Comparison of canine urine specific gravity measurements between various refractometers in a clinical setting
Abstract
Background
Veterinary facilities might use multiple refractometers and individuals to measure urine specific gravity (USG). Previous comparison studies show conflicting results. Furthermore, the clinical significance of measurement differences and interobserver variabilities has not been assessed.
Objectives
We aimed to determine statistically and clinically significant differences between four refractometers in measuring canine USG and subsequent categorization of urine concentrations and azotemia and determine the variability between different observers performing USG measurements.
Methods
Fifty-nine specimens were included for the USG measurements with four refractometers by different observers. Each refractometer pair was compared using Spearman's rank correlation, Bland-Altman difference plots, and Deming regression analyses. Calculated bias was compared to set performance goals. Interobserver agreement was evaluated, and intraclass correlation coefficients were used to determine differences in the categorization of urine concentrations and azotemia (prerenal or renal).
Results
There was excellent correlation (rs = .99-1.00) between refractometers. All comparisons involving R4 showed significant constant and proportional biases. Mean bias met the clinical performance goals for all refractometers, except for comparisons with R4, where up to 17 results were outside the allowable bias. There was almost perfect agreement (rs = .999) between observers and excellent agreement (ICC = .96-.99) for the classification of urine concentrations. In azotemic patients (22%), there was perfect agreement (ICC = 1.00) for the categorization of azotemia.
Conclusions
In most cases, three of the refractometers evaluated in this study can be used interchangeably at all USG values, without affecting clinical decision-making. Multiple observers did not significantly affect decision-making.
1 INTRODUCTION
Refractometers are commonly used and invaluable tools for assessing urine in clinical practice. Previous studies in dogs have shown the suitability of urine specific gravity (USG) as a proxy for measuring urine concentration, based on its strong agreement with urine osmolality (gold standard).1-3 Urine specific gravity measurements can also assist in differentiating between renal and nonrenal azotemia, which helps guide clinicians in their diagnostic workups.4
In clinical practice, different refractometers might be used at different time points for analyzing urine from the same patient. Refractometers intended for USG measurements could differ in terms of the manufacturer, means of measurement (optical vs digital), conversion factors, specificity for veterinary species (refractometers produced for use in people vs those developed for use in a particular species, such as in dogs), and measuring range. Furthermore, in multi-veterinarian practices and practices employing veterinary nurses or technical staff, different individuals might read USG measurements in the same patient.
Previous studies have shown conflicting results regarding the appropriateness of using different refractometers interchangeably in dogs.3, 5, 6 One study comparing a veterinary digital refractometer (designed for use in cats) with a medical optical refractometer, found an unacceptable negative proportional bias in the digital refractometer when measuring the USG of canine urine.5 These findings are similar to those of another study comparing a combination of five veterinary (dog and cat specific) and medical digital and optical refractometers to a reference method.6 A negative proportional bias was found in all refractometers compared with the estimated specific gravity from dried total solids and was greatest in the cat-specific refractometers (both optical and digital) when measuring the USGs of canine urine.6 In contrast, a study in dogs, that compared a medical digital and optical refractometers showed that, in 91.5% of the dogs, USG measurements differed by <0.002 units on the USG scale and concluded that these refractometers could be used interchangeably.3 Furthermore, these studies either involved only one observer to reduce variability3 or cited possible interobserver variability as a study limitation when more than one observer was included.5, 6
The aims of this study were first, to compare USG readings of four different refractometers using standard method comparison statistics; second, to determine whether any differences were clinically significant and relevant by comparing the refractometers in their classification of urine concentration categories and types of azotemia; and third, to determine the magnitude of variability between observers. We hypothesized that the refractometers would show significantly different readings to each other over a range of USGs and, subsequently, that azotemic patients would be classified differently by the various refractometers. Furthermore, we hypothesized that significant variability would exist between observers.
