Canine melanocytes: Immunohistochemical expression of melanocytic markers in different somatic areas
Abstract
enBackground
Melanoblasts originate in the neural crest from where they migrate to peripheral tissues and differentiate into melanocytes. Alteration during melanocyte development and life can cause different diseases, ranging from pigmentary disorders and decreased visual and auditory functions, to tumours such as melanoma. Location and phenotypical features of melanocytes have been characterised in different species, yet data on dogs are lacking.
Objective
This study investigates the expression of melanocytic markers Melan A, PNL2, TRP1, TRP2, SOX-10 and MITF in melanocytes of selected cutaneous and mucosal surfaces of dogs.
Animals
At necropsy, samples from five dogs were harvested from oral mucosa, mucocutaneous junction, eyelid, nose and haired skin (abdomen, back, pinna, head).
Materials and Methods
Immunohistochemical and immunofluorescence analyses were performed to assess marker expression.
Results
Results showed variable expression of melanocytic markers in different anatomical sites, particularly within epidermis of haired skin and dermal melanocytes. Melan A and SOX-10 were the most specific and sensitive melanocytic markers. PNL2 was less sensitive, while TRP1 and TRP2 were seldomly expressed by intraepidermal melanocytes in haired skin. MITF had a good sensitivity, yet the expression often was weak.
Conclusions and Clinical Relevance
Our results indicate a variable expression of melanocytic markers in different sites, suggesting the presence of subpopulations of melanocytes. These preliminary results pave the way to understanding the pathogenetic mechanisms involved in degenerative melanocytic disorders and melanoma. Furthermore, the possible different expression of melanocyte markers in different anatomical sites could influence their sensitivity and specificity when used for diagnostic purposes.
Résumé
frContexte
Les mélanoblastes proviennent de la crête neurale, d'où ils migrent vers les tissus périphériques et se différencient en mélanocytes. L'altération des ménanocytes durant leur développement et leur vie peut induire différentes maladies : des troubles pigmentaires et des fonctions visuelles et auditives, ainsi que tumeurs telles que le mélanome. La localisation et les caractéristiques phénotypiques des mélanocytes ont été précisées dans différentes espèces, mais ces données manquent chez le chien.
Objectif
L'expression des marqueurs mélanocytaires Melan A, PNL2, TRP1, TRP2, SOX-10 et MITF est étudiée dans des échantillons de peau et de muqueuses de chiens.
Matériels et méthodes
Durant l'autopsie de cinq chiens, des échantillons de muqueuse buccale, de jonction cutanéo-muqueuse, de paupière, de nez et de peau velue (abdomen, dos, pavillon, tête) ont été prélevés ; des analyses immunohistochimiques et d'immunofluorescence ont été réalisées pour évaluer l'expression des différents marqueurs.
Résultats
Les marqueurs mélanocytaires sont exprimés de façon variable selon les différents sites anatomiques, en particulier au sein de l'épiderme de la peau velue et des mélanocytes dermiques. Melan A et SOX-10 sont les marqueurs mélanocytaires les plus spécifiques et les plus sensibles. PNL2 apparait moins sensible, tandis que TRP1 et TRP2 sont rarement exprimés par les mélanocytes intraépidermiques de la peau velue. MITF avait une bonne sensibilité, mais l'expression était souvent faible.
Conclusions et pertinence clinique
Les marqueurs mélanocytaires sont exprimés de façon variable dans différents sites anatomiques, suggérant la présence de sous-populations mélanocytaires. Ces résultats préliminaires ouvrent la voie à la compréhension des mécanismes pathogéniques impliqués dans les maladies mélanocytaires dégénératives et le mélanome. De plus, ces éventuelles différences d’expression des marqueurs mélanocytaires selon le site anatomique pourraient influencer la sensibilité et la spécificité des analyses utilisant ces marqueurs à visée diagnostique.
Resumen
esIntroducción
los melanoblastos se originan en la cresta neural desde donde migran a los tejidos periféricos y se diferencian en melanocitos. La alteración durante el desarrollo y la vida de los melanocitos puede causar diferentes enfermedades, que van desde trastornos pigmentarios y disminución de las funciones visuales y auditivas, hasta tumores como el melanoma. La ubicación y las características fenotípicas de los melanocitos se han caracterizado en diferentes especies, pero faltan datos sobre perros.
Objetivo
Este estudio investiga la expresión de los marcadores melanocíticos Melan A, PNL2, TRP1, TRP2, SOX-10 y MITF en melanocitos de superficies cutáneas y mucosas seleccionadas de perros.
Materiales y Métodos
durante las necropsias, se obtuvieron muestras de cinco perros de mucosa oral, unión mucocutánea, párpado, nariz y piel con pelo (abdomen, espalda, pabellón auricular, cabeza); Se realizaron análisis inmunohistoquímicos y de inmunofluorescencia para evaluar la expresión de los marcadores.
Resultados
Los resultados mostraron una expresión variable de marcadores melanocíticos en diferentes sitios anatómicos, particularmente en la epidermis de la piel con pelos y en melanocitos dérmicos. Melan A y SOX-10 fueron los marcadores melanocíticos más específicos y sensibles. PNL2 fue menos sensible, mientras que TRP1 y TRP2 rara vez se expresaron en melanocitos intraepidérmicos en la piel con cabello. MITF tenía una buena sensibilidad, pero la expresión a menudo era débil.
Conclusiones y relevancia clínica
Nuestros resultados indican una expresión variable de marcadores melanocíticos en diferentes sitios, lo que sugiere la presencia de subpoblaciones de melanocitos. Estos resultados preliminares inician el camino para comprender los mecanismos patogénicos implicados en los trastornos melanocíticos degenerativos y el melanoma. Además, la posible expresión diferente de los marcadores de melanocitos en diferentes sitios anatómicos podría influir en su sensibilidad y especificidad cuando se utilizan con fines de diagnóstico.
