Effects of butorphanol versus dexmedetomidine sedation on intradermal allergen and histamine responses in dogs with atopic dermatitis
Abstract
enBackground
Sedation is commonly used during intradermal testing (IDT). Morphine and its derivatives have long been avoided because of their histamine-releasing effects.
Hypothesis/Objectives
Butorphanol, an opioid agonist/antagonist, will not adversely affect IDT in dogs.
Animals
Ten client-owned dogs diagnosed with atopic dermatitis.
Methods
Dogs were randomized to be sedated with butorphanol (0.4 mg/kg) or dexmedetomidine (5 μg/kg). Routine IDT along with intradermal injections of various dilutions of histamine were performed on the lateral thorax, followed 7 days later by the alternative sedative and IDT on the opposite side. The injection sites were subjectively scored and objectively measured by one investigator, blinded to the sedatives, and compared between groups.
Results
When the mean wheal diameters from the objective measurements of all antigens, including saline and histamine dilutions, were compared, butorphanol was associated with significantly smaller reactions than dexmedetomidine (P = 0.0001). There was a high level of agreement between sedatives when positive reactions subjectively scored as ≥3+ were compared (κ = 0.91). When mean wheal diameters of histamine at concentrations of 1:100,000, 1:400,000, 1:1,600,000 and 1:6,400,000 were compared, there were no significant differences between sedative types. Moreover, the percentage agreement when comparing subjective interpretation of all histamine dilutions between sedations was high (κ = 0.90). However, there was only 69% agreement beyond chance when objective and subjective interpretations of all antigens were compared between sedative groups.
Conclusions
Although butorphanol resulted in significantly smaller wheal size in comparison to dexmedetomidine, it did not affect the overall subjective interpretation of the results of IDT.
Résumé
frContexte
Une sédation est fréquemment utilisée pour la réalisation des tests intradermiques (IDT). La morphine et ses dérivés ont longtemps été évités à cause de leurs effets de relargage d'histamine.
Hypothèses/Objectifs
Le butorphanol, un agoniste/antagoniste opioïde, n'aurait pas d'effet sur les IDT chez le chien.
Sujets
Dix chiens de propriétaires atteints de dermatite atopique.
Méthodes
Les chiens ont été au hasard sédatés avec le butorphanol (0.4 mg/kg) ou de la dexmédétomidine (5 μg/kg). Des IDT de routine avec des injections intradermiques de dilutions variées d'histamine ont été réalisées sur le thorax latéral, suivies, 7 jours plus tard par la sédation alternative et des IDT sur le côté opposé. Les sites d'injection étaient notés subjectivement et mesurés objectivement par un investigateur, en aveugle pour les sédatifs et comparés entre les groupes.
Résultats
Quand les diamètres moyens des papules des mesures objectives de tous les antigènes, y compris les dilutions saline et l'histamine, étaient comparés, le butorphanol était associé avec des réactions significativement plus petites qu'avec la dexmédétomidine (P = 0.0001). Il y avait un niveau élevé de corrélation entre les sédatifs quand les réactions positives étaient notés subjectivement à ≥ 3 + étaient comparés (κ = 0.91). Quand les diamètres des papules d'histamine à des concentrations de 1:100,000, 1:400,000, 1:1,600,000 and 1:6,400,000 étaient comparées, il n'y avait aucune différence significative entre les types de sédation. En outre, le pourcentage de corrélation en comparant l'interprétation subjective de toutes les dilutions d'histamine entre les sédations était élevé (κ = 0.90). Cependant, il y avait seulement 69% de chance de corrélation quand les interprétations objectives et subjectives de tous les antigènes étaient comparées entre les groupes de sédatifs.
Conclusions
Bien que le butorphanol entraine des papules significativement plus petites en comparaison de la dexmédétomidine, cela n'affecte pas l'interprétation subjective globale des résultats d'IDT.
Resumen
esIntroducción
la sedación se utiliza con frecuencia durante las pruebas alérgicas intradermales (IDT). La morfina y sus derivados se han evitado desde hace mucho tiempo debido a sus efectos liberadores de histamina.
Hipótesis/Objetivos
el butorfanol, un opioide agonista/antagonista, no afectaría de forma negativa la IDT en perros.
Animales
diez perros de propietarios privados diagnosticados con dermatitis atópica.
