rice, wheat, corn, soy, beetroot and brewers' yeast). Following the methodology employed for the LPT in human subjects,15 we established the positivity threshold (i.e. the cut-off value) for the canine test as 3.0, the value corresponding to the mean plus three standard deviations of all 240 SIs obtained in laboratory beagles.
The LPT was subsequently performed in 25 pet dogs coming to their veterinary surgeon for routine interventions using 28 different food extracts plus Bos d 7. In these dogs, the SIs ranged from 0.8 to 2.9 (average and median 1.7); none of the SIs was higher than the proposed threshold of 3.0 for any of the tested food allergens.
Performance of the LPT in dogs with delayed food allergy
Finally, the 28 dogs with delayed FA were tested with the LPT. The number of allergens used for stimulation varied among dogs, as the number of co-cultures performed varied to better match food items inducing flares during OFC (range 13–30, average 26, median 27).
In contrast to healthy beagles and client-owned pets, all 28 dogs with delayed FA had at least one positive LPT with an SI ≥3.0. The number of food items with an SI above the threshold ranged from 2 to 10 (average 5.1, median 5.0). The LPT positivity rates to the various ingredients are shown in Figure 1; the five main food extracts inducing a positive LPT were chicken and turkey (both 46%), beef (39%) as well as corn (38%) and duck (36%).
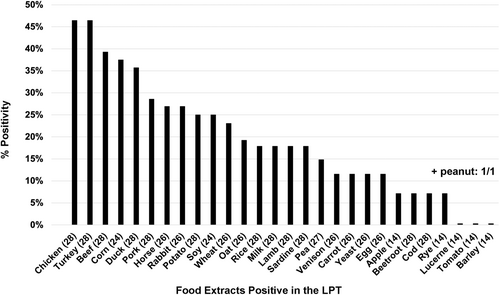
In 10 dogs, allergens were tested in duplicates (291 values). The coefficients of variation (CV%) between duplicate cell counts on D5 ranged from 0.5% to 20.4% (average 4.8%, median 4.3%); overall, there was little variation between duplicate cell counts, as only 14 of 291 allergen pairs (4.8%) had a CV% >10%.
Despite the limitations of a relatively small dataset, this LPT reliably discriminated between healthy dogs and those with delayed FA and demonstrated excellent sensitivity and specificity.
Correspondence between OFC and LPT results
Altogether, 57 food ingredients with a positive LPT matched some of the 68 food sources identified as causing a delayed flare (≥6 h) after OFC (84%) (Table S1). The agreement between the OFC and LPT was full (i.e. all culprit food ingredients causing a late flare had a corresponding positive LPT) in 18 of the 28 dogs (64%); it was partial (i.e. only some of the culprit ingredients were positive in the LPT) in the other 10 (36%) (Table S1). In each one of these 28 food-allergic dogs, the LPT also was positive for food extracts for which an OFC had not been done, so the clinical relevance of these results was not assessable.
IL-2 serum concentrations in the co-culture supernatant
In the three healthy dogs whose PBMCs were cultured with nine allergens over 5 days, SIs (i.e. fold-changes) of IL-2 concentrations over those cultured without allergens reached a maximum of 2.4 on D4 and D5. The co-cultures of cells of dogs with delayed food allergies with allergens inducing a negative LPT (SI < 3.0) had a maximal IL-2 SI of 2.9 on the same final 2 days. By contrast, as early as D1, the IL-2 SIs from co-cultures of cells from allergic dogs with food allergens inducing a positive LPT were >3.0 for eight of 28 allergens (29%); by D5, all 28 offending food allergens had IL-2 SIs ranging from 3.5 to 24.8 (average 7.6, median 6.3) (Figure 2a). Altogether, the IL-2 SIs were significantly different over time (Friedman ANOVA, p < 0.0001), being significantly higher each day compared to the preceding one, except for between D4 and D5 when the IL-2 concentrations had stabilised.
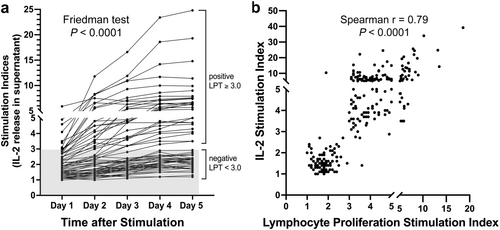
Finally, the correlation between both positive and negative LPT and IL-2 SIs was highly significant (Spearman r = 0.79; p < 0.0001), suggesting that both cell proliferation and IL-2 release were likely to be the consequences of the same allergen-driven stimulation of T cells (Figure 2b).