Zusammenfassung
deHintergrund
Melanoblasten stammen aus der Neuralleiste, von der sie ins periphere Gewebe auswandern und zu Melanozyten ausdifferenzieren. Veränderungen während der Entwicklung der Melanozyten und ihres Lebenslaufs können verschiedene Krankheiten bedingen, von Pigmentunregelmäßigkeiten und verminderten visuellen und auditorischen Funktionen bis hin zu Tumoren wie Melanome. Die Lokalisation und die phänotypischen Merkmale wurden bei verschiedenen Spezies beschrieben, beim Hund gibt es jedoch bisher keine Daten.
Ziel
Diese Studie untersucht die Exprimierung von Melanozytenmarkern Melan A, PNL2, TRP1, TRP2, SOX-10 und MITF an Melanozyten von selektiven kutanen und mucokutanen Oberflächen von Hunden.
Materialien und Methoden
Bei der Nekropsie wurden Proben aus der oralen Mukosa, mucokutanen Übergängen, Augenlid, Nase und behaarter Haut (Bauch, Rücken, Pinna, Kopf) von fünf Hunden genommen; immunhistochemische und Immunfluoreszenz-Analyse wurde durchgeführt, um eine Markerexprimierung zu erfassen.
Ergebnisse
Die Ergebnisse zeigten eine unterschiedliche Exprimierung der Melanozytenmarker an verschiedenen anatomischen Stellen, vor allem innerhalb der Epidermis der behaarten Haut und der dermalen Melanozyten. Melan A und SOX-10 waren die spezifischsten und sensitivsten Melanozytenmarker. PNL2 waren weniger sensitiv, während TRP1 und TRP2 selten an intraepidermalen Melanozyten der behaarten Haut exprimiert wurden. MITF zeigte eine gute Sensibilität, obwohl die Exprimierung oft nur schwach war.
Schlussfolgerungen und klinische Relevanz
unsere Ergebnisse zeigten eine variable Exprimierung der Melanozytenmarker an verschiedenen Stellen, was auf das Auftreten unterschiedlicher Subpopulationen der Melanozyten hinweist. Diese vorläufigen Ergebnisse ebnen den Weg für ein Verständnis der pathogenen Mechanismen, die bei degenerativen Melanozyten-Veränderungen und beim Melanom eine Rolle spielen. Weiters könnte die möglicherweise unterschiedliche Exprimierung der Melanozytenmarker an verschiedenen anatomischen Stellen ihre Sensibilität und Spezifität beeinflussen, wenn sie für diagnostische Zwecke eingesetzt werden.
要約
ja背景
メラノブラストは神経堤で発生し、そこから末梢組織へ移動してメラノサイトに分化する。メラノサイトの発生・生育過程における変化は、色素障害、視覚・聴覚機能の低下からメラノーマなどの腫瘍に至るまで、様々な疾患の原因となる可能性がある。メラノサイトの位置や表現型は、様々な動物種で明らかにされているが、犬に関するデータは不足している。
目的
本研究の目的は、犬の皮膚・粘膜のメラノサイトにおけるメラノサイトマーカーMelan A, PNL2, TRP1, TRP2, SOX-10, MITFの発現を検討することであった。
材料と方法
剖検時に、5頭の犬から口腔粘膜、粘膜皮膚接合部、眼瞼、鼻および有毛皮膚(腹部、背部、耳介、頭部)のサンプルを採取し、免疫組織化学および免疫蛍光解析を行い、マーカーの発現を評価した。
結果 その結果、解剖学的な部位によってメラノサイトマーカーの発現が異なり、特に有毛皮膚表皮内および真皮のメラノサイトの発現にばらつきがあることが判明した。Melan AおよびSOX-10は、最も特異的で感度の高いメラノサイトマーカーであった。PNL2は感度が低く、TRP1およびTRP2は有毛皮膚内の表皮内メラノサイトでほとんど発現していなかった。MITFは感度が高いが、発現が弱い場合が多い。
結論と臨床的意義
今回の結果は、部位によってメラノサイトマーカーの発現が異なることを示し、メラノサイトの亜集団の存在を示唆するものであった。これらの予備的な結果は、変性メラノサイト障害やメラノーマに関わる発症メカニズムの理解に道を開くものである。さらに、解剖学的部位によってメラノサイトマーカーの発現が異なる可能性があることから、診断目的で使用する場合には、その感度や特異性に影響を与える可能性がある。
摘要
zh背景
黑素细胞起源于神经嵴,从那里迁移到周围组织并分化为黑素细胞。黑素细胞发育和生活过程中的变化会导致不同的疾病,从色素性疾病、视觉和听觉功能下降,到黑色素瘤等肿瘤。黑色素细胞的位置和表型特征已经在不同的物种中得到了表征,但关于犬的数据仍然缺乏。
目的
本研究研究黑素细胞标志物黑素A、PNL2、TRP1、TRP2、SOX-10和MITF在犬皮肤和粘膜表面黑素细胞中的表达。
材料和方法
尸检时,从五只犬的口腔粘膜、粘膜皮肤连接处、眼睑、鼻子和毛发皮肤(腹部、背部、耳廓、头部)采集样本;进行免疫组织化学和免疫荧光分析以评估标记物的表达。
结果
结果显示黑素细胞标记物在不同解剖部位的表达不同,尤其是在毛发皮肤的表皮和真皮黑素细胞内。黑素A和SOX-10是最特异和敏感的黑素细胞标志物。PNL2较不敏感,而TRP1和TRP2仅在毛发皮肤表皮内黑素细胞中表达。MITF具有良好的敏感性,但表达往往较弱。
结论和临床相关性
我们的结果表明,不同部位的黑素细胞标志物表达不同,表明存在黑素细胞亚群。这些初步结果为理解退行性黑素细胞疾病和黑色素瘤的发病机制铺平了道路。此外,当用于诊断目的时,黑素细胞标记物在不同解剖部位的可能不同表达可能会影响其敏感性和特异性。
Resumo
ptContexto
Os melanoblastos se originam da crista neural, de onde eles migram para os tecidos periféricos e se diferenciam em melanócitos. Alterações durante o desenvolvimento e ao longo da vida dos melanócitos pode causar diferentes doenças, desde alterações pigmentares e redução das funções auditivas e visuais, a tumores como o melanoma. Localização e características fenotípicas dos melanócitos têm sido detalhadas em várias espécies, mas em cães os dados ainda são escassos.
