Effects of a 0.3% cholesterol diet and a 20% fat diet on plasma lipids and lipoproteins in Quaker parrots (Myiopsitta monachus)
Abstract
Background
Lipid disorders are common in captive psittacine birds, but associated changes in blood lipids and lipoproteins have not been well characterized. The Quaker parrot is prone to dyslipidemia and has been extensively used as an experimental model.
Objectives
We aimed to study the effects of a 0.3% cholesterol diet and a 20% fat diet on plasma lipids and lipoproteins in Quaker parrots.
Methods
Two crossover studies were performed with each diet. During each study, 12 parrots were divided into two groups fed the treatment or control diet for 2 weeks. After a 2-month wash-out period, the groups were reversed. At the end of each period, plasma lipidomics and lipoprotein profiling were performed. Data were analyzed by univariate tests adjusted for false discovery rates, volcano plots, and enrichment analyses.
Results
The cholesterol diet induced changes in many plasma lipids and lipoproteins. Total cholesterol and cholesteryl esters were significantly and markedly elevated. Ceramides were the second subclass of lipids that were elevated. Several glycerophosphocholines, sphingomyelins, and one diacylglycerol were also significantly elevated, albeit to a lesser magnitude. All lipoproteins were elevated, with the greatest increase seen in non-HDL. The fat diet mainly resulted in a decrease in plasma glycerolipids and an increase in acylcarnitines. Lipoprotein plasma levels remained unchanged.
Conclusions
Quaker parrots fed a 0.3% cholesterol diet showed profound and complex dyslipidemic changes that could be used to further study lipid disorders and their management in psittacine birds. A 20% fat diet higher in n-6 polyunsaturated fatty acids did not lead to dyslipidemia.
1 INTRODUCTION
Psittaciformes are prone to dyslipidemia and various lipid disorders, such as atherosclerosis, hepatic lipidosis, obesity, and fatty tumors.1-4 However, the types of plasma lipid and lipoprotein changes associated with these disorders have been poorly reported, even when just considering simple tests such as plasma total cholesterol and triglycerides. This likely stems from the difficulties in diagnosing and connecting these conditions concurrently to plasma dyslipidemic changes in large cohorts of birds with similar preanalytic factors, such as postprandial states, diets, and the occurrence of vitellogenesis in females, all of which can profoundly influence plasma lipids in birds. In addition, few clinical lipidologic tests are commercially available in psittacine birds and are typically restricted to simple tests such as total cholesterol, triglycerides, and unvalidated lipoprotein assays run on traditional laboratory analyzers.
To circumvent these difficulties and increase knowledge on the characterization, epidemiology, diagnosis, and treatment of psittacine dyslipidemia and associated disorders, experimental models are essential tools. These models allow control of analytical and biological variability, permit validation of some diagnostic tests on the experimental species models, and facilitate induction of different levels of dyslipidemia or related lipid disorders, all of which are needed to test the accuracy of various diagnostic tests. Various psittacine species have been used to induce dyslipidemia, atherosclerosis, or hepatic lipidosis, and include budgerigars (Melopsittacus undulatus),5 Quaker parrots (Myiopsitta monachus),6-8 and macaws (Ara spp.).9 These conditions were induced with diets containing 1%-2% cholesterol, fructose, or animal fat dietary items. Spontaneous models of dyslipidemia, such as in Hispaniolan Amazon parrots (Amazona ventralis), have also been used.10 Of these psittacine models, the Quaker parrot has been the most widely used as the species is prone to spontaneous dyslipidemia,2 hepatic lipidosis, and other lipid disorders,1 and readily develops hypercholesterolemia, lipoprotein abnormalities, and atherosclerosis with increased cholesterol feeding.6 In addition, novel clinical lipidology methods such as advanced lipoprotein profiling11 and clinical lipidomics12 have been introduced in this species by the authors. These laboratory techniques will seek to both increase the tools available to understand lipid disorders in parrots and promote the search for novel plasma biomarkers and therapeutic targets.
Plasma lipidomics and advanced lipoprotein profiling have not been used in psittacine birds with lipid disorders, as they are not yet straightforward to implement clinically. Therefore, using these techniques on experimentally induced lipid disorders is a first step in comprehensively characterizing blood lipid changes in psittacine birds and their interpretation in association with various conditions. Most diet-induced dyslipidemias in parrots were produced with 1%-2% cholesterol feeding (regular parrot diet is poor in cholesterol, close to 0%), which led to excessive and nonphysiologic hypercholesterolemia. While this high dietary cholesterol was highly effective in inducing atherosclerosis quickly, the results on blood changes were less comparable to spontaneous dyslipidemia and transferable to the study of clinical diagnostic tests. The high-fat diets previously used were of animal sources such as lard or beef tallow, which are not typically found in parrot diets and have different fatty acid profiles than plant-based sources of fat such as vegetable oils and seeds.