2 MATERIALS AND METHODS
2.1 Patients
This prospective study was a method comparison study, based on previously published guidelines for method comparison in the clinical laboratory.7 Urine specimens were collected from canine patients presented to the Onderstepoort Veterinary Academic Hospital (OVAH), University of Pretoria, from May 2017 to September 2018. The dogs were presented with a variety of historical and clinical findings warranting, in all cases, a complete urinalysis and determination of serum creatinine concentration as part of routine diagnostic procedures. No urine specimens or serum samples were collected exclusively for use in this study. There was no selection for breed, age, sex, presenting complaint, or final diagnosis. However, patients receiving fluid therapy or with macroscopic hematuria or pigmenturia at the time of urine and/or blood collection were not included in the study. The study was approved by the Animal Ethics Committee of the University of Pretoria in May 2017 (certificate number V036-17).
2.2 Study specimens
Urine was collected via free-flow, catheterization, or cystocentesis as part of a routine diagnostic workup. Complete urinalyses were performed in the clinic within 30 minutes of collection and included organoleptic evaluations (color, turbidity, and odor), USG determinations, urine dipstick examinations, and microscopic evaluations of urinary sediments. Surplus urine (≥1 mL) from this procedure was submitted to the clinical pathology laboratory for use in the study. Urine specimens were considered for inclusion if a complete urinalysis was performed by the attending clinician, and if serum creatinine concentration was determined within 2 hours of urine collection. All the results of a complete urinalysis were recorded and submitted with the specimen. Specifically included in this record was the turbidity of the specimen and the dipstick results for protein, glucose, and heme protein. Each submitted specimen was stored at 2-8°C for up to 12 hours8 until the USG could be measured, which was usually at the end of the working day. Blood for serum creatinine concentrations was collected either at the same time or within 2 hours of urine collection.
2.3 Specimen analysis
Four refractometers were used to measure the USG (Table 1). All the selected instruments were hand-held optical refractometers used by final-year veterinary and veterinary nursing students and clinicians across various hospital clinics (Figure 1). Additionally, all four refractometers were designed for USG measurements in human urine, and none were veterinary specific. The refractometers were calibrated to 1.000 using distilled water at room temperature before each measurement, as recommended.9 A group of six observers took part in the study, and for each specimen, two observers from this group were randomly selected to perform the analyses. The observers included certified laboratory technologists, residents, and specialist clinical pathologists. The urine specimens were left to warm to room temperature (22-25°C) before analyses. Urine specimens were resuspended, and USGs were determined by placing one to two drops of room temperature, noncentrifuged urine on each of the four refractometers. The USG was measured on each instrument by two independent observers, in the same room, and one after the other, using the same drop(s) of urine. The observers were blinded to the USG measured in the complete urinalyses and to the USG measurements determined by the other observer. Concentrated urine specimens with a USG beyond the analytical range of the refractometer were assigned the value of the upper limit of the measurement. Dilution of concentrated urine specimens reportedly yields spuriously elevated USG values, and for this reason, dilutions were not performed.6 Serum creatinine concentrations were measured with a modified Jaffe method using an automated chemistry analyzer (Cobas Integra 400 Plus, Roche Products [Pty] Ltd.). Serum creatinine concentrations measured beyond the analytical range of the assay (>1300 μmol/L or >14.7 mg/dL) were achieved using automatic dilutions.
| Refractometer study number |
Refractometer manufacturer Refractometer model |
Temperature compensation | USG measurement range |
|---|---|---|---|
| R1 |
Atago MASTER-SUR/NM |
No | 1.000-1.060 |
| R2 |
American Optical TS Meter (TS-B/10400) |
Yes | 1.000-1.035 |
| R3 |
Bellingham and Stanley The Field Refractometer (21-24) |
Yes | 1.000-1.040 |
| R4 |
JorVet Protein/Specific Gravity refractometer (J-0351) |
Yes | 1.000-1.050 |
- Abbreviation: R, refractometer.