Objetivo
Este estudo investiga a expressão dos marcadores melanocíticos Melan A, PNL2, TRP1, TRP2, SOX-10 e MITF nos melanócitos de determinadas superfícies cutâneas e mucosas em cães.
Materiais e métodos
À necrópsia, foram coletadas amostras de mucosa oral, junção mucocutânea, pálpebra, nariz e pele pilosa (abdômen, dorso, orelha, cabeça); para se avaliar a expressão dos marcadores, realizou-se imuno-histoquímica e imunofluorescência.
Resultados
Os resultados demonstraram expressão de marcadores melanocíticos em diferentes sítios anatômicos, particularmente na epiderme da pele pilosa e nos melanócitos dérmicos. Melan A e SOX-10 foram os marcadores melanocíticos mais específicos e sensíveis. PNL2 foi menos sensível, enquanto TRP1 e TRP2 foram raramente expressados por melanócitos intraepidérmicos na pele pilosa. MITF apresentou boa sensibilidade, apesar de a sua expressão ser frequentemente fraca.
Conclusões e relevância clínica
Nossos resultados indicam uma expressão variável de marcadores melanocíticos em diferentes regiões, sugerindo a presença de subpopulações de melanócitos. Estes resultados preliminares embasam a compreensão dos mecanismos patogenéticos envolvidos nas doenças melanocíticas degenerativas e no melanoma. Além disso, a possível diferença na expressão de marcadores melanocíticos em diferentes sítios anatômicos pode influenciar a sua sensibilidade e especificidade quando utilizado para diagnóstico.
INTRODUCTION
Melanocytes are neural crest-derived cells, which migrate to peripheral tissues (e.g. epidermis, hair follicle, mucosa, cochlea, iris, mesencephalon), where they synthesise melanin pigment.1 Melanin is important in skin and hair pigmentation, protects skin and uvea from solar radiation and contributes to endolymph formation in the cochlea.2 Therefore, melanocytic disorders can manifest as syndromic diseases whereby hypopigmentation, blindness and deafness coexist.3 Also, melanocytic tumours comprise multiple biologically distinct categories, which differ in terms of site of origin (cutaneous versus mucosal), clinical and histological presentation, biological behaviour, causative role of UV radiation and genetic mutations, in both humans and animals.
Much of the information on melanocytes is gathered from studies conducted on humans, rabbits and mice.4-6 However, the biology of canine melanocytes remains largely unknown. With the exception of rare reports, the veterinary scientific literature does not provide extensive studies focused on the characterisation of these cells, with some rare exceptions.7
In humans, the melanocyte density in the epidermis (expressed as melanocyte: keratinocyte ratio)8, 9 is 1:10, with about 1200 melanocytes/mm2 of human skin, independently of ethnicity.9 Nevertheless, the number of melanocytes varies in different anatomical districts. It, therefore, has been hypothesised that an unequal distribution of melanocytes may partially explain the site-specific incidence of some pathological conditions, such as melanoma, offering perspectives not only on the aetiology of this tumour,10 but also on hyper/hypopigmentation disorders.11
Recently, different canine diseases have been proposed as possible spontaneous models for human counterparts, such as vitiligo, Vogt–Koyanagi–Harada (VKH) syndrome and melanoma.12-16
Nevertheless, given that knowledge on canine melanocytes remains unexplored or, at least, sparse, further studies to unveil their biology are recommended both for a better characterisation of canine diseases and for an accurate comparison among dogs and humans. Additionally, canine mucosal melanoma has been identified as a possible valuable model for the rare human mucosal melanoma, which, in both species, is a very aggressive disease with a high metastatic rate, no efficient therapies and poor prognosis.15-17 However, data are still lacking and further studies are required to confirm the strength of this model.14 To the best of the authors' knowledge, the presence of melanocytes in different somatic areas of dogs has never been assessed systematically in large studies and different breeds; moreover, the expression of different melanocytic markers in different anatomic regions has never been reported.
The aim of the present study was to investigate the expression of different markers, namely Melan A, PNL2, TRP1, TRP2, SOX-10 and MITF, in melanocytes within cutaneous and mucosal surfaces, evaluating their co-expression and the distribution of melanocytic populations in different anatomical sites.
MATERIALS AND METHODS
Case selection
Samples were harvested from five dogs, with no clinically reported dermatological or mucosal lesions, submitted for necropsy after owner's consent. Samples (two to three) were collected from different cutaneous and mucosal sites, less than 3 h after the death of the dog. Sampling was performed harvesting an 8 mm punch biopsy (measured parallel to the epithelial surface) to guarantee a proper evaluation. In the present study samples were collected from: oral mucosa (internal aspect of the cheek), oral mucocutaneous junction (lateral upper lip the canine tooth level), eyelid (lower right eyelid), nose, pinna (tip), ventral abdomen (cranial to the umbilical scar, on the median line), back (lumbar area, on the median line) and head (between the pinnae, on the median line).