The purpose of this study was to quantify and characterize the dyslipidemia caused by two diets, a high cholesterol diet at 0.3% and a diet doubling the standard fat content of parrot pellets. Another objective was to characterize a parrot model of experimental dyslipidemia for future research on psittacine lipid disorders. A 0.3% dietary cholesterol content was selected to induce dyslipidemia more comparable with spontaneous dyslipidemia and reduce the likelihood of atherosclerosis induction. A 20% dietary fat content was selected to assess the effect of a moderate increase in dietary fat and for practical purposes for the proper extrusion of the pellets made with oil supplementation of the control diet.
We hypothesize that the cholesterol diet would induce an increase in total cholesterol, cholesteryl esters (CEs), non-HDL lipoproteins, and have other effects on the lipidome and that the fat diet would induce changes in plasma glycerolipids, fatty acyls, and also other lipid classes.
2 MATERIALS AND METHODS
2.1 Animals
Twelve 3-year-old Quaker parrots (Myiopsitta monachus) were used for this study. The parrots were captive bred and hand raised at the Hagen Avicultural Research Institute (QC, Canada) and were maintained as a research colony since 1 year of age. The parrots included six males and six females; sex was confirmed by DNA testing on blood samples. The parrots were housed together at the University of Guelph – Central Animal Facility in a large stainless-steel aviary with food and water provided ad libitum and fed a pelletized diet (Tropican 2 mm pellets, Hagen Inc., Baie d'Urfée, QC, Canada). They were considered healthy and free of spontaneous dyslipidemia based on a recent physical examination, and plasma biochemistry and lipoprotein panels. In addition, these birds were tested for avian chlamydiosis and avian bornavirus by PCR. Animal utilization protocols (AUPs) were approved for this research by the University of Guelph - Animal Care Committee (AUP#3875 and AUP#4035).
2.2 Experimental diets
For this study, two experimental diets were custom made by Hagen Inc., Baie d'Urfée, QC, Canada and based on a Quaker parrot maintenance diet (Tropican 2 mm pellets). For the “cholesterol” diet, a cholesterol powder (>92.5%, from sheep wool, Sigma-Aldrich, Oakville, ON, Canada) was blended with the raw flour materials prior to extrusion at a level of 0.3%. For the “fat diet,” sunflower oil was added during the pellet extrusion and coating processes to approximately double the fat content of the regular pellet from 10% to 20% fat.
The control diet (maintenance diet) and two experimental diets were analyzed to verify their contents. For nutritional diet analysis, a 100 g sample of each of the three pelleted parrot diets was submitted to an independent laboratory (SGS Canada Inc. Agriculture and Food, Mississauga, ON, Canada). Total fat was analyzed by an acid hydrolysis method (SGS-Canada, test QAM-105), total cholesterol composition was obtained using a gas chromatographic method (SGS-Canada, test QAM-118), and moisture by forced air oven (SGS-Canada, test QAM-103). The cholesterol diet was confirmed to contain 0.316% cholesterol on a dry matter basis, and the fat diet was confirmed to contain 20.3% fat on a dry matter basis. The control diet contained 0.002% cholesterol and 11.4% fat on a dry matter basis. The fatty acid profile of the control diet was also previously reported.12 The diets were stored refrigerated in the dark until use.
2.3 Dietary trials
Two different crossover dietary trials were conducted on 12 birds that received both treatments in each trial. For the first trial, the Quaker parrots were randomized using computer software (R, version 4.0.4, R Foundation for Statistical Computing, Vienna, Austria) into two groups of six parrots (three males and three females). One group was fed the cholesterol diet while the other was fed the control diet for 2 weeks. After a wash-out period of 2 months, the groups were reversed and fed the other diet for another 2 weeks. The order was randomized per group. After another wash-out period of 7 months, the second trial using the fat diet was performed in an identical manner. Long wash-out periods were selected between diet changes to allow enough time for plasma lipids to return to baseline levels. The experimental diet feeding duration was considered sufficient to cause significant dyslipidemia while avoiding lipid-accumulation lesions in arteries and liver, based on prior information from the timing of blood cholesterol changes and atherosclerotic lesion development associated with cholesterol feeding in this species. No treats or fruits were allowed during the feeding trials. As birds were housed in groups of 6, individual food intake was not measured; however, weight was monitored once weekly.