2.4 Data analysis
2.4.1 Interobserver agreement and method comparison
All data were tested for normality using the Kolmogorov-Smirnov normality test. Interobserver agreement for each refractometer was evaluated by calculating the Spearman's rank correlation coefficient for duplicate readings. The coefficient of variation (CV) of duplicate measurements was used to determine interobserver CVs.
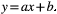
A significant constant bias was identified if the confidence interval of the regression line y-intercept did not include zero, and a significant proportional bias was identified if the confidence interval of the regression line slope did not include 1.0.7
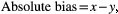
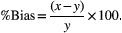
This bias was compared with the performance goals for analytical bias drawn from two sources: (a) derived from biological variation in humans—0.27%10; and (b) consensus reached by a panel of small animal internal medicine specialists at the OVAH—0.002 USG units.
2.4.2 Determination of clinically relevant differences in the classification of urine concentrations and azotemia
For each refractometer, patients were placed into four defined concentration categories according to the mean of the duplicate USG readings. These categories were as follows: highly concentrated (USG > 1.030), moderately concentrated (USG of 1.013-1.029), isosthenuric (USG of 1.008-1.012), and hyposthenuric (USG < 1.008) urine.4, 11
Patients were also categorized as azotemic and nonazotemic. Azotemia was defined as a serum creatinine concentration greater than the laboratory upper reference limit of 109 μmol/L (1.2 mg/dL). The subset of azotemic patients was further classified according to the USG findings for each refractometer as follows: prerenal azotemia (USG ≥ 1.030) or renal azotemia (USG < 1.030).11
The level of reliability (agreement and correlation) between refractometers for each concentration and azotemia category was determined using the intraclass correlation coefficient based on a mean rating (k = 2), absolute agreement, and two-way model.12 The correlation coefficients were interpreted as follows: <.50 is poor; .50-.75 is moderate; .75-.9 is good, and >.90 is excellent reliability.12
Statistics were performed using MedCalc for Windows (version 16.4.3) (MedCalc Software). Statistical significance was set at a P < .05.
3 RESULTS
3.1 Patients and specimen analyses
Surplus urine was obtained from 60 canine patients. Information regarding signalment (breed, age, and sex) of the dogs included in this study is provided in Table S1. One urine specimen was excluded due to the presence of macroscopic pigmenturia, and 59 were included. Most of the specimens were clear in appearance (42/59; 71%) and the rest showed varying degrees of turbidity (17/59; 29%). The median USGs, using the mean of the duplicate readings, as measured by each refractometer, is shown in Table 2. The median pH of the specimens was 6 (range 5-8). Only 4 (7%) of the specimens were glucosuric, and 46 (78%) of the specimens were proteinuric, although the majority of the proteinuric specimens (29/46; 63%) showed only a 1+ on the protein patch of the urine dipstick. Thirty-one (53%) of the specimens showed hematuria, hemoglobinuria, or myoglobinuria, as measured by the blood patch on the urine dipstick. However, none of these specimens showed macroscopic pigmenturia or hematuria, based on the exclusion criteria for this study.
| Refractometer label | Median USG | IQR |
|---|---|---|
| R1 | 1.024 | 1.015-1.042 |
| R2 | 1.023 | 1.015-1.035 |
| R3 | 1.021 | 1.014-1.040 |
| R4 | 1.024 | 1.016-1.050 |
- Abbreviation: R, refractometer.
Thirteen (22%) of the enrolled patients were azotemic and had serum creatinine concentrations ranging from 113 to 1703 μmol/L (1.3-19.3 mg/dL).
3.2 Data analysis
3.2.1 Interobserver agreements and method comparisons
All data showed non-Gaussian distributions (P < .05). Perfect to almost perfect agreement between observers was determined for all refractometers (rs = .998-1.000, P < .0001). The calculated interobserver CVs were 0.03% for R2, 0.04% for R1 and R3, and 0.05% for R4.