Pigmentation evaluation
The presence of pigmentation was evaluated macroscopically on epithelium and hair both before and after inclusion in paraffin and sectioning. Subgrossly, the degree of pigmentation was semiquantitatively evaluated on the epidermis/mucosal surface and assigned one of the following values: 0, pink/whitish skin with no spots; 1, light brown skin with no spots; 2, dark brown skin with no spots; 3, black skin with no spots; P, patchy, because of the presence of spots or irregular distribution of pigment. The same grades were assigned for the evaluation of hair coat pigmentation: 0, white hair; 1, light brown hair; 2, dark brown hair; 3, black hair; P, patchy, because of the presence of spots or different coloration of hair.
Histological and immunohistochemical evaluation
From all samples a routinely stained haematoxylin & eosin slide was prepared. Five micrometre sections were cut from formalin-fixed and paraffin-embedded samples and mounted on poly-l-lysine-coated slides, which then were dewaxed and dehydrated. Immunohistochemical evaluation was performed on serial sections with antibodies reported to be cross-reactive in canine species, raised against Melan A,18 PNL2,18 TRP1, TRP2, SOX-10 and MITF. Positive controls were obtained from canine melanomas for Melan A, PNL2, TRP1, TRP2 and MITF, while mammary gland was used as control for SOX-10.19 AEC was used as a chromogen for its red colour, to differentiate from melanin. Bleaching was not performed. Negative controls were run omitting the primary antibody and incubating control sections with TBS. IBA1 immunolabelling was performed, following protocols reported previously, in selected cases to differentiate melanocytes from melanin-laden macrophages.20 Immunohistochemical protocols and antibodies used in this study are summarised in Table 1.
| Antigen | Clone | Antigen retrieval | Dilution | Distributor |
|---|---|---|---|---|
| Melan A | A103-M27C10-M29E3 (mouse) | TRIS-Edta buffer, pH 9.0 | 1:150 | Abcam |
| PNL2 | PNL2 (mouse) | Citrate buffer, pH 6.0 | 1:150 | Santa Cruz |
| TRP1 (TYRP1/gp75) | Polyclonal rabbit | TRIS-Edta buffer, pH 9.0 | 1:100 | LSbio |
| TRP2 | Polyclonal rabbit | Citrate buffer, pH 6.0 | 1:250 | Abcam |
| SOX-10 | A-2 (mouse) | TRIS-Edta buffer, pH 9.0 | 1:200 | Santa Cruz |
| MITF | C5/D5 (mouse) | TRIS-Edta buffer, pH 9.0 | 1:75 | Merck |
For each antibody, the presence of positive cells was assessed within the cutaneous/mucosal epithelium, in the hair bulb and follicular wall and in the other cutaneous/mucosal structures (e.g. glands, nerves, dermis).
- The location within epidermis (basal/suprabasal);
- The shape (bright round, oval, fusiform or dendritic cells);
- The presence of intracytoplasmic melanin pigment.
Immunofluorescence
Double immunofluorescence was performed on selected samples from nose, oral mucosal and haired skin. The co-expression of melanocytic markers was assessed pairing antibodies with similar antigen retrieval methods. For this purpose, primary antibodies previously described for immunohistochemical evaluation (TRP1, SOX-10 and MITF) were co-localised with anti-Melan A conjugated with Alexa Fluor 594, while TRP2 was co-localised with anti-PNL2 conjugated with Alexa Fluor 488 antibodies (Santa Cruz). SOX10 and TRP1 also could be co-localised, yet co-localisation of TRP1 and TRP2 was not possible, both being primary antibodies raised in rabbit. After dewaxing and dehydrating, the slides were incubated with a 3% hydrogen peroxide methanol solution. Antigen retrieval was performed as reported for immunohistochemical evaluation and primary antibodies were incubated overnight at 4°C with the appropriate concentration. After washing with tris-buffered saline, a secondary goat anti-mouse conjugated with a red fluorochrome (Goat Anti-Mouse IgG H&L-Alexa Fluor 647) and with a 1:200 dilution, was applied for 2 h at room temperature (RT). After a careful wash, a protein block step was performed before incubation with Melan A and PNL2 fluorochrome-conjugated antibodies. To co-localise SOX-10 and TRP1, after incubation of TRP1, a secondary anti-rabbit antibody conjugated with a fluorochrome (Goat Anti-Rabbit IgG H&L-Alexa Fluor 594; Abcam) was used. Finally, a drop of aqueous mounting medium containing DAPI (Abcam) was added and slides were incubated for 5 min at RT, before coverslip mounting. Negative controls were run omitting the primary antibodies and incubating the sections with phosphate-buffered saline.
Samples were evaluated using an Olympus BX51 fluorescent microscope equipped with a Nikon DS-Qi2Mc camera. NIS-ELEMENTS D software was used for image acquisition and analysis.