Métodos
los perros fueron distribuidos al azar para ser sedados con butorfanol (0,4 mg/kg) o dexmedetomidina (5 μg/kg). Se realizó la IDT de forma rutinaria así como inyecciones intradérmicas de varias diluciones de histamina en la parte lateral del tórax, seguido 7 días después por la administración del otro sedante y la IDT en el lado opuesto del tórax. Los puntos de inyección fueron evaluados de forma subjetiva y medidos de forma objetiva por un investigador que desconocía el tipo de sedante, y se compararon entre grupos.
Resultados
cuando se compararon los diámetros medios de los abones mediante medición objetiva de todos los antígenos, incluyendo el suero salino y las diluciones de histamina, el butorfanol se asoció significativamente con reacciones mas leves que la dexmedetomidina (P = 0,0001). Hubo un alto paralelismo entre los agentes sedantes cuando se compararon las evaluaciones subjetivas como ≥3 + (κ = 0,91). Cuando se compararon los diámetros medios de los abones provocados con histamina a concentraciones 1:100.000, 1:400.000, 1:1.600.000 y 1:6.400.000 no hubo diferencias significativas entre los dos sedantes. Y lo que es más, el porcentaje de acuerdo cuando se compararon las interpretaciones subjetivas de todas las diluciones de histamina entre ambos sedantes fue alta (κ = 0,90). Sin embargo hubo solo un 69% de acuerdo mas que simple azar cuando las interpretaciones objetivas y subjetivas de todos los antigenos se compararon entre los grupos con los dos sedantes.
Conclusiones e importancia clínica
aunque la administración de butorfanol resultó en la formación de abones más pequeños en comparación con la dexmedetomidina, esto no afectó la interpretación subjetiva global de los resultados de la IDT.
Zusammenfassung
deHintergrund
Es ist üblich, bei der Durchführung eines Intradermaltests (IDT) eine Sedierung zu verwenden. Morphine und ihre Derivate wurden dabei aufgrund ihrer Histamin-freisetzenden Wirkung lange vermieden.
Hypothese/Ziele
Butorphanol, ein Opioid Agonist/Antagonist, beeinflusst den IDT bei Hunden nicht.
Tiere
Zehn Hunde in Privatbesitz, die mit atopischer Dermatitis diagnostiziert worden waren.
Methoden
Die Hunde wurden zufällig eingeteilt und entweder mit Butorphanol (0,4 mg/kg) oder Dexmedetomidin (5 μg/kg) sediert. Es wurden routinemäßige IDTs zugleich mit intradermalen Injektionen verschiedener Verdünnungen von Histamin am lateralen Thorax durchgeführt. Dieses Prozedere wurde 7 Tage später mit dem jeweils anderen Sedativum und dem IDT auf der anderen Thoraxseite durchgeführt. Die Injektionsstellen wurden subjektiv beurteilt und objektiv von einem Untersucher gemessen, der gegenüber den Sedativa geblindet war, und es wurde ein Vergleich zwischen den Gruppen durchgeführt.
Ergebnisse
Beim Vergleich der durchschnittlichen Größe der objektiven Messungen der Quaddeln aller Antigene, inklusive der Kochsalzlösung und der Histaminverdünnungen, wurden bei Butorphanol signifikant geringere Reaktionen als bei Dexmedetomidin (P = 0,0001) beobachtet. Es bestand eine große Übereinstimmung zwischen den Sedativa beim Vergleich von positiven Reaktionen, die subjektiv mit ≥3 + verglichen wurden (κ=0,91). Die durchschnittlichen Quaddelgrößen der Histaminkonzentrationen 1:100.000, 1:400.000, 1:1,600.000 und 1:6,400.000 zeigten keine signifikanten Unterschiede zwischen den verwendeten Sedativa. Darüber hinaus war die prozentuelle Übereinstimmung beim Vergleich der subjektiven Interpretation aller Histaminverdünnungen zwischen den Sedativa hoch (κ=0,90). Trotzdem bestand nur eine 69%ige Übereinstimmung, wenn objektive und subjektive Interpretationen aller Antigene zwischen den verschiedenen Sedativa Gruppen verglichen wurden.
Schlussfolgerungen
Obwohl bei Butorphanol signifikant kleinere Quaddeln im Vergleich zu Dexmedetomidin entstanden, hat das die allgemeine subjektive Interpretation der Ergebnisse der IDTs nicht beeinflusst.
