Hematologic and biochemical reference intervals and blood cell morphology in South American pit vipers (Bothrops pubescens)
Abstract
Background
The maintenance of snakes in captivity for venom extraction and antivenom production is essential due to the high incidence of snake envenomation in Latin America and the Caribbean. Hematology and biochemistry are valuable in the laboratory evaluation of these animals, which indirectly improves their health and welfare.
Objectives
The objective of this study was to determine the reference intervals for hematologic and plasma biochemistry variables in Bothrops pubescens snakes kept in captivity and to examine sex variation.
Methods
Blood samples from 20 healthy B pubescens snakes were collected for hematologic and biochemistry evaluations and compared between sexes. The sample analysis consisted of a complete blood count using manual methods previously described, and blood cell morphology evaluation. Plasma biochemistry consisted of albumin, uric acid, aspartate aminotransferase, creatine kinase, and total plasma protein measurements.
Results
Hematologic and biochemical variables were demonstrated to be different when compared with previously published values. A difference between the sexes was not observed for B pubescens.
Conclusions
Given that many illnesses can lead to laboratory alterations, knowledge about the reference intervals of healthy captive animals is essential to evaluate the health status and correct management of these snakes.
1 INTRODUCTION
Approximately 70 000 snakebites occur each year in Latin America and the Caribbean, caused mainly by encounters with viperids of the Bothrops genus.1, 2 Currently, the only effective treatment is the administration of antivenom. The significant number of snakebite envenomations provides justification for the maintenance of captive snakes to be used for venom extraction in the production of antivenom, as well as other scientific studies.3
Knowledge and general management of diseases are crucial for providing adequate care for animals in captivity. Laboratory tests provide support for clinical suspicions, making disease control and prevention easier. Only a few studies on the hematology of snakes have been published, despite the importance of health management in captive animals used for venom extraction.3-5
Some species of the Bothrops genus have hematologic variables already published.4-7 However, no published work for Jararaca-pintada (Bothrops pubescens Cope 1870), a pit viper from the state of Rio Grande do Sul (Southern Brazil) and Uruguay8 used in the venom pool for bothropic antivenom production,9 was found. The objective of this study was to determine reference intervals (RIs) for hematologic and plasma biochemical variables from B pubescens snakes kept in captivity and compare values between sexes.
2 MATERIALS AND METHODS
2.1 Sample collection
In March 2019, 20 healthy adult specimens of B pubescens (8 males and 12 females, previously sexed by probing) with good nutritional status were selected. All snakes were maintained in captivity at the Zoobotanical Foundation of Rio Grande do Sul (FZB-RS) for venom extraction and used for antivenom production. They were held in fiberglass enclosures with a controlled temperature (18-25°C) and fed monthly with live rodents.
Peripheral blood samples were taken during the morning. All animals were fasted for 14 days before collection by the ventral coccygeal vein. The snakes were physically restrained, and the blood was collected with a 3 mL syringe and 0.45 mm × 13 mm needle, with a total volume of 400 µL for each snake. Blood was immediately stored in lithium heparin microtubes and refrigerated for a maximum of 4 h until analysis at the Veterinary Clinical Analysis Laboratory (LACVET) of the Federal University of Rio Grande do Sul (UFRGS), Brazil. The study followed international standards on animal welfare and was approved by the Brazilian Institute of Environment and Renewable Natural Resources (Sisbio protocol 65588) and the ethics committee of the UFRGS (protocol 35930).
2.2 Hematologic and biochemical analyses
RBC and WBC counts were determined manually using a hemocytometer (Neubauer chamber) with a Natt and Herrick stain.10 The packed cell volume (PCV) was obtained with the microhematocrit method after centrifugation for 5 min at 11 500g (Thermo Scientific Heraeus Pico 17). Hemoglobin concentration (Hb) was determined by spectrophotometric analysis using a hemoglobinometer (HemoCue Hb 301, Fresenius). Indices, such as the mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), were calculated using standard formulas.11
Blood smears with anticoagulated samples were stained with a Wright-Giemsa stain (Sigma-Aldrich). A trained veterinary clinical pathologist evaluated the blood films using light microscopy to assess blood cell morphology, detect the presence of blood parasites, and obtain a WBC differential count. Leukocytes were morphologically classified as heterophils, azurophils, basophils, and lymphocytes.12
After performing the CBC, heparinized blood samples were centrifuged for 5 min at 1000g to obtain plasma. Biochemistry variables, such as albumin (Alb), uric acid (UA), aspartate aminotransferase (AST), and creatine kinase (CK), were measured using commercial enzyme kinetic kits in an automated analyzer (CM-200, Wiener Lab). The total plasma protein (TTP) concentration was estimated by refractometry.