RESULTS
Case selection and macroscopical evaluation
The five dogs selected for this preliminary investigation were a chow chow, a Labrador retriever, a Siberian husky, a mixed English setter and a mixed breed dog. The five animals showed a variable degree of pigmentation of both skin/mucosa and of hair coat in the sampled areas. The results of macroscopical evaluation are reported in Tables 2 and 3, respectively.
| N. | Breed | Pigm. | Melan A | PNL2 | TRP1 | TRP2 | SOX-10 | MITF | |
|---|---|---|---|---|---|---|---|---|---|
| Oral Mucosa | 1 | Chow chow | 2 | + | + | + | + | + | + |
| 2 | Mixed breed | 2 | + | ± | ± | ± | + | + | |
| 3 | Labrador retriever | 2 | + | + | + | + | + | + | |
| 4 | English setter | P | − | − | ± | − | − | − | |
| 5 | Siberian husky | P | ± | − | ± | ± | ± | ± | |
| Oral MCJ | 1 | Chow chow | 3 | + | + | + | + | + | + |
| 2 | Mixed breed | 3 | + | + | + | + | + | + | |
| 3 | Labrador retriever | 3 | + | + | + | + | + | + | |
| 4 | English setter | 2 | + | + | + | + | + | + | |
| 5 | Siberian husky | 3 | + | + | + | + | + | + | |
| Eyelid | 1 | Chow chow | 2 | + | + | + | + | + | + |
| 2 | Mixed breed | 3 | + | + | + | + | + | + | |
| 3 | Labrador retriever | 3 | + | + | + | + | + | + | |
| 4 | English setter | 2 | + | + | + | + | + | + | |
| 5 | Siberian husky | 3 | + | + | + | + | + | + | |
| Nose | 1 | Chow chow | 2 | + | ± | + | ± | + | + |
| 2 | Mixed breed | 3 | + | ± | + | ± | + | + | |
| 3 | Labrador retriever | 2 | + | ± | + | ± | + | + | |
| 4 | English setter | 2 | + | ± | + | ± | + | + | |
| 5 | Siberian husky | 3 | + | + | + | ± | + | + | |
| Abdomen | 1 | Chow chow | 1 | − | − | − | − | + | − |
| 2 | Mixed breed | 2 | + | − | − | − | + | + | |
| 3 | Labrador retriever | 1 | + | + | − | + | + | + | |
| 4 | English setter | 2 | + | + | − | + | + | + | |
| 5 | Siberian husky | 2 | + | + | + | + | + | + | |
| Back | 1 | Chow chow | 1 | + | − | − | − | + | + |
| 2 | Mixed breed | 1 | + | − | − | − | + | + | |
| 3 | Labrador retriever | 1 | + | − | − | − | + | + | |
| 4 | English setter | P | ± | − | + | − | − | + | |
| 5 | Siberian husky | P | − | − | − | − | ± | − | |
| Pinna | 1 | Chow chow | 1 | + | + | + | − | + | + |
| 2 | Mixed breed | 2 | + | + | − | − | + | + | |
| 3 | Labrador retriever | 1 | + | + | − | − | + | + | |
| 4 | English setter | 2 | + | + | + | − | + | − | |
| 5 | Siberian husky | 3 | + | + | + | − | + | − | |
| Head | 1 | Chow chow | 1 | + | − | − | − | + | − |
| 2 | Mixed breed | 1 | − | − | − | − | + | − | |
| 3 | Labrador retriever | 1 | + | ± | + | − | + | ± | |
| 4 | English setter | 2 | + | − | + | − | + | ± | |
| 5 | Siberian husky | 2 | − | − | − | − | + | − |
- Note: Pigm., degree of pigmentation evaluated macroscopically on the epithelial surface (0/1/2/3 and P, patchy); expression of melanocytic markers is expressed as present (+), absent (−) or patchy (±).
| N. | Breed | Pigm. | Melan A | PNL2 | TRP1 | TRP2 | SOX-10 | MITF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | W | B | W | B | W | B | W | B | W | B | W | ||||
| Perioral | 1 | Chow chow | 1 | − | + | − | − | − | − | − | − | − | + | − | − |
| 2 | Mixed breed | 3 | + | + | + | + | + | + | + | + | + | + | + | + | |
| 3 | Labrador retriever | 1 | + | + | + | + | − | + | − | + | − | + | − | + | |
| 4 | English setter | P | − | − | − | + | − | + | − | + | − | + | − | + | |
| 5 | Siberian husky | 0 | − | + | − | + | − | + | − | − | − | + | − | + | |
| Periocular | 1 | Chow chow | 1 | + | + | − | + | − | + | − | − | − | + | − | + |
| 2 | Mixed breed | 3 | + | + | + | + | + | + | + | + | + | + | + | + | |
| 3 | Labrador retriever | 1 | − | + | + | + | − | + | − | + | − | + | − | + | |
| 4 | English setter | P | + | + | + | + | + | + | + | + | + | + | + | + | |
| 5 | Siberian husky | P | + | + | + | + | − | + | − | + | + | + | − | + | |
| Abdomen | 1 | Chow chow | 1 | − | − | + | − | − | − | − | − | + | + | − | − |
| 2 | Mixed breed | 1 | − | − | − | − | − | − | − | − | + | + | − | + | |
| 3 | Labrador retriever | 1 | + | + | − | − | − | + | − | − | + | + | − | + | |
| 4 | English setter | P | − | + | − | − | − | − | − | − | + | − | + | + | |
| 5 | Siberian husky | 0 | + | + | + | + | − | − | + | − | + | + | + | + | |
| Back | 1 | Chow chow | 1 | + | + | − | + | − | + | − | + | + | + | − | + |
| 2 | Mixed breed | 2 | + | + | + | + | − | + | + | + | + | + | − | + | |
| 3 | Labrador retriever | 1 | + | + | − | + | − | + | − | + | + | + | − | + | |
| 4 | English setter | P | − | + | − | + | − | − | − | + | − | + | − | + | |
| 5 | Siberian husky | 3 | + | + | + | + | + | − | + | + | + | + | − | + | |
| Pinna | 1 | Chow chow | 1 | + | + | + | − | + | − | − | − | + | + | − | + |
| 2 | Mixed breed | 3 | + | − | + | − | + | − | − | − | + | + | + | + | |
| 3 | Labrador retriever | 1 | + | − | + | − | + | − | + | − | + | + | + | + | |
| 4 | English setter | P | + | + | + | − | + | + | + | − | + | + | + | − | |
| 5 | Siberian husky | 3 | + | + | + | − | + | − | + | − | + | + | + | − | |
| Head | 1 | Chow chow | 1 | + | + | + | − | + | − | − | − | + | + | − | + |
| 2 | Mixed breed | 2 | + | − | − | − | + | − | − | − | + | + | − | + | |
| 3 | Labrador retriever | 1 | + | + | + | − | + | − | + | − | + | + | + | + | |
| 4 | English setter | P | + | − | + | − | + | − | + | − | + | + | + | + | |
| 5 | Siberian husky | P | + | + | + | − | + | − | + | − | + | + | + | + | |
- Note: Pigm, degree of pigmentation of hair coat (0/1/2/3 and P, patchy); expression of melanocytic markers is expressed as present (+), absent (−).