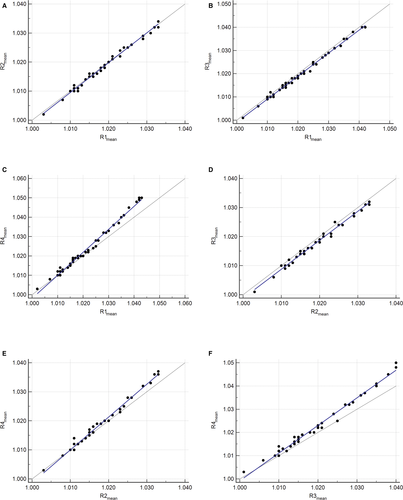
Using the mean of the duplicate readings, there was an excellent correlation between each pair of refractometers (Table 3). Bland-Altman difference plots and Deming regression plots comparing each pair of refractometers are shown in Figures 2 and 3, respectively. The final number of specimens included in each comparison analysis, after the removal of highly concentrated specimens, is indicated in Table 3. Statistically significant negative constant and positive proportional biases were identified for all comparisons with R4 (Table 3). No statistically significant bias was identified between the other three refractometer pairs (R1 vs R2, R1 vs R3, and R2 vs R3) (Table 3). Mean bias estimates from the Bland-Altman difference plots were <0.002 for all comparisons except R1 vs R4 and R3 vs R4 (Figure 2).
| First refractometer | Second refractometer | n | Correlation (rs) | P-value | y-intercept | 95% CI (y-intercept) | Slope | 95% CI (slope) |
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | 39 | .990 | <.0001 | −0.026 | −0.064 to 0.012 | 1.026 | 0.988-1.063 |
| R3 | 44 | .994 | <.0001 | 0.009 | −0.016 to 0.033 | 0.991 | 0.967-1.014 | |
| R4 | 46 | .996 | <.0001 | −0.189 | −0.232 to −0.146 | 1.187 | 1.145-1.229 | |
| R2 | R3 | 39 | .992 | <.0001 | 0.001 | −0.034 to 0.037 | 0.997 | 0.962-1.032 |
| R4 | 39 | .990 | <.0001 | −0.132 | −0.176 to −0.088 | 1.131 | 1.088-1.174 | |
| R3 | R4 | 44 | .994 | <.0001 | −0.188 | −0.247 to −0.129 | 1.188 | 1.130-1.246 |
- Abbreviation: R, refractometer.
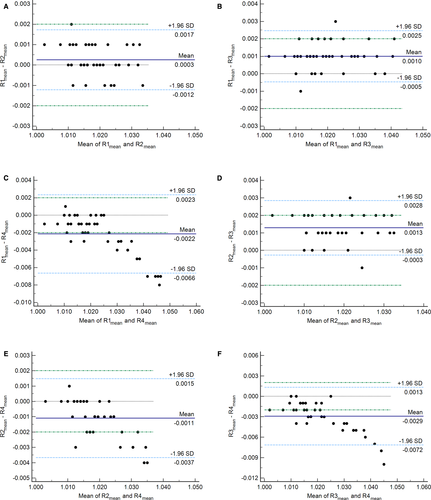
When comparing the calculated bias to both of the set performance goals, a clinically unacceptable positive bias was present between R1 vs R4 and R2 vs R4 at a USG value of 1.030, and between R3 vs R4 at USG values of 1.020 and 1.030 (Table 4). All other comparisons met the set performance goals at all USG values.