Abstract
chAbstract
jaIntroduction
Canine atopic dermatitis (AD) is a common pruritic skin disorder. Intradermal testing (IDT) is considered an important diagnostic test in selecting allergens for the formulation of allergen-specific immunotherapy.1 Sedation is routinely administered during IDT to restrain dogs adequately and minimize stress. Previous studies have evaluated numerous commonly used sedatives and sedative combinations for use in IDT, including ketamine–zolazepam, ketamine–diazepam, tiletamine–zolazepam, xylazine–atropine, medetomidine, propofol, oxymorphone and isoflurane.2-8
Medetomidine was shown not to inhibit responses to intradermally injected histamine in normal dogs;7 therefore, it can be extrapolated that the same holds true for dexmedetomidine, the dextrorotatory enantiomer of medetomidine,9 which is routinely used in clinical settings for IDT and commercially available and labelled for use in dogs in the USA. Dexmedetomidine is reportedly as safe as medetomidine; it may offer improved sedation and analgesic effects.10
Opioids have not been recommended for IDT because they may cause mast cell degranulation and histamine release, leading to increased vascular permeability and increased smooth muscle contraction, with the potential for adverse effects on the results of IDT.3-5 To date, there is only one reported clinical study evaluating the effects of opioid sedation on IDT results, in which oxymorphone (a pure opioid receptor agonist) resulted in a significant decrease in subjective scores and wheal diameter when compared with xylazine in flea-allergic dogs.4
A previous study in anaesthetized dogs evaluated the effects of morphine and butorphanol on pulmonary resistance, arterial blood pressure and venous plasma histamine levels following rapid intravenous (i.v.) injections. The i.v. injection of morphine at 3 mg/kg caused increased plasma histamine levels, airway constriction and arterial hypotension; butorphanol administered at 0.75 mg/kg did not produce airway constriction or elevations in plasma histamine concentrations. In addition, although the mean arterial pressure was decreased with butorphanol, it was markedly less than that for morphine.11
Butorphanol is a κ opioid receptor agonist and μ opioid receptor antagonist9 and is frequently used as a sedative in companion animals. Although generally avoided for IDT, its use is sometimes needed to enhance the level of sedation. To the authors' knowledge, there are no reported studies demonstrating the influence of butorphanol on IDT. The purpose of the present study was to evaluate the effects of butorphanol versus dexmedetomidine sedation on IDT in dogs with AD.
Materials and methods
Animals
The sample size was estimated based on a mean of 6 mm wheal for the control group with a standard deviation of 2.5 mm, based on a similar previous study.12 In order to identify a clinically important difference of 3 mm or greater between the experimental (butorphanol) and control (dexmedetomidine) groups with an 80% probability at the 0.05 level of significance, a total of 10 dogs were necessary for this study.
Between January and May 2012, 10 dogs with a calm disposition, diagnosed with AD and candidates for IDT, completed this prospective study. Client consent was obtained for all participants. Diagnosis of AD was based on the dog's history and clinical signs and the exclusion of other pruritic skin disorders, including cutaneous adverse food reactions, parasitic diseases (Sarcoptes scabiei and Demodex spp,) and flea-allergy dermatitis. Dogs with secondary bacterial pyoderma or Malassezia dermatitis were treated with appropriate antimicrobial and antifungal therapy, when needed, for at least 4 weeks before testing; however, dogs were permitted to participate as long as the lesions did not interfere with the performance or interpretation of IDT.
Appropriate drug withdrawal periods were enforced for medications commonly used in the symptomatic therapy of AD known to inhibit type I hypersensitivity reactions.13 Injectable corticosteroids were withdrawn within the previous 8–12 weeks, oral corticosteroids within the previous 4 weeks and topical corticosteroids within the previous 3 weeks. In addition, dogs were not permitted to have received antihistamines or products or diets that contained ω3/ω6 fatty acids within the previous 2 weeks. The study was approved by the University of Tennessee Institutional Animal Care and Use Committee.