- Abbreviations: B, hair bulb; W, follicular wall.
Melan A
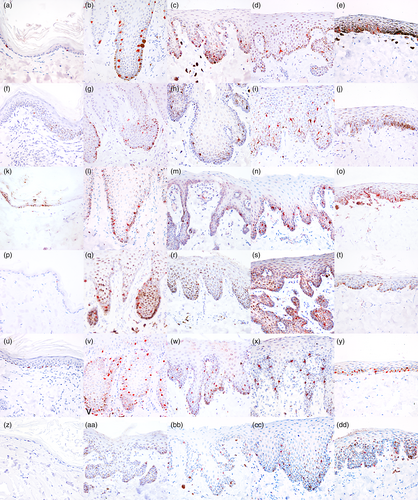
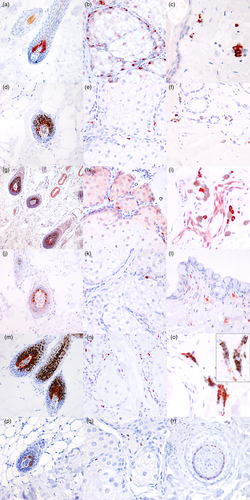
Melan A immunolabelling was observed as a granular cytoplasmic reaction. The reaction was strong and specific, and no signs of background stain were present. Melan A positive cells were observed in the basal and suprabasal layers of the epithelium in mucosal, junctional, nasal and cutaneous samples (Table 2; Figure 1a–e). One of the dogs (Siberian husky, Case 5) showed mucosa with a macroscopical patchy pigmentation. In this case, corresponding to the macroscopically evident patcahy pigmentation (P), there were areas with numerous Melan A+ melanocytes alternating with areas where no positive cells could be observed. A strong expression of this marker also was seen in the hair bulbs and in the infundibula of hair follicles (Table 3; Figure 2a). In hair bulbs, where pigmentation was prominent with H&E visualisation, the expression of Melan A was more intense and located in a higher number of cells. Nevertheless, the expression of Melan A also could be observed in scattered cells in those samples where hair pigmentation was not histologically evident. In one of the samples from the abdominal skin (Case 4), a mild hyperplasia of the epidermis was seen, associated with multifocal, mild lymphocytic and plasmacellular infiltrates in the superficial dermis; in this case, the number of melanocytes appeared to be increased. Generally, no melanocyctes were observed in sebaceous glands, except for Meibomian glands and periocular sebaceous glands, where occasional Melan A+ cells were observed among the basal cells (Figure 2b). Only in one case (Case 1) were Melan A+ cells present near apocrine glands in the dermis and these were interpreted as dermal melanocytes (Figure 2c).
PNL2
The expression of PNL2 was seen as a finely granular, cytoplasmic immunolabelling. The expression of this marker was variable and sometimes very faint, with an occasional uneven distribution of the immunoreactivity in the cytoplasm. Positive cells were seen in the basal and suprabasal layers of the epithelium (Table 2; Figure 1f–j). In a similar way to Melan A, patchy-pigmented oral mucosa showed areas with the presence of PNL2+ melanocytes alternating with areas with no detectable cells. Occasionally, in homogeneously pigmented epithelia such as the nose and the mucosal junctions, PNL2+ melanocytes were present with a multifocal distribution, unlike the regular presence of immunoreactive melanocytes seen for other markers, particularly Melan A and SOX-10. The immunolabelling was stronger in follicular melanocytes, in both hair bulb (Table 3; Figure 2d) and follicular wall. Scattered PNL2+ cells were present in the basal compartment of Meibomian glands (Figure 2 e), yet, in a similar way to Melan A, not observed in sebaceous glands of the other cutaneous areas sampled for this study. Dermal melanocytes were negative in all samples examined, including for Case 1 (Figure 2f).
TRP1
The immunolabelling for TRP1 was cytoplasmic and coarsely granular; the intensity of the labelling was variable, from moderate to strong. In eyelid and oral mucocutaneous junctions (Figure 1k–o), the marker's expression was strong and similar to what observed with Melan A, while no TRP1+ intraepithelial melanocytes were present in most of the samples harvested from the cutaneous sites (Table 2). As for hair bulbs (Figure 2g) and follicular walls (Table 3), the immunolabelling was strong and specific, and apparently increased with the quantity of melanin-containing cells. In all of the samples from haired skin, sparse TRP1+ positive round cells, characterised by moderate amounts of cytoplasm and rounded nucleus, were observed in particular in the superficial dermis. Rare immunolabelled cells were observed in the basal compartment of Meibomian and periocular sebaceous glands (Figure 2h). Dermal melanocytes did not label with TRP1, yet some positive cells were seen among negative melanocytes (Figure 2i) and were interpreted as mast cells.