2.3 Statistical analysis
All statistical analyses used to define the RIs were performed according to the ASVCP guidelines13, 14 using MedCalc Statistical Software (version 14.8.1, MedCalc Software bvba). Before establishing RIs, the hematologic and biochemical variables were assessed for normal distributions, and outliers were identified. The Shapiro-Wilk test was performed to confirm a normal distribution after histogram evaluation, according to ASVCP guidelines. Non-parametric data were transformed using the Box-Cox power transformation method and re-evaluated for normality. After data normalization, outliers were verified using Tukey's interquartile fences, but exclusion was not necessary. The RIs were determined based on the parametric method. Variables were described by mean, minimum, and maximum values, and RIs with 90% confidence intervals. An independent t-test was used to compare the effect of sex on hematologic and biochemical variables. Values were considered statistically significant at P < 0.05.
3 RESULTS
B pubescens RBCs (Figure 1A) were elliptical with eosinophilic cytoplasm and a basophilic, irregular, and condensed nucleus. Fewer numbers of polychromatophilic RBCs were observed. These cells were smaller and rounder, with a higher nucleus-to-cytoplasm ratio and more basophilic cytoplasm than the mature RBCs. Thrombocytes were also found individually or in aggregates. They were frequently elliptical with a light blue cytoplasm and a rounded, basophilic, condensed nucleus (Figure 1A).
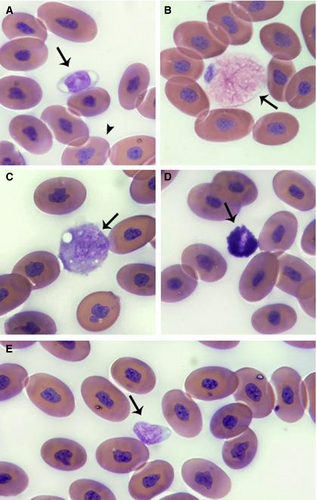
WBCs presented with different morphologic characteristics for each cell type, which were divided into four cell types. Heterophils were round with abundant cytoplasm, elongated small eosinophilic granules, and a basophilic rounded nucleus (Figure 1B). Azurophils were round, with azurophilic granules and occasional vacuoles in the cytoplasm; the nuclei were eccentric and round (Figure 1C). Basophils were small and spherical cells with dense basophilic cytoplasmic granules, which sometimes obscured the nucleus (Figure 1D). Lymphocytes were the most frequently observed WBC and characterized as small cells, with a rounded basophilic nucleus and a high nuclear-to-cytoplasmic ratio (Figure 1E). No cells resembling monocytes, morphologic alterations, viral inclusions, or blood parasites were observed.
Significant differences between sexes in hematologic (Table 1) and biochemical variables of B pubescens (Table 2) were not observed in our study. General hematologic (Figure 2) and biochemistry (Figure 3) values are shown as the mean and standard deviation with ranges (Table 3).
| Variables | Males | Females | P-value |
|---|---|---|---|
| RBC (106/µL) | 0.67 (0.04) | 0.61 (0.13) | 0.251 |
| Hb (g/dL) | 8.15 (1.24) | 7.92 (1.51) | 0.734 |
| PCV (%) | 27.63 (3.04) | 27.58 (3.17) | 0.978 |
| MCV (fL) | 412.32 (39.70) | 463.92 (62.63) | 0.065 |
| MCHC (%) | 29.38 (1.47) | 28.71 (4.18) | 0.913 |
| WBC (103/µL) | 8.49 (3.02) | 12.02 (4.29) | 0.072 |
| Hetero (103/µL) | 0.91 (0.49) | 1.55 (1.12) | 0.278 |
| Lymph (103/µL) | 5.87 (2.64) | 8.96 (3.36) | 0.052 |
| Azuro (103/µL) | 1.46 (0.99) | 1 (0.78) | 0.194 |
| Baso (103/µL) | 0.25 (0.21) | 0.51 (0.53) | 0.140 |
- Abbreviations: Azuro, azurophils; Baso, basophils; Hb, hemoglobin; Hetero, heterophils; Lymph, lymphocytes; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; PCV, packed cell volume; RBC, red blood cells; WBC, white blood cells.