Experimental design
A prospective, randomized, single-blind, crossover study design was used. Following a predetermined computer-generated randomization scheme, dogs were assigned to receive butorphanol or dexmedetomidine as sedation for IDT. A minimum of at least 7 days separated each IDT to eliminate any possible residual drug effects and allow for owner convenience. The primary investigators interpreting the IDT results were blinded to the treatments. Each dog received butorphanol (Torbugesic®; Fort Dodge Animal Health, New York, NY, USA) at the dosage of 0.4 mg/kg i.v. or dexmedetomidine (Dexdomitor®; Pfizer Animal Health, New York, NY, USA) at the dosage of 5 μg/kg i.v. Each dog was given the randomly assigned sedative by the dermatology technician, shaved over the lateral thorax and covered with a surgical drape before the evaluators entered the room and during the IDT to ensure that the primary investigators were unaware of the sedative used. When presented for the second test, the opposite lateral thorax was used. Upon completion of the IDT and interpretation of the results for each procedure, the primary investigators were excused from the area to remain blinded to the sedative used. Dogs that received dexmedetomidine were administered the reversal agent atipamezole (Antisedan®; Pfizer Animal Health) at the dosage of 0.05 mg/kg intramuscularly at the completion of IDT interpretation.
Intradermal testing
For each procedure, the hair coat on the lateral thoracic wall was clipped using a number 40 clipper blade, and a felt tip pen was used to mark each injection site at ~1.5–2 cm intervals. A volume of 0.05 mL of 56 geographically appropriate allergens, as well as sterile saline and histamine as negative and positive controls, respectively, in a predetermined order, was injected intradermally by the primary investigator using 1 mL syringes with 26 gauge needles. The antigens comprised 34 pollens, eight moulds, two house dust mite species, a storage mite and various miscellaneous environmental allergens. The testing concentrations of most pollens, environmental allergens and moulds were 2000 PNU/mL, while the mites varied from 1:5000 to 1:10,000 w/v. Additionally, four quadrupling dilutions of histamine (from 1:100,000 to 1:6,400,000) were administered at the end of the IDT. Approximately 15 min after completion of the IDT, the primary investigator scored each injection site, first subjectively and then objectively, for wheal formation; this was followed by subjective scoring by an experienced investigator to prevent any major discrepancies in scoring. The subjective measurements of the primary investigator were used for analysis unless there was a discrepancy of more than 2+. Subjective scores were classified as either negative (score 0, 1 or 2) or positive (score 3 or 4).14 Sterile 0.9% saline was used as the negative control and graded as 0, while histamine phosphate (1:100,000 w/v) was used as the positive control and graded as 4+. Each objective score was determined by taking an average of measurements of length and width for each wheal diameter in millimetres using a transparent ruler. An objective measurement was considered positive if it was greater than or equal to the mean diameter of histamine and saline reactions.
Statistical analysis
All analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC, USA). Sample size calculation was performed using the procedure [PROC POWER]. A mixed-model ANOVA [PROC MIXED] was used to test the effect of sedative (butorphanol and dexmedetomidine) on the overall mean wheal size for all allergens. Sedation, dog week and antigen were included as class variables and dog as a random variable in the model. Independent variables included sedative, antigen, week and the interaction between sedation and week. A second mixed model was used to compare the mean wheal size among each of the four dilutions of histamine. Sedation, antigen and week were included as class variables in this model, and sedative, antigen, week and the interaction between sedation and antigen and between sedation and week were included as independent variables. A multiple range test according to the method of Tukey was used to adjust for multiple levels of the independent variables in each model. The fit of both models to the data was evaluated by comparing the model residuals with a normal distribution. The assessment of normality was evaluated with the test statistic of Shipiro–Wilk. Data are reported as least squares means ± 1 SEM.
The agreement beyond chance between sedatives for subjective assessment of positive and negative wheal responses for all allergens except saline and histamine and for objective (positive or negative) versus subjective responses was evaluated with the κ statistic [PROC FREQ]. Additionally, the same procedure was used to compare the objective and subjective responses among sedatives when evaluating the four dilutions of histamine. A P value of ≤0.05 was used to determine statistical significance in all tests.
Results
Fifteen client-owned dogs diagnosed with AD were enrolled into the study, although only 10 dogs completed the study. The remaining five dogs were removed due to inadequate sedation provided by the butorphanol to allow for complete intradermal testing. The 10 dogs consisted of four spayed females and six castrated males aged 2–12 years (median, 3.5 years). The following dog breeds composed the study population: shepherd cross-bred (n = 2), Labrador cross-bred (n = 2), Staffordshire terrier (n = 2), and one each of Staffordshire cross-bred, terrier cross-bred, boxer cross-bred and Chinese crested. Body weights ranged from 7.0 to 32.6 kg (median, 20.65 kg). No adverse effects were noted at any time before, during or after the study.