TRP2
Immunolabelling for TRP2 was strong and coarsely granular, with a cytoplasmic distribution. The intensity of the staining was unpredictably variable, being strong and neat in some cases, in particular on the nose, oral mucocutaneous junction and eyelid junction (Table 2; Figure 1q,rt, respectively), while in haired skin and oral mucosa (Figure 1p,s), the immunolabelling often was faint. Generally, immunolabelling was stronger in hair bulbs (Table 3; Figure 2j) than in intraepithelial melanocytes. In a similar way to PNL2, the immunolabelling of this marker showed a multifocal distribution in all cases in some macroscopically heavily pigmented samples, such as the nose (Table 2). Rare positive cells were observed in the basal compartment of Meibomian glands (Figure 2k). Dermal melanocytes did not label with TRP2, yet in some samples, TRP2+ cells were seen in the dermis (Figure 2l). Morphologically these cells were characterised by a moderate quantity of cytoplasm and interpreted as histiocytes.
SOX-10
SOX-10 expression appeared as a strong and diffuse nuclear immunolabelling, regularly expressed in both haired skin, mucocutaneous junctions and oral mucosa (Figure 1u–y). The immunolabelling of the nucleus was sharp and strong and allowed the rapid and solid identification of positive cells. Besides, this neat immunolabelling granted a better identification of melanocytes also in highly pigmented sites, such as the hair bulbs (Table 3; Figure 2m). All positive cells within the epithelia and in hair follicles were morphologically compatible with melanocytes, being characterised by scant clear cytoplasm or filled with melanin pigment and showing dendritic prolongations of the cytoplasm. Positive cells among the basal component of Meibomian glands (Figure 2n) were frequently observed; on other samples from haired skin, no positivity was assessed yet, differently from the other markers, rare positive nuclei were observed in the cutaneous samples from the head of Case 1. Dermal melanocytes were positive (Figure 2o). The same cells were negative for the macrophagic marker IBA1 (inset in Figure 2o). (Positivity also was observed in dermal nerves and in myoepithelial cells of apocrine glands and of mammary gland, that were sampled together with the abdominal skin.)
MITF
Immunolabelling of MITF was seen as a finely granular nuclear reactivity. Often, the immunoreactivity was faint when compared to the other markers, yet nuclear expression was helpful particularly in highly pigmented samples. In a similar way to PNL2, TRP1 and TRP2, melanocyte immunolabelling with MITF was more rarely observed within haired epithelia (Table 2; Figure 1z), while it was more prominent in samples harvested from mucocutaneous junctions (Figure 1bb, dd), oral mucosa (Figure 1cc) and, even if weaker, from the nose (Figure 1aa).
The labelling of melanocytes in hair bulbs was more intense and clearer when compared to the intraepithelial samples (Table 3; Figure 2p). Rare positive cells were observed in the basal compartment of Meibomian glands (Figure 2q). Moreover, the inner root sheet showed a moderate-to-intense immunolabelling in hair follicles (Figure 2r). Dermal melanocytes showed a variable positivity. MITF+ cells were scattered in the superficial to mid-dermis; these cells were characterised by rounded shape, a moderate amount of cytoplasm and a central rounded nucleus, morphologically similar to mast cells.
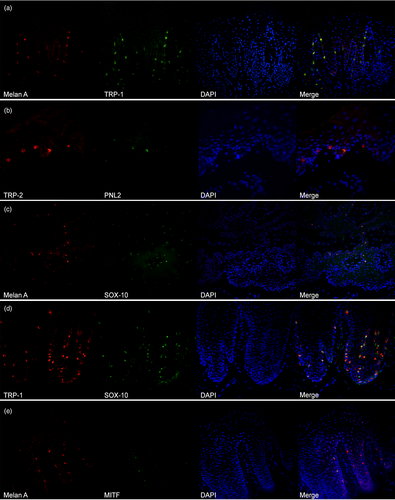
Melanocytic marker co-expression
Melan A showed a clear and moderate-to-strong expression and an almost total co-localisation with TRP1 on oral mucosa and nose (Figure 3a). PNL2 confirmed a lower sensitivity in detecting melanocytes and the signal often was weaker. The co-localisation was possible only with TRP2, because both antibodies required antigen retrieval with citrate buffer. TRP2 was more sensitive than PNL2 in detecting melanocytes (Figure 3b). Melan A and SOX-10 showed an almost complete co-localisation (Figure 3c), with SOX-10 being more sensitive and, as a consequence of the nuclear staining, easier for operators to recognise. On the nose and mucosal sites, SOX-10 showed a sensitivity, which was similar to TRP1 (Figure 3d). Co-localisation of Melan A and MITF confirmed that the latter was less sensitive; in a similar way to immunohistochemical findings, the signal was weak (Figure 3e).
DISCUSSION
The expression of melanocytic markers in canine species has never been assessed in a dedicated study, despite numerous markers already being used in diagnostic environments, particularly for neoplastic lesions, such as amelanotic melanomas.21-24
Melan A is considered one of the best and most specific markers for melanocytes in different species, dogs included.7, 25-27 Results from our study confirm the specificity and the usefulness of Melan A for the recognition of intraepidermic melanocytes, both in immunohistochemical and immunofluorescence evaluation. Also, Melan A proved useful for the recognition of dermal melanocytes, particularly in the dermis of the chow chow, where the histiocytic/macrophagic origin of the cells also was ruled out using IBA1. Being a cytoplasmic antigen, Melan A may not be the best immunohistochemical marker for a quantitative evaluation of melanocytes, as a result of its possible interference with cytoplasmic melanin-pigmented granules. In these cases, in fact, a nuclear marker, such as SOX-10 should be preferred as observed in other studies.28 Melan A also was confirmed as a good melanocytic marker when tested with immunofluorescence and is considered, together with SOX-10, the most sensitive marker for the detection of melanocytes in our samples. These two markers showed an almost complete co-localisation, with SOX-10 being more sensitive and, as a consequence of the nuclear staining, associated with easier recognition by the operator.