| First refractometer | Second refractometer | Bias | First refractometer USG measurement | ||
|---|---|---|---|---|---|
| 1.010 | 1.020 | 1.030 | |||
| R1 | R2 | Measured USG | 1.010 | 1.021 | 1.031 |
| Absolute bias | 0.000 | 0.001 | 0.001 | ||
| Bias (%) | 0.03 | 0.05 | 0.08 | ||
| R3 | Measured USG | 1.010 | 1.020 | 1.030 | |
| Absolute bias | 0.000 | 0.000 | 0.000 | ||
| Bias (%) | −0.01 | −0.02 | −0.03 | ||
| R4 | Measured USG | 1.010 | 1.022 | 1.034 | |
| Absolute bias | 0.000 | 0.002 | 0.004 | ||
| Bias (%) | −0.01 | 0.17 | 0.35 | ||
| R2 | R3 | Measured USG | 1.008 | 1.018 | 1.028 |
| Absolute bias | −0.002 | −0.002 | −0.002 | ||
| Bias (%) | −0.20 | −0.20 | −0.20 | ||
| R4 | Measured USG | 1.010 | 1.022 | 1.033 | |
| Absolute bias | 0.000 | 0.002 | 0.003 | ||
| Bias (%) | 0.03 | 0.16 | 0.28 | ||
| R3 | R4 | Measured USG | 1.012 | 1.024 | 1.036 |
| Absolute bias | 0.002 | 0.004 | 0.006 | ||
| Bias (%) | 0.19 | 0.37 | 0.55 | ||
- Abbreviation: R, refractometer.
3.2.2 Determination of clinically relevant differences in the classification of urine concentrations and azotemia
Urine specimens over a range of USG values were included in the study so that each concentration category was represented (Table S2). The overall agreement and correlation between all the refractometers for categorization of urine concentration was excellent (ICC = .988; 95% confidence interval [CI] = 0.982-0.992). The agreement and correlation between each pair of refractometers for the categorization of urine concentrations were also excellent (Table 5). Across all six refractometer pairs, there were a total of 18 discordant concentration classifications: nine highly concentrated vs moderately concentrated, six moderately concentrated vs isosthenuric, and three isosthenuric vs hyposthenuric. The agreement and correlation between all refractometers for the categorization of azotemia was perfect (ICC = 1.00).
| First refractometer | Second refractometer | Correlation (ICC) | 95% CI (ICC) |
|---|---|---|---|
| R1 | R2 | .986 | .976-.992 |
| R3 | .993 | .989-.996 | |
| R4 | .964 | .934-.979 | |
| R2 | R3 | .978 | .963-.987 |
| R4 | .977 | .961-.986 | |
| R3 | R4 | .956 | .921-.975 |
- Abbreviation: R, refractometer.
4 DISCUSSION
This study found no clinically significant differences between three of the four medical optical refractometers in their measurement of USGs. The fourth refractometer, R4, showed clinically significant differences at higher USG values when compared with the three other refractometers. In the majority of cases, and in all azotemic cases, there was no clinically significant difference between observers and between the refractometers in their classification of patients into urine concentration categories. This study differs from others conducted in dogs in that all the refractometers compared were medical optical hand-held refractometers, were different models to those previously compared and, were not veterinary specific (digital or optical).3, 5, 6 In two previous studies, one optical and one digital refractometer (one veterinary specific and one medical) were compared with one another.3, 5 Both found that the digital refractometer measured lower USG values than the optical refractometer, but in the study using a medical digital refractometer, these differences were within acceptable limits.3, 5 Tvedten et al compared five different refractometers—three medical optical refractometers and two veterinary-specific digital refractometers—in dogs and cats. The authors found that the refractometers reported different results from one another.6 Our study aimed to compare the refractometers currently used in the clinics of our hospital to determine whether the same was true, specifically to determine if clinically significant differences that could affect decision-making were present. As no digital or species-specific refractometers were used in the hospital clinics, these types of refractometers were not included in this study, and to our knowledge, the chosen refractometers have not been included in any previous method comparison studies.
The urine specimens included in this study varied in their physical and chemical properties, and a wide range of USG measurements was analyzed. No specimen dilutions were performed, based on Tvedten et al's findings that dilution resulted in spurious increases in USG.6 Although this study aimed only to compare refractometers and not to determine accuracy or precision, there are no studies, to the authors’ knowledge, that assess whether this spurious increase in USG is predictable and equal in magnitude across various refractometers. Additionally, this study aimed to assess clinically relevant implications of differences between refractometers and, in dogs, the degree that urine is concentrated above 1.030 does not provide clinically relevant information.