Food allergen-specific IgE
In three dogs (1, 6 and 20), the PAX revealed elevated concentrations (≥28.0 ng/mL) of IgE against food extracts or components of the same food source that triggered a late flare during OFC. Because these dogs also had a positive LPT, they were thus suspected of having a combination of IgE- and cell-mediated FA (i.e. a mixed FA; Table S1). In three other dogs (10, 11 and 21), the PAX was positive for allergens expected to IgE-cross-react with food items that induced a flare during OFC (Table S1); these also could represent examples of mixed FA. In dogs 4, 8, 16 and 22, the PAX detected IgE against some food allergens for which an OFC had not been performed, and the clinical relevance of such sensitisation was thus unknown (Table S1).
DISCUSSION
In this paper, we present the development and suitability of an LPT that uses the co-culture of canine PBMCs with food allergens to help recognise dogs with delayed FA; this LPT also permits the identification of culprit foods causing late flares after OFC.
In the 2023 European guidelines on the diagnosis of IgE-mediated FA in humans, this type of food reaction is reported to have signs recurring within 2 h of the consumption of offending items. In rare situations, for example, when anaphylaxis occurs after the ingestion of mammalian meats because of IgE binding to galactose-alpha-1,3-galactose (i.e. alpha-gal), there can be a delay of ≤6 h before signs eventually develop.3
That most food-allergic dogs in this series have a late flare of signs after an OFC is not surprising, as this predominance of long TTF has been reported before. Indeed, in a recent review, <10% of 234 dogs with FA had a flare on the first day after provocation.16 In another report of 46 dogs with FA, the median TTF was 12 h (range 1.5 h to 10 days), with only 11 of these dogs (24%) having a TTF of <6 h after provocation.17 This relative rarity of immediate signs after OFC was speculated before as most canine FAs having an underlying cell-mediated pathogenesis.16
The main signs observed in this series of dogs with delayed signs after OFC are identical to those reported in recent reviews on canine FA. Indeed, AD has been reported in 13%–100% of dogs with FA,18 while, conversely, an FA is diagnosed in between 10% and 50% of dogs with AD.19 Likewise, diarrhoea is, by far, the most common noncutaneous sign of canine FA. At the same time, vomiting is seen relatively less commonly.20 Of importance is that, in humans, these manifestations are not typical of IgE-mediated FA and instead are mostly seen in those with cell-mediated or mixed pathogenesis.1, 3
Most dogs with delayed FA experienced signs after consuming more than one food source, which could be the consequence of cross-reactivities between allergens found in different foods (e.g. between cod and salmon, beef and lamb, or duck and chicken). The ingredients that triggered delayed reactions during OFC were generally similar to those reported previously in dogs with FA.21 There were some relative changes in their prevalence, however, which could indicate a recent increase in the use of lower-priced ingredients (such as chicken and corn) in commercial dog foods. Of interest is that one dog exhibited signs after eating peanuts, a rarely reported event;22 the LPT confirmed the peanut sensitisation.
The LPT described herein is not the first test to use the ability of food allergens to stimulate T-cell-rich PBMCs in dogs. A 2004 study described a ‘lymphocyte stimulation test, LST’, in 11 dogs suspected of FA.7 In the LST, PBMCs were cultured for 3 days with extracts of food allergens at 5 μg/mL, and the incorporation of radioactive tritiated thymidine measured the lymphocyte blastogenesis; an SI of ≥2.0 was considered positive.7
The subsequent cellular tests used in dogs with FA are probably best described as ‘lymphocyte activation tests, LAT’, as they quantify, by flow cytometry, the expression of an activation marker, the IL-2 receptor CD25, in CD4-positive helper T lymphocytes.8-11, 23, 24 In these articles, the PBMCs were cultured with food allergens for 49-11, 23, 24 to 68 days, and the concentration of food allergens used for stimulation was either 59-11, 23, 24 or 10 μg/mL.8 In the LAT, the cut-off value was set as 1.1%, the highest percentage of CD4+/CD25low expressing lymphocytes among PBMCs of five healthy laboratory beagles.