Despite being one of the most commonly used markers for melanocytic tumours,22, 27 PNL2 did not show satisfactory results in our study, showing less intense immunolabelling and revealing fewer melanocytes compared to other markers. This result could be the result of a lower expression of the protein itself, which could result in a weaker immunostaining reactivity, less easily recognisable both with immunohistochemical and immunofluorescence evaluation. Moreover, PNL2 appears not to be sensitive in the detection of dermal melanocytes.
The expression of TRP1 in dermal cells could identify dermal melanocytes, yet in our study positive cells were morphologically compatible with mast cells and TRP1 positivity was not co-expressed with other melanocytic markers in this cellular population. To the best of the authors' knowledge, TRP1 is not expressed in mast cells, and therefore, this result could be interpreted as a nonspecific reaction of the antibody used in this study, likely to have been caused by its polyclonal origin. Currently there are not many monoclonal antibodies available and specific for canine species, and hence, this result should be reassessed with more specific antibodies, preferably targeting specific canine antigens.
Scattered TRP2+cells were observed in the dermis, yet the expression of this marker did not appear to co-localise with TRP1. Also in this case a nonspecific cross-reaction can be hypothesised. Because of the shape of the TRP1+ dermal cells, the complete lack of pigmentation and lack of cross-reaction with the other antibodies, they could be interpreted as histiocytes/resident macrophages, scattered in the dermis and positive for IBA1 immunolabelling.
SOX-10 is a common marker used to highlight melanocytes in both neoplastic and non-neoplastic lesions in humans,28-30 and it is recommended for sentinel lymph node assessment in melanoma cases.31 In humans, no difference has been noted, quantitatively, between SOX-10 and immunostaining for Melan A,32 in agreement with the findings herein. To the best of the authors' knowledge, this is the first study assessing the normal expression of SOX-10 in canine epithelia and dermis, although some recent data are available regarding its expression in tumours.24, 33 The expression of SOX-10 in mammary gland, also used in the present study as a positive control, previously has been reported in both mouse and human,19, 34 where it plays important regulatory roles in promoting both stem- and EMT-like properties in mammary stem cells.34
MITF is another commonly used marker in human medicine and it has shown to be more effective in identifying melanocytes in tumour lesions, rather than in chronic sun-damaged skin, when compared to Melan A.35 Moreover, it has been suggested as an adjunctive marker that could help in the diagnosis of metastatic tumours that are suspicious for melanoma, yet negative for other melanocytic markers.36 In dogs, the expression of this marker has been demonstrated in melanomas, but data on MITF expression in this species is scarse.23 It is worthy of note that in our study, MITF expression was observed in the nuclei of cells from the inner root sheath (IRS), as was reported in mice.37
The presence of melanocytes, confirmed by immunohistochemical evaluation, within the basal compartment of Meibomian glands, may be the reason for the presence of a frequent strong melanisation of tumours arising from these structures, when compared to tumours arising from sebaceous glands.38 Accordingly, the presence of SOX-10+ cells was observed only seldomly in sebaceous glands in occasional samples from the head of one of our cases. It could be postulated that the presence of melanin in Meibomian tumours could be due to a proliferation of a non-neoplastic population of melanocytes, as has been reported in human basal cell tumours, in which the proliferation of melanocytes has been confirmed in different studies.39
The presence, particularly in the dermis, of melanocytes expressing different sets of proteins, may indicate the presence of melanocyte subpopulations; these may be at the origin of different melanocytic tumours or melanocytic disorders. Moreover, results from our study suggest the presence of a strong variability among subjects, probably breed-related. For example, dermal melanocytes were confirmed only in a chow chow, which could justify the predisposition towards the development of particular degenerative and neoplastic diseases in some breeds. Nevertheless, the dermal melanocyte subpopulation hypothesis needs to be substantiated by further studies with larger study groups.
One of the limitations of this study is the restricted number of dogs and breeds tested. Although a supposed interbreed variability is supported by our results, nevertheless, it should be tested and supported on a larger scale, possibly by comparing black-coated dogs, which are known to be more predisposed to the development of melanocytic tumours,16, 17 with light-coated breeds. Another point that should be addressed is the implementation of antibodies, with the development of monoclonal antibodies specific for canine species. This probably would reduce the possibility of suspected nonspecific immunolabelling as observed with TRP1 and TRP2.
CONCLUSIONS
Melan A and SOX-10 appear to be the most sensitive and specific markers for melanocytes in canine samples. Moreover, the immunostaining appears sharp and more easily identifiable when compared to the other markers. These data could help in the characterisation of melanocytes in dogs and lay the foundations for a better understanding of the pathogenesis of melanocyte-related diseases.
AUTHOR CONTRIBUTIONS
Ilaria Porcellato: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; visualization; data curation. Margherita Orlandi: Methodology; validation; visualization; writing – review and editing; investigation; formal analysis; data curation. Adriana Lo Giudice: Investigation; methodology; data curation; validation; writing – review and editing. Monica Sforna: Validation; writing – review and editing; visualization. Luca Mechelli: Supervision; project administration; resources; writing – review and editing. Chiara Brachelente: Funding acquisition; conceptualization; investigation; methodology; validation; formal analysis; writing – review and editing.
ACKNOWLEDGEMENTS
The authors would like to thank ECVD for supporting this study with a Training Grant (2020). Open Access Funding provided by Universita degli Studi di Perugia within the CRUI-CARE Agreement.
FUNDING INFORMATION
This study was self-funded.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest have been declared.