| Variables | Males | Females | P-value |
|---|---|---|---|
| UA (mg/dL) | 2.11 (0.83) | 1.69 (0.57) | 0.218 |
| Alb (g/L) | 20.63 (2.39) | 18.58 (6.4) | 0.423 |
| AST (U/L) | 23.00 (14.24) | 22.83 (14.91) | 0.916 |
| CK (U/L) | 310.38 (49.80) | 281.67 (60.31) | 0.304 |
| TPP (g/L) | 50.50 (7.19) | 50.67 (10.27) | 0.970 |
- Abbreviations: Alb, albumin; AST, aspartate aminotransferase; CK, creatine kinase; TPP, total plasma protein; UA, uric acid.
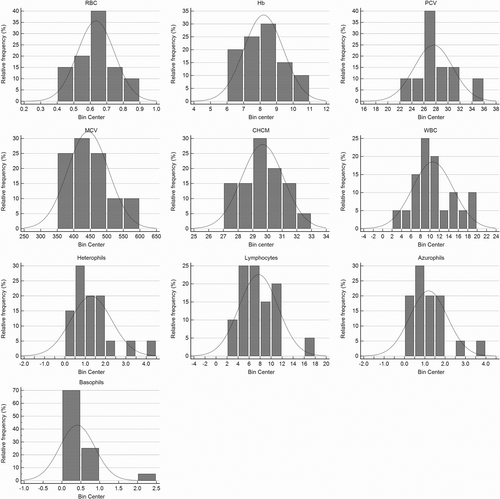
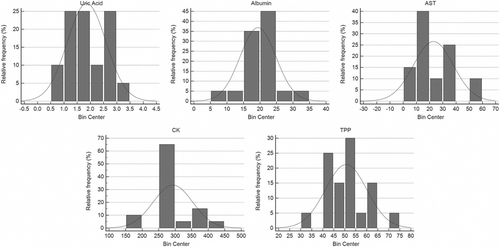
| Variables | Mean | Min-Max | Lower RI (90% CI) | Upper RI (90% CI) |
|---|---|---|---|---|
| RBC (106/µL) | 0.63 | 0.46-0.83 | 0.41 (0.34-0.49) | 0.85 (0.78-0.92) |
| Hb (g/dL) | 8.01 | 4.50-10.7 | 5.17 (4.23-6.11) | 10.85 (9.91-11.78) |
| PCV (%) | 27.60 | 23.00-34.00 | 21.32 (19.25-23.40) | 33.88 (31.80-35.95) |
| MCV (fL) | 443.28 | 350.64-565.21 | 322.25 (282.22-362.27) | 564.31 (524.29-604.34) |
| MCHC (%) | 29.62 | 15.51-32.96 | 23.29 (0-26.33) | 32.49 (31.69-33.20) |
| WBC (103/µL) | 10.60 | 3.70-19.80 | 2.15 (0-4.94) | 19.06 (16.26-21.85) |
| Hetero (103/µL) | 1.04 | 0.12-4.21 | 0.15 (0.06-0.32) | 4.00 (2.67-5.79) |
| Lymph (103/µL) | 7.72 | 2.07-17.03 | 0.80 (0-3.09) | 14.65 (12.36-16.93) |
| Azuro (103/µL) | 0.97 | 0.07-3.89 | 0.08 (0.01-0.2) | 3.54 (2.46-4.89) |
| Baso (103/µL) | 0.27 | 0-2.07 | 0 (0-0.03) | 1.60 (0.98-2.46) |
| UA (mg/dL) | 1.86 | 0.8-3.1 | 0.42 (0.05-0.89) | 3.30 (2.82-3.77) |
| Alb (g/L) | 19.40 | 7.00-31.0 | 8.79 (5.28-12.30) | 30.01 (26.50-33.52) |
| AST (U/L) | 18.10 | 7.00-56.00 | 5.52 (3.82-8.01) | 75.22 (45.48-129.25) |
| CK (U/L) | 293.27 | 164.00-423.00 | 176.00 (136.91-214.91) | 409.58 (371.20-447.88) |
| TPP (g/L) | 50.60 | 32.00-74.00 | 32.17 (26.07-38.26) | 69.03 (62.94-75.13) |
- Abbreviations: Alb, albumin; AST, aspartate aminotransferase; Azuro, azurophils; Baso, basophils; CK, creatine kinase; Hb, hemoglobin; Hetero, heterophils; Lymph, lymphocytes; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; PCV, packed cell volume; RBC, red blood cells; TPP, total plasma protein; UA, uric acid; WBC, white blood cells.