2.4 Sample collection
The parrots were fasted overnight prior to sample collection. Blood was collected and stored according to guidelines for plasma lipidomics.13 To minimize stress and exertion, birds were captured in the dark, and blood was collected within 2-5 minutes following capture. A 1 mL blood sample was collected from the right jugular vein of each parrot under manual restraint using a 3 mL syringe connected to a 26 g needle. Then, 0.8 mL of blood was transferred to a heparinized tube without a serum separator (BD Microtainer, Becton and Dickinson, Mississauga, ON, Canada) and 0.2 mL to an EDTA tube (BD Microtainer). Tubes were inverted a minimum of five times and placed on ice. Blood was centrifuged for 10 minutes at 1500g, and approximately 0.5 mL of plasma was harvested and aliquoted in cryovials. The plasma was stored at −80°C until shipping on dry ice to the various analytical laboratories.
2.5 Lipidomics
A targeted metabolomics/lipidomics panel was performed using the Biocrates Life Sciences MxP Quant 500 kit (Biocrates Life Sciences, Innsbruck, Austria) at the Analytical Facility for Bioactive Molecules (The Hospital for Sick Children, Toronto, Canada). Heparinized plasma was analyzed for 630 metabolites using this kit, but only lipid analytes were included in this study. The lipidomics panel included 14 bile acids, 12 nonesterified fatty acids (FAs), carnitine and 39 acylcarnitines, 14 lysophosphatidylcholines (LPC), 76 phosphatidylcholines (PC), 15 sphingomyelins (SM), 28 ceramides (Cer), 8 dihydroceramides [Cer(d18:0)], 19 hexosylceramides (HexCer), 9 dihexosylceramides (Hex2Cer), 6 trihexosylceramides, 22 CEs, 44 diacylglycerols (DG), and 242 triacylglycerols (TG, triglycerides). The panel represented lipids from five lipid classes (sterol lipids, fatty acyls, glycerophospholipids, glycerolipids, and sphingolipids).
To minimize contamination, solvents used were of LC–MS grade and glassware was triple rinsed with Milli-Q water, isopropanol, and methanol 100% (Sigma-Aldrich, Oakville, ON, Canada). Samples, standards, and controls (10 μL) were added to the Biocrates 96-well filter plate with internal standard, dried under nitrogen gas, and then extracted with methanol 100%, as per kit instructions. Extracted samples were analyzed by LC/MS/MS using the Biocrates supplied HPLC column on an Agilent 1290 HPLC system (Agilent Technologies: Santa Clara, CA, USA) coupled to a Sciex Q-Trap 5500 mass spectrometer (Sciex, Framingham, MA, USA) both in positive and negative polarity. Data were acquired using Analyst (v.1.6.3) and quantified using MetIDQ (v.9.7.1-DB110-Oxygen 2893). Calculated concentrations were reported in μM.
Lipid species were reported using the LipidMaps short-hand nomenclature14 whenever practical.
2.6 Lipoprotein profile
Advanced lipoprotein profiling (Liposearch panel) was performed on the EDTA plasma at Skylight Biotech Inc., Akita, Japan. The technique used gel permeation high-performance liquid chromatography to separate lipoproteins by size prior to chemical analyses, as previously described in mammals and birds.11, 15, 16 The lipoprotein panel included total cholesterol concentration, total triglycerides concentration, four main lipoprotein classes [portomicrons, very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein HDL)] and 20 subclasses. Lipoprotein particle sizes were also obtained for the main classes as calculated from cholesterol plots using a proprietary algorithm.16 The lipoprotein profiling technique was previously reported in Quaker parrots.11
2.7 Statistical analysis
Each dietary trial was analyzed separately, using an identical statistical workflow.
For lipidomics datasets, data were first filtered with analytes with more than 20% of the values above or below the limits of quantitation or limits of detection or noninformative variables removed.
To explore broad changes in the plasma lipidome, the fold changes (concentrations means of the experimental diet/concentration means of the control diet) between groups were plotted by lipid subclasses. Differences in individual lipids between dietary groups were then assessed using a series of paired t tests with an alpha of 0.05 (P < 0.05) and a false discovery rate (FDR) of 0.05 (q < 0.05) for significance. The most important changes in the plasma lipidome were identified on volcano plots, which graph the fold changes against the statistical significance of the various lipids by lipid subclasses. A cut-off value of 1.5-fold change was used. This allowed the identification of variables with a high magnitude of changes that were also statistically significant.