Two of the above-mentioned previous studies excluded specimens that were turbid or had active urine sediments.3, 6 Specimen turbidity is the result of suspended particles such as cells and is not expected to affect the refractive index of urine. One of the studies also excluded glucosuric or significantly proteinuric urine (≥2+ on a urine dipstick).3 Glucosuria and significant proteinuria are both known to spuriously increase USGs, although these conditions do not appear to significantly affect the relationship between USG and urine osmolality in dogs.1, 3 Because the aim of this study was not to assess refractometer accuracy but to determine how the refractometers compared to one another, conditions that could spuriously affect the USGs were not excluded. Macroscopic pigmenturia or hematuria, on the other hand, can cause difficulty in reading an exact USG measurement. For the purposes of this study, exact measurements were needed, resulting in one specimen with macroscopic pigmenturia being excluded.
Three of the instruments were temperature compensated, and one (R1) was not. As the temperature of a solution increases above a certain threshold, the refractive index decreases due to the expansion of solutes.9 Temperature compensation is a means of measurement standardization so that only solute types and concentrations will affect the refractive index and, thus, the measured USGs. According to the manufacturer of R1, the temperature threshold above which USG could decrease is 20°C.13 In this study, varying biases in USGs were found between R1 and the other three refractometers (R2, R3, and R4). If the temperature of the specimens analyzed by R1 affected the USG, one would expect a significant constant positive bias in all three temperature-compensated refractometers compared with R1. On the contrary, the biases found were insignificant for R2 and R3; and positive (constant and proportional) only for R4. Thus, the absence of temperature compensation is unlikely to have affected comparisons including R1 but cannot be ruled out as a factor contributing to the differences seen.
There was no difference between observers in their measurement of USG. Two previous studies comparing the performance of different refractometers ensured only one observer analyzed the specimens to reduce inter-reader variability.3, 14 In addition, other previous studies mentioned inter-reader variability as a study design limitation but this variability was not measured.5, 6 In clinical practice, various individuals such as veterinary nurses, veterinary technologists, and veterinarians may be reading USG values, and keeping observers consistent is often not possible. To replicate this scenario, we used six different observers with various levels of experience and knowledge, and perfect to almost perfect agreement was found between them. Apart from the subjectivity when reading USG values that fall between two lines of measurement and those where there is no clear line of demarcation, analysis of USG is largely objective, which makes this result unsurprising.
The agreement between each pair of refractometers was excellent to perfect. Using regression analysis, statistically significant constant and proportional biases were identified between all the refractometer pairs, which included R4. These positive biases were considered clinically relevant at higher USG values, with the greatest bias when R4 was compared with R3. This indicates that the bias, particularly the proportional bias, will affect clinical decisions. The mean systematic bias between all other refractometer pairs (whether negative or positive) was never greater than the set performance goals, and in some pairs, was undetectable (Table 4). Furthermore, the mean difference between most refractometer pairs was <0.002 (Figure 2), except for R1 vs R4 and R3 vs R4, where it was >0.002. This is likely due to the proportionally larger differences seen at USG values above 1.030. This trend is not apparent when evaluating R2 vs R4, because of the narrower measuring range of R2 (up to 1.035), which resulted in more highly concentrated specimens being removed for statistical analysis.