Subjective scores
Allergens
There was a high level of agreement between sedative groups (κ = 0.91) when the proportions of positive and negative subjective allergen scores were evaluated (Table 1). Of the 10 dogs and 560 paired allergens evaluated, 16 subjective interpretations were not in agreement. Fifteen observations from three dogs were found to be positive (i.e. ≥3+) in the dexmedetomidine sedation group while negative (i.e. ≤2+) in the butorphanol group. Only one observation was reported positive in the butorphanol group while negative in the dexmedetomidine group. One dog had 11 of 16 discrepancies, one had three of 16, and two other dogs had one each. Overall, six of 16 subjective interpretation discrepancies were initially positive, then graded as zero.
- a Positive, ≥3+; and negative, 0, 1+ or 2+.
Histamine concentrations
There was a high level of subjective score agreement between both sedative groups (κ = 0.90). Of the 40 subjective measurements between both sedative groups, there were only two that disagreed. This occurred in the 1:1,600,000 histamine dilution, where the score was positive with dexmedetomidine sedation and negative with butorphanol sedation.
Objective scores
Allergens
When the mean wheal diameters from the objective score measurements of all the allergens were compared between sedative groups, there was a statistically significant difference between butorphanol (9.21 ± 0.69) and dexmedetomidine (9.75 ± 0.69; P < 0.0001), with butorphanol having a slightly smaller wheal diameter.
Histamine concentrations
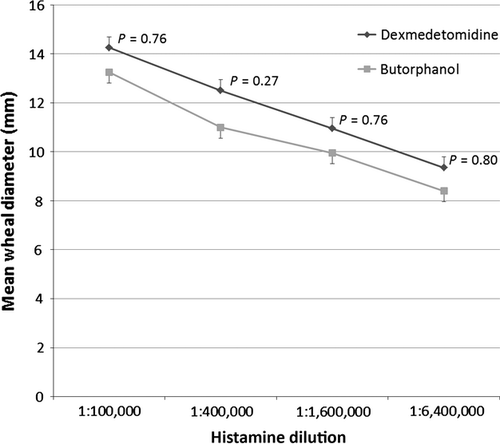
The mean wheal diameters of all histamine concentrations were not statistically significantly different between groups (Figure 1), although sedation with butorphanol resulted in slightly smaller wheal diameters.
Comparison of subjective versus objective interpretation of allergen results
When the subjective interpretation of allergen results was compared with the objective interpretation using the κ coefficient, there was a fair to good percentage of agreement (κ = 0.65) beyond chance between sedation groups (Table 2). Butorphanol and dexmedetomidine exhibited 64 and 72% agreement between subjective and objective interpretations, respectively.
- a Positive, ≥3+; and negative, 0, 1+ or 2+.
Comparison between weeks of testing
There were no significant differences in median wheal diameters for allergens, saline and all histamine dilutions between the weeks of testing (P = 0.79).
Discussion
Intradermal testing is frequently performed with the goal of identifying and selecting specific allergens for immunotherapy in dogs with AD. Although considered minimally painful, sedation is routinely performed to minimize stress and to provide adequate restraint. While morphine and other opioids have long been avoided due to their histamine-releasing effects, results of this study showed that sedation with butorphanol at 0.4 mg/kg significantly decreased objective wheal sizes in comparison to sedation with dexmedetomidine but did not affect overall subjective interpretation of IDT reactions.
Dogs with atopic dermatitis were selected for this study because it was thought that allergen reactions would be more applicable in a clinical setting. However, for uniformity and to maximize useful information from each dog, we included four quadrupling dilutions of histamine to enhance the value of this study.
The present study showed a high level of agreement between sedative groups when positive reactions subjectively scored as ≥3+ were compared. Subjective scoring was defined as 3+ or greater because, in the authors' opinions, scores of 1+ and 2+ are generally not reproducible. Additionally, to improve consistency between the first and second readers of the IDT as well as to account for the time elapsed during the subjective and objective measurements, where a 2+ reaction was more likely to fade, a 3+ or greater reaction was defined as a positive response.