To assist in functional interpretation, an unweighted lipid class enrichment analysis was performed as well as a pathway analysis using BioPAN except for CEs and acylcarnitines (not included in BioPAN).17 Lipid names were standardized and subclasses automatically imputed using RefMet18 and following the LipidMaps classification system.14 The enrichment ratio was calculated as the ratio of the number of significant lipid species by class over the number of detected lipid species by class divided by the ratio of the total number of significant lipids over the total number of detected lipids. An enrichment ratio over 1 means that a lipid class/subclass has more significant lipids than what would be expected if the frequency was equal among lipid classes/subclasses. Significant pathways were assessed using paired t tests in BioPAN. In addition, for important lipids identified on volcano plots that increased with treatment diets, disease enrichment analysis to explore the reported association between these lipid species and specific diseases was performed using the literature mining web-based software LipiDisease on August 14, 2021.19
Analytes of the lipoprotein panels were compared between the dietary trial treatments by a series of Wilcoxon signed-rank tests for paired data with P-values adjusted for an FDR of 0.05. To assess shifts in LDL and HDL subfraction distributions, percentages were used instead of absolute values.
R was used for statistical analysis using the R-package ggplot2 for statistical graphing.20
3 RESULTS
No side effects of the experimental diets were observed, and both experimental diets were well accepted by the birds with no clinically significant decrease in weight observed (more than 10% change). One bird died prior to the fat diet trial as it suffered a tongue injury caused by a conspecific that led to fatal hemorrhage. In addition, one bird was excluded from the control treatment in the cholesterol trial, and a different bird was excluded from the control group in the fat trial. Both birds presented with severe hypertriglyceridemia (>33 mmol/L) likely caused by vitellogenesis in a female in the first trial (two eggs were laid during that trial with high VLDL levels) and postprandial hyperlipidemia in a male in the second trial (evidenced by elevations in plasma portomicrons and VLDLs, the bird likely consumed fallen pellets on the cage floor prior to blood sampling).
3.1 Cholesterol trial
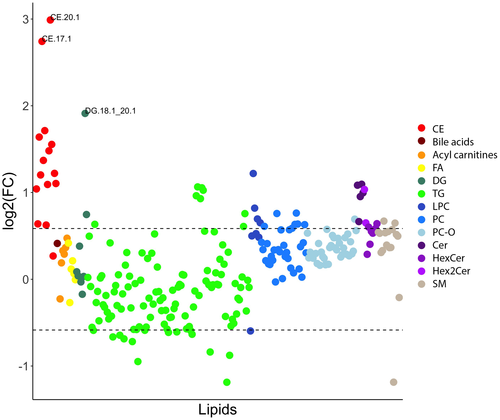
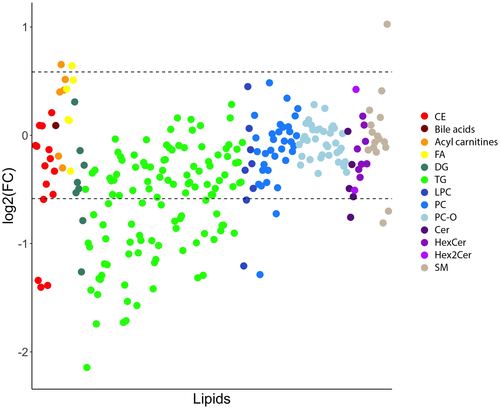
Birds fed the cholesterol diet had an elevation of many plasma lipid species belonging to all lipid classes, with a higher magnitude of elevation observed in CEs and ceramides (Figure 1). Many plasma triacylglycerols were also decreased in birds fed this diet.
On the univariate analysis, many lipid species were significantly increased compared with birds on the control diet. Eleven CEs (all q < 0.036), one DG (DG 18:1_20:1, q = 0.0186), three LPCs (in C17 or C18, all q < 0.041), 13 PCs (all q < 0.045), eight PC-Os (all q < 0.044), three Cers (all q < 0.044), three HexCers (all q < 0.044), and six SMs (all q < 0.028) were significantly increased.