No cases for R1 vs R2 and only one case each for R1 vs R3 and R2 vs R3 were outside the maximum allowable clinically relevant difference as determined by the Bland-Altman difference plots (Figure 2). These findings are similar to those previously reported, where 91.5% of specimens showed mean differences in USG values of ≤0.002 between a medical optical and digital refractometer.3 It can be concluded that R1, R2, and R3 compare favorably with one another and can be used interchangeably with no clinically significant differences. Conversely, comparisons with R4 (R1 vs R4, R2 vs R4, and R3 vs R4) exhibited substantially more cases outside the maximum allowable clinically relevant difference of 0.002 (15, 6, and 17 cases, respectively) (Figure 2). In all these cases, the USG values measured by R4 were higher than the other refractometer. Of these cases, two dogs for R2 vs R4, and three dogs for R1 vs R4 and R3 vs R4, would have had a discordant result around the clinical decision cut-off of 1.030. In the remaining cases, the classification and clinical interpretation were not affected. Based on the discordant results and clinically significant biases in comparisons involving R4, it can be concluded that this instrument should not be used interchangeably with the other refractometers in the present study.
These conclusions regarding interchangeability differ from those made by Tvedten et al, who concluded that, with increasing USG values, the results of all five refractometers included in their study did not increase consistently compared with the reference method and with one another.6 Thus, these instruments could not be used interchangeably.6 However, when only evaluating the three refractometers with the same specifications as the refractometers used in the present study, it was determined that all three had a mean bias of ≤0.002 (positive or negative) compared with the reference method.6 Therefore, it is possible that these medical optical refractometers compared similarly to each other, but no method comparison statistics were performed between the refractometers, only between the refractometers and the reference method.6
Lastly, the agreement between the refractometers in their categorization of patient urine concentrations and azotemia was excellent to perfect. To the authors’ knowledge, this is the first study that has assessed the differences in refractometers based on the clinical significance of results. This finding does not, however, diminish the value of repeated USG measurements. One USG value should not be used in isolation and further diagnostics and follow-up measurements are necessary to confirm the categorizations of urine concentrations and azotemia. The number of discordant results between R4 and the other three refractometers strengthens this recommendation. These discordant results could lead to misclassification and, if not followed up, could result in misdiagnoses and incorrect patient management.
A limitation of this study is that the complete urinalyses were performed by several different people. The number of proteinuric, glucosuric, and turbid specimens might have been different had one individual performed all complete urinalyses, as there is a degree of subjectivity involved when assessing these variables. Another limitation is that analyses with warmer urine or at ambient temperatures greater than 25°C were not performed, and so conclusions regarding how the refractometers compared in warmer conditions could not be drawn. Also, only a small number of azotemic dogs were included. Further studies are needed to confirm the similarity of refractometers in their categorization of azotemic patients. Lastly, the minimum recommended sample size of 40 for method comparison studies7 was not achieved in the comparisons with R2 (39 specimens included). Because specimens were collected prospectively and were not excluded based on the degree of urine concentration, many urine specimens concentrated beyond the measuring range of R2 were initially included. Nevertheless, the comparisons that included R2 show similar trends to the other comparisons and the range ratio, an additional indicator for the appropriate number of samples to include,7 calculated from a range of USG values of 1.003-1.033 is 11. This ratio was high, indicating that small sample sizes are sufficient to obtain acceptable results.7
In conclusion, refractometer comparisons that included R1, R2, and R3 did not show clinically relevant differences in most cases, and these refractometers can be used interchangeably at all USG values. In contrast, R4 should not be used interchangeably with the other three refractometers, despite its excellent to perfect correlation in the categorization of urine concentrations and azotemia. The reason for this conclusion is based on the number of discordant results, the number of cases outside the maximum allowable clinical difference, and the failure to meet the performance goals at USG values ≥ 1.020. Additionally, given that interobserver variability is negligible, regardless of the observer's level of experience, measurements by different observers will also not show clinically relevant differences in almost all cases. These findings have positive implications in clinical practice, where several different refractometers could be used by different observers during a patient's hospitalization and at follow-up visits.
ACKNOWLEDGMENTS
The authors are grateful to the students, veterinary nurses, and clinicians working at the Onderstepoort Veterinary Academic Hospital for supplying samples for this study and to Mrs Carien Muller (University of Pretoria) and Dr Zandri Whitehead (University of Pretoria), who assisted the authors in USG measurement.
CONFLICT OF INTEREST
The authors state that there is no conflict of interest.