When diminishing concentrations of histamine at 1:100,000, 1:400,000, 1:1,600,000 and 1:6,400,000 were evaluated, there were no significant differences in mean wheal diameters or subjective scores between the sedative groups. This observation, along with a high level of agreement between subjective interpretations of the two sedative groups showed that overall subjective interpretation during IDT was not affected by sedation with butorphanol.
The overall mean wheal diameters from the objective score measurements of allergens did show significantly smaller reactions in dogs sedated with butorphanol when compared with dogs sedated with dexmedetomidine. The mean decrease in diameter, however, was only 0.54, and unlikely to be clinically relevant.15
Factors resulting in variability in intradermal allergen testing results were considered in the design of this study. Blinding and randomization of sedation aimed to eliminate bias from knowledge of the sedative used; however, the standardized order of allergen injections remained a source of possible bias. A minimum of 1 week between each IDT was selected to minimize any potential drug effect and to prevent the induction of a type I hypersensitivity. A previous investigation evaluating the cumulative effects of IDT found that after three consecutive weeks of repeated IDT, nonatopic dogs began to produce positive reactions.16 In the present study, comparison between objective median wheal measurements of all reactions from week 1 and week 2 found no statistical differences, making induction of hypersensitivity unlikely. Although every effort was made to deliver a total volume of 0.05 mL of each allergen and each serial dilution of histamine intradermally, it is possible that some antigens were injected subcutaneously, which would result in a decreased wheal-and-flare response and smaller objective measurements. The site of injection and antigen reactivity can also be affected because skin thickness generally decreases dorsally to ventrally on the trunk.17 When IDT was performed on the opposite side of the dog, antigen injections could have been performed in reverse order to account for the variation. Additionally, a small degree of variability between testing of each dog is also acknowledged.8 Any of these factors may have influenced the statistically significant smaller reactions found in the butorphanol-treated dogs when compared with dexmedetomidine. However, a treatment effect of butorphanol was considered most likely to be responsible for the reduced reactions seen with this sedative, although the biological basis for this response is unknown.
Hubbard and White18 recently highlighted the lack of standardization and variability within the subjective scoring method for IDT and suggested that the objective scoring system may be helpful in providing a point of reference for inexperienced individuals when learning to grade IDT. The present study showed an overall 69% agreement beyond chance between objective and subjective scores, which is considered fair to good. These results question the validity of solely using the objective scoring method.
Butorphanol is commonly used in small animal practice as an analgesic and pre-anaesthetic agent. Previous studies evaluating overall sedation suggested a possible synergistic effect when using butorphanol in combination with an α2 agonist,19 and the combination produced greater sedation and analgesia at lower doses than dexmedetomidine or butorphanol alone.20 Moreover, another study evaluating sedative protocols for canine hip radiography found dexmedetomidine in combination with butorphanol to be safe and to provide clinically sufficient sedation and muscle relaxation.21 This added level of sedation could further decrease patient discomfort and stress encountered during skin testing. Owing to its partial antagonistic affinity at the μ opioid receptor,22 butorphanol displaces the agonist from the opiate receptor and prevents receptor activation,23 which could possibly decrease or block mast cell degranulation and subsequent histamine release. This makes the results of the present study promising, in that butorphanol did not show a clinically significant difference in subjective response when compared with dexmedetomidine. As speculated with ciclosporin, which may block less than 100% (~50–80%) of histamine release,12 this study indicates that butorphanol sedation will not inhibit strong wheal-and-flare responses.
In conclusion, although butorphanol resulted in significantly smaller wheal size in comparison to dexmedetomidine, it did not affect subjective interpretation of the IDT, as noted by the high level of agreement between subjective scores. Further studies evaluating lower doses of butorphanol in combination with dexmedetomidine are warranted because of its potential beneficial effects on sedation of dogs for intradermal testing. However, when used alone butorphanol resulted in poor sedation. Five study subjects were excluded due to inadequate sedation in this study, and therefore butorphanol alone cannot be recommended for sedation for IDT. Careful interpretation should be considered, though, because weak positive reactions (i.e. 1+, 2+), may be suppressed or become negative with use of butorphanol sedation.
Acknowledgements
The authors would like to thank Jacqueline Bryan Watson for assistance with patient recruitment, Laura McDougal for assistance with data entry and Jacqueline Davis for her technical contribution to the study.