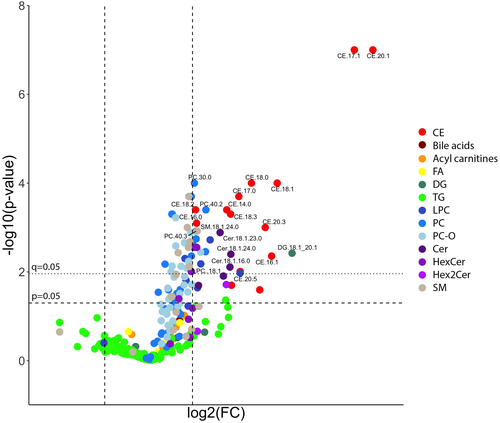
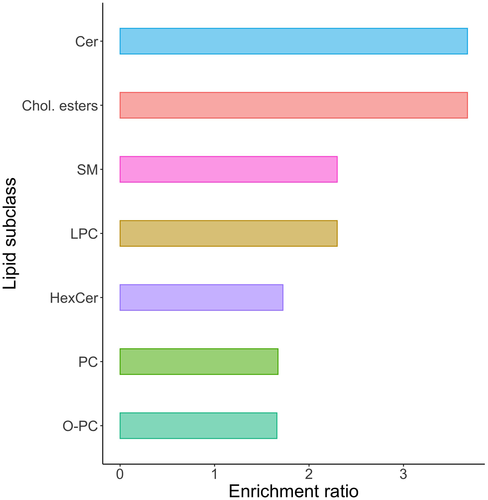
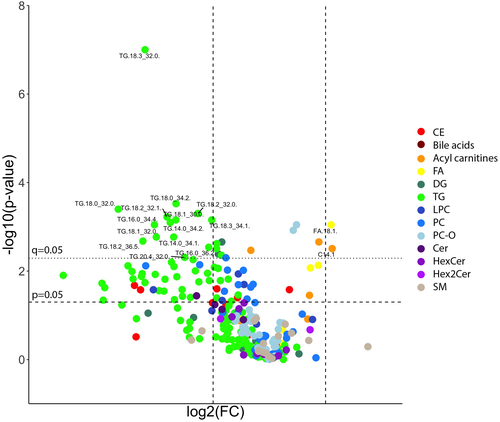
On the volcano plots, the most impactful features increasing with the cholesterol diet were CEs, which were the most significant lipids, and the lipids with the greatest magnitudes of increase (Figure 2). One of the DGs (DG 18:1_20:1) was also significant with markedly increased levels. Ceramides were the second lipid subclass with a significant increase in magnitude. A few phospholipid, glycerolipid, and sphingolipid species were also found to be important, but the overall change in these lipids was lower than the other subclasses. Enrichment analysis showed that several sphingolipid subclasses, CEs, and several phospholipid subclasses were overrepresented in the plasma lipidome changes when compared with other lipid categories (Figure 3). CE and Cer were more than three times, and SM and LPC were more than two times likely to be significantly elevated than other lipid types. Pathway analyses (excluding CEs and acylcarnitines) did not identify significantly overexpressed biosynthetic pathways; however, SM synthesis from Cer tended to be upregulated, and PC synthesis from DG tended to be downregulated. Of the lipids identified as significantly increased on the volcano plots, only cholesteryl oleate (CE 18:1, 3.3-fold change of 3.3, q = 0.005) was matched on LipiDisease with several citations, especially as it pertained to atherosclerosis, hyperlipidemias, coronary artery disease, and hypercholesterolemia. The set of lipids significantly increased by more than 1.5-fold was also significantly associated with atherosclerosis with the disease enrichment analysis; primarily CE 16:0, CE 18:1, and CE 18:3 having the highest number of hits on literature mining.