4 DISCUSSION
As observed in B pubescens, RBCs with irregular nuclei have been previously described in snakes from Viperidae and Elapidae families.15, 16 Furthermore, a small number of immature RBCs (non-quantified) was observed. This finding is related to the young specimens during ecdysis and suggested that the final step of RBC maturation occurs in peripheral circulation.17
Regarding the RBC series, when compared with previous studies, the RBC count was similar in Bothrops alternatus, Bothrops jararacussu, and Bothrops diporus,5 and was higher in Bothrops ammodytoides, Bothrops moojeni, Bothrops leucurus, and Bothrops jararaca.4-7 The mean PCV was higher than other Bothrops species but relatively similar to a viperid, Vipera ammodytes.18 The MCV mean was close to B moojeni and B leucurus values, indicating that these species have RBCs of similar sizes.5, 6 Hemoglobin and MCHC means obtained were equivalent to B leucurus and lower than values for B ammodytoides, B alternatus, B jararacussu, and B diporus,4-6 demonstrating that not all species from the same genus have similar hematologic variables.
Considering the WBC variables, the WBC count was similar to other captive Bothrops snakes4-6 and higher than B jararaca and female B leucurus.7 As previously observed in Bothrops snakes, Naja naja and Crotalus adamanteus, lymphocytes were the most prevalent WBC followed by azurophils,4-7, 12, 16 although for some snakes, higher relative azurophil counts have been seen.19 In our study, eosinophilic-like cells similar to eosinophils in stained blood smears were not observed, as in other hematologic studies for snakes.12 Previous studies reported eosinophils in some snake species,16, 19, 20 although researchers have classified them as differentiated heterophils based on specific stains.12, 21 To confirm WBC types and differentiate eosinophils and heterophils in some snake species, further studies using flow cytometry or cytochemical staining techniques should be considered.
Although the quantification of albumin in reptiles is more accurate with protein electrophoresis, in the present study, we used low-cost techniques and techniques that were accessible to the laboratories in our region.22 Our biochemical results were similar to those of other species of Bothrops snakes7 and males of Naja naja maintained in environmental captivity.16 However, the influence of external factors could not be assessed in the present study because the snakes were kept in captivity.
In reptiles, blood parasites are considered incidental findings, given that many are nonpathogenic and have been commonly detected in recently captured healthy animals.23 However, stressful conditions, such as poor husbandry and disease, are considered factors that could cause alterations in the CBC.11, 22 In the present study, the absence of blood parasites was expected, given that the sampled animals were captive and their environment made vector-borne parasite transmission difficult. The absence of vector-borne organisms suggests that our results were closer to physiologic conditions.
The sex influence was not seen in B pubescens, in contrast with that observed in Pituophis ruthveni.24 However, the difference between sexes due to hormonal influences must be investigated in snakes to improve the interpretation of laboratory results.
In this study, limitations, such as a small number of animals and the lack of comparison between captive and free-living animals, seasons, temperature, and biological stage, were encountered. In addition, for this study, cytochemical staining could be valuable to confirm the cell types, although the stain used in this study was effective in differentiating all nucleated cells. Further studies with B pubescens and other venomous snake species should be carried out to address these issues.
Our results could be used as RIs for the interpretation of hematologic and biochemical tests for captive male and female B pubescens since the variables of this snake species do not seem to be influenced by sex.
DISCLOSURE
The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.