In contrast to the LST and the LAT, our test directly counts cells after a 5-day culture with food allergens. Consequently, the test described in this paper appropriately deserves the denomination of a ‘proliferation test’. Because lymphocytes represent the main cell category among canine PBMCs, and only T and B cells are capable of proliferating and secreting IL-2 after antigen contact, this test is thus appropriately described as a bona fide ‘lymphocyte proliferation test or LPT’. The main advantages of the LPT compared to other tests are the direct determination of cell counts, the lack of use of radioactive isotopes and the lack of need for an expensive flow cytometer.
The LAT and LPT measure distinct aspects of lymphocytes' response to food allergens, yet their results are not interchangeable and might lack concordance. Therefore, it is vital to use proper terminology that accurately reflects the unique features of each cellular test rather than conflating them all under the term ‘LPT’. Using a similar denomination would imply uniformity among tests that are inherently diverse and yield different values.
This article on the use of LPT in food-allergic dogs offers some new insights relevant to veterinary surgeons. First, it has the novel finding that food items eaten by healthy laboratory beagles do not activate their PBMCs. Second, we showed that the LPT yielded values below the chosen positivity threshold for all tested foods in healthy pet dogs eating a variety of diets. Third, this is the first time that dogs with FA are separated based on their post-OFC TTF and suspected underlying immune mechanisms. For this reason, we limited the study of the LPT to dogs with delayed reactions after OFC, those suspected of having a cell-mediated FA. Consequently, as all healthy dogs had SI below the threshold and all dogs with delayed FA had at least one food with an SI above it, this LPT currently has both a 100% sensitivity and specificity for the identification of delayed FA in dogs. Finally, this is only the second time after the previously mentioned LST7 in which cellular test results are compared with those of the OFC, with both the LST and our LPT identifying most offending foods (about 80%).
The ability of the LPT to help recognise dogs with delayed FA and identify food components causing flares is not surprising in the light of most dogs with FA exhibiting delayed reactions after food ingestion, an observation suggestive of cell-mediated pathogenesis. Such a mechanism would explain why the PAX only occasionally detected serum IgE specific for culprit food allergens. Furthermore, a dichotomy of pathogenesis for canine FA—as for the human counterpart—could account, on its own, for the historically poor performance of IgE sensitisation tests (serological, intradermal or prick tests) to diagnose an FA that might not always involve allergen-specific IgE.6
Our results in dogs with delayed FA do not exclude that dogs with immediate reactions after OFC also might have a positive LPT to culprit allergens, as T cells help mediating the B-cell secretion of IgE; further studies are ongoing to clarify this matter.
This study's main limitation is that the determination of the TTF after OFC relied on pet owners observing clinical signs at home instead of being assessed by a veterinary surgeon. This could have led to the underreporting of immediate reactions (especially if mild) shortly after OFC, potentially resulting in the misinterpretation that these dogs only suffered from delayed FA. This concern supports the performance of in-clinic OFCs when pets are suspected of having IgE-mediated, immediate food allergies based on their clinical signs and history. A second limitation is that the LPT yielded positive SIs to some food extracts against which the dog had not been recognised as being allergic beforehand; as OFCs were not done with most of these ingredients, it is not known if these results were true or false positives.
CONCLUSIONS
In summary, the LPT described herein was useful in identifying dogs with delayed FAs, those suspected of having a cell-mediated pathogenesis. Additionally, this test reasonably predicted whether or not food items triggered a flare of signs ≥6 h after oral ingestion. Such a test should be a good complement to existing serological tests designed to identify food allergen-specific IgE in immediate FAs. Additional studies are needed to verify these results in a higher number of dogs with delayed FAs, and to compare LPT results with those of other techniques designed to test food sensitisations in such patients.
AUTHOR CONTRIBUTIONS
Carlos Fernandez-Lozano: Conceptualisation; methodology; investigation; validation; resources; writing—review and editing. Ana Mas-Fontao: Conceptualisation; resources; writing—review and editing. Silvia T. Auxilia: Resources; writing—review and editing. Marie Welters: Resources; writing—review and editing. Alla Olivrī: Resources; writing—review and editing. Ralf S. Mueller: Resources; writing—review and editing. Thierry Olivry: Conceptualisation; methodology; data curation; validation; formal analysis; supervision; visualisation; project administration; writing—original draft.
ACKNOWLEDGEMENTS
The authors are grateful to the veterinary surgeons who provided blood samples of dogs with food allergies used in this study.
FUNDING INFORMATION
Self-funded.
CONFLICT OF INTEREST STATEMENT
AMF and TO are employed by Nextmune; CFL is a former employee of this company.