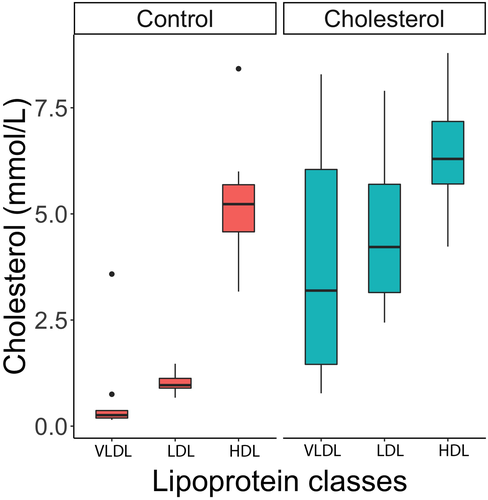
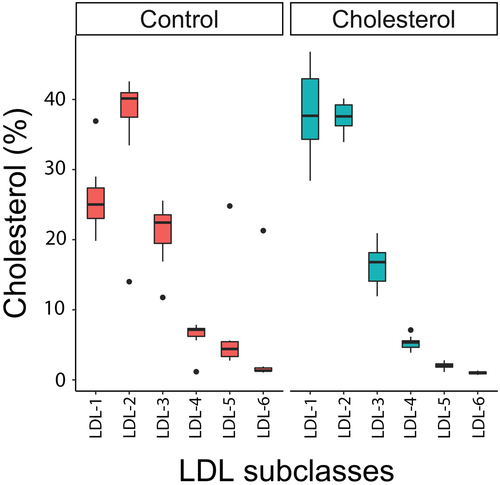
The total cholesterol concentration was significantly increased about 2.3 times in parrots fed the cholesterol diet (q = 0.005) while the total triglycerides was unchanged (q = 0.16) compared with those fed the control diet (median [interquartile range]: control diet = 6.9 (0.9) mmol/L, cholesterol diet = 15.9 (8.9) mmol/L). For the lipoprotein panels, all fractions (VLDL, LDL, HDL) (Figure 4) and particle numbers for the different lipoproteins were significantly increased (all q < 0.042). However, there was a profound shift in the LDL/HDL ratio (from a median of 0.18 to 0.64, 3.5-fold increase) as LDLs increased more than HDLs. Within the LDLs, mild changes in subtype distributions were evident, with a higher proportion of LDL-1 (q = 0.004) and lower proportions of LDL-5 (q = 0.004) and LDL-6 (q = 0.004) in parrots fed on the cholesterol diet compared with those fed the control diet (Figure 5). The HDL subfraction distribution did not change with diet (all q > 0.05).
3.2 Fat trial
For most birds fed the fat diet, a decrease in plasma TG species with minor changes in other lipid categories is shown in Figure 6.
On univariate analysis, many lipid species were significantly different from the birds on the control diet. Two acylcarnitines (C14:1, C14:8, all q < 0.039), one fatty acid (oleic acid: FA 18:1, q = 0.022), and two PC-Os (PC-O 36:3, PC-O 38:5, all q < 0.026) were increased in birds fed the fat diet. Conversely, three DGs (including 16:1, 18:1, or 18:2 fatty acyl chains, all q < 0.048) and 15 TGs (all q < 0.039) were decreased in birds fed the fat diet.
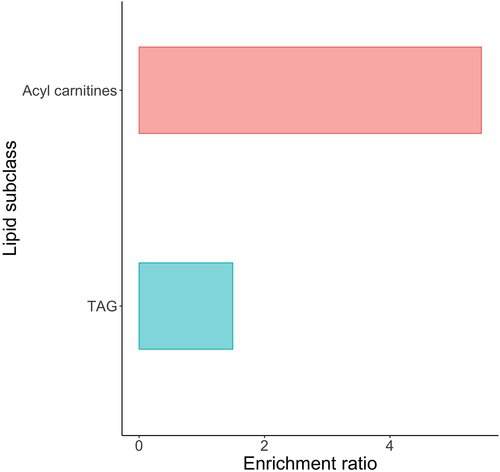
On volcano plots, the most impactful features that decreased with the fat diet were TGs, which were the most significant and greatest changing lipids (Figure 7). One FA (oleic acid: FA 18:1) and one acylcarnitine (C14:1) were important lipids following an opposite trend. Enrichment analysis showed that acylcarnitines and TGs were overrepresented in the plasma lipidome changes compared with other lipid categories (Figure 8). Pathway analysis (excluding CEs and acylcarnitines) identified two significantly overexpressed biosynthetic pathways, upregulated SM synthesis from Cer (P = 0.049), and upregulated PC synthesis from DG (P = 0.046). Not enough elevated lipids with the fat diet were significant to perform a disease enrichment analysis.
No significant changes in total cholesterol, total triglycerides, lipoprotein concentrations, and particle numbers were seen between diets (all q > 0.05). Within LDLs, there were mild changes in subtype distribution with a higher proportion of LDL-1 (q = 0.004) and lower proportions of LDL-5 (q = 0.004) and LDL-6 (q = 0.004) when on the fat diet. HDL subfraction distributions did not change with diet (all q > 0.05).
4 DISCUSSION
These two diet trials provide comprehensive information on changes in plasma lipids and lipoproteins associated with cholesterol feeding, as well as baseline fat level doublings of a standard pelleted diet for psittacine birds.
The cholesterol diet trial used a lower dietary cholesterol content at 0.3% than a similar study previously reported in the Quaker parrot that used a 1% cholesterol diet.6 In the previous report, the main objective was to induce atherosclerotic lesions. Higher dietary cholesterol concentrations led to a mean plasma cholesterol increase of 40-50 mmol/L, which are well beyond what is typically seen in spontaneous hypercholesterolemia (between 10 and 30 mmol/L).2, 21 However, cholesterol was fed for a longer period of 2-8 months in that study, and the plasma cholesterol plateaued at about 3-4 months. In the present study, the 0.3% cholesterol diet was fed for only 2 weeks and reached a level of 15.9 mmol/L, which is closer to spontaneous hypercholesterolemia in parrots. When calculating the plasma cholesterol of Quakers fed the 1% cholesterol diet in the previous study at the 2-week time point, the total cholesterol was actually similar to our present study of about 14.6 mmol/L. Therefore, it is possible that plasma cholesterol could have continued to increase with a longer feeding of 0.3% cholesterol and in the absence of longer feeding durations; it is unknown whether a plateau was reached at a lower level or if it would have continued to increase. As the Quaker parrots used in this study were part of a long-term research colony, we did not want to induce irreversible arterial lesions with longer feeding durations or higher dietary cholesterol concentrations. As total cholesterol is made of about 2/3 of CEs, it is not surprising that many plasma CEs were elevated on the cholesterol diet. Most CE species were elevated except for CEs in C22.
As expected, all lipoproteins were elevated, especially non-HDL lipoproteins. Lipoproteins are the main carriers of plasma lipids, and LDLs are particularly rich in free cholesterol and CEs (~40%-50%).22 Therefore, even if HDL is the main lipoprotein in the plasma of Quaker parrots,11 LDL was expected to increase markedly with cholesterol feeding. While this has been previously reported in this species,6 a gold standard technique for lipoprotein measurements was not used to document this lipoprotein shift. In addition, LDL subtype measurements showed that LDL particles tended to shift to larger, less dense LDLs. These lipoprotein changes (marked increases in VLDL and LDL, shifts in LDL/HDL ratios, and larger LDLs) were also documented in spontaneous dyslipidemia in Quaker parrots,23 showing the potential value of this diet-induced model to study dyslipidemia in psittacine birds.
The other lipid class impacted by cholesterol feeding was the ceramides. The ceramides had a similar enrichment ratio as the CEs, and several ceramide species were identified on the volcano plot. Most ceramides are carried by VLDLs and LDLs,22 which were markedly elevated in this study. The three main significant ceramides contained saturated fatty acid chains, namely Cer(d18:1/16:0), Cer(d18:1/23:0), and Cer(d18:1/24:0). Of these three ceramides, only Cer(d18:1/16:0) with palmitic acid is a minor ceramide species in Quaker parrots.12 It is noteworthy that certain ceramides, such as Cer(d18:1/16:0) and ceramide ratios, including Cer(d18:1/16:0) and Cer(d18:1/24:0), are frequently associated with cardiovascular diseases.24, 25 Cholesterol feeding impacted other lipid classes than ceramides, which showed broader effects on overall lipid metabolism. These included glycerophosphocholines and sphingolipids. Whether these changes are seen in parrots with spontaneous dyslipidemia remains to be seen, but it is likely that the plasma lipidome is altered beyond just sterol lipids.
The fat diet trial used a 20% fat diet compared with the 10% fat of the control diet. While the fat content was doubled, this is not considered a high-fat diet, as experimental high-fat diets in rodents typically have around 60% fat instead of the normal rodent diet of 10% (similar to parrots).26 It was technically challenging to further increase the fat percentage of the parrot pellets by adding sunflower oil without changing the pellet texture. In addition, a sunflower seed diet (about 50% fat) was attempted several times in this parrot colony, but the parrots were unwilling to consume the seeds. In this study, the fat diet did not cause significant dyslipidemia, invalidating our hypothesis. However, several TGs were decreased on the fat diet, and some acylcarnitines were also increased. Sunflower oil is rich in n-6 polyunsaturated fatty acids (PUFAs), mainly linoleic acid (FA 18:2), and the standard pelletized diet of these parrots had a fatty acid profile dominated by oleic and linoleic acids in equal proportion.12 Polyunsaturated fatty acids have largely had beneficial effects on human dyslipidemia, including n-3 and n-6 PUFAs.27, 28 In humans, a moderate amount of linoleic acid has been associated with a reduction in blood total cholesterol, LDL, and triglycerides compared with a diet rich in saturated and monounsaturated fatty acids.27, 28 However, it is well recognized that n-3 and n-6 PUFAs have opposing effects on metabolic functions as n-6 PUFA-derived eicosanoids have pro-inflammatory functions, but this aspect was not investigated in our study.27, 29 In the Quaker parrots used in this study, the increase intake of n-6 PUFAs in the form of linoleic acid appeared to decrease blood triglycerides and lead to larger, more buoyant LDLs. In a previous dietary study of Quaker parrots supplemented with either 10% flaxseeds (high in n-3 PUFAs) or 10% sunflower seeds (high in n-6 PUFAs), no plasma total cholesterol or triglyceride changes were seen.8 HDL subfraction changes were seen in that study, whereas LDL subfraction changes were seen in our study. In another study on the same species administered different dietary supplementation levels (low to high) with alpha-linolenic acid, no changes were seen in total cholesterol and triglycerides.7 Other studies in different bird species primarily focused on dietary n-3 PUFAs; therefore, it is difficult to compare those studies with the results of this study. In cockatiels, an increase in the dietary intake of n-3 PUFAs induced a decrease in plasma cholesterol and triglycerides.30 In a cohort of parrots studied postmortem, the tissue content of alpha-linolenic acid (FA 18:3) was negatively associated with the presence of atherosclerotic lesions.31 In chickens, increasing the dietary n-3 or n-6 PUFA content led to a decrease in plasma triglycerides.32 The effects of different n-3 and n-6 PUFA levels on plasma lipids in parrots warrant further investigations.
In addition, two acylcarnitines in C14 were also significantly increased on the fat diet, and acylcarnitines were identified as the most likely lipid type to change on the enrichment analysis. Acylcarnitines play an essential role in fatty acid transport to mitochondria and peroxisomes for fatty acid oxidation.33 Therefore, the observed increase in plasmatic acylcarnitines in Quaker parrots on the fat diet may be linked to an increase in fatty acid oxidation that would go along with the observed decrease in plasma triglycerides. Lastly, plasma intermediates use was upregulated to make SMs and PCs in birds on the fat diet. Since increased plasma lipid intermediates, such as ceramides and DGs, have been associated with several lipid-related conditions,24, 34 this is another beneficial effect of higher n-6 PUFA content in the fat diet fed to these parrots.
A limitation of this study was that several lipid species were not measured as the targeted profile was restricted by the MxP Quant 500 panel. In particular, other similar glycerophospholipid and lysoglycerophospholipid types, such as PCs, LPCs, oxidized lipids, and lipid mediators, were not measured and could be important lipids in dyslipidemia and cardiovascular diseases.35, 36 However, this targeted metabolomics kit was very comprehensive and allowed us to obtain a broad snapshot of the lipidome with a single assay; a comprehensive lipidome requires using different panels, methodologies, and occasionally, different laboratories, as demonstrated when reporting the plasma lipidome of the Quaker parrot.12 Furthermore, increasing the analytic number of a 12-parrot sample size would cause decreased statistical power, which is already a problem in lipidomics studies of animals.
In conclusion, we determined that the cholesterol diet model can be used to study clinical pathologic techniques, perform agreement studies on lipidologic assays, and test various therapeutic options in parrots with dyslipidemia. Regarding the fat diet study, further research is needed to investigate the effect of a larger increase in fat content, as well as the effects of various n-6 and n-3 dietary supplementations. A hypertriglyceridemic model using specific drugs (eg, poloxamer 407)37 instead of dietary induction may also be more effective at causing transient elevations in plasma triglyceride concentrations for short-term studies and should also be investigated.
ACKNOWLEDGMENTS
Funding for this study was provided by the Morris Animal Foundation (grant D19ZO-301) and the OVC Pet Trust Foundation. This publication has not been reviewed or endorsed by the Morris Animal Foundation, and the views expressed do not necessarily reflect the views of the Foundation, its officers, directors, affiliates, or agents. The authors also thank Hagen Inc. for providing and manufacturing the experimental diets and supporting the Quaker parrot colony at the OVC and, in particular, Benoît Choquet for formulating the diets. The authors thank the Analytical Facility of Bioactive Molecules, the Hospital for Sick Children, Toronto, Canada, for performing the analysis. Finally, the authors thank the staff at the University of Guelph - Central Animal Facility for their excellent care of the Quaker parrot colony and also Dr. Sara Gardhouse (Kansas State University) for her initial help with this project.