Morphologic and quantitative evaluation of bone marrow aspirates from Hispaniolan Amazon parrots (Amazona ventralis)
Abstract
Background
Bone marrow aspirate assessments provide valuable information about hematopoietic status and hematologic disease. Hematopoietic cell differentials and morphologies have been anecdotally described in psittacines, but quantitative studies are lacking.
Objectives
We aimed to determine differential cell counts and calculate granulocyte:erythroid (G:E) ratios in bone marrow aspirates from Hispaniolan Amazon parrots and report representative morphologies of the hematopoietic cells.
Methods
Bone marrow aspirates were collected from 32 clinically healthy, captive, parrots. Peripheral blood was obtained for CBCs. Bone marrow differential cell counts (%) were determined by counting 500 cells on modified Wright's-stained smears. G:E ratios were calculated. Representative images of hematopoietic cells at all stages of development were taken.
Results
Of the 32 parrots sampled, 17 bone marrow samples were of sufficient cellularity and quality for evaluation. Erythroid cells comprised 68.9 ± 8.6% (total ± SD) of the hematopoietic cells and consisted primarily of early- and late-stage polychromatophilic rubricytes (43.6 ± 2.1% of total erythroid cells). Granulocytic cells comprised 28.1 ± 3.8% of the hematopoietic cells and consisted primarily of mature and band heterophils (11.9 ± 5.2% and 6.5 ± 3.4%, respectively, of total granulocytic cells). A unique morphologic finding in avian progranulocytes was the presence of multiple different granules. The G:E ratio was 0.4 ± 0.2 (median 0.4, range 0.1-0.9). Thrombocyte lineage cells could not be reliably identified and were not counted. CBC results were largely within expected limits.
Conclusions
The low G:E ratios observed could be normal in this species; however, these ratios could be affected by factors related to sampling and cell identification. These findings will be a valuable resource for the diagnostic evaluation of clinical bone marrow samples from Hispaniolan Amazon parrots and could serve as a general reference for psittacine bone marrow evaluation.
1 INTRODUCTION
Microscopic examination of bone marrow aspiration and core biopsy samples is the primary method of evaluating the hematopoietic system of vertebrate species. Bone marrow aspirates are optimal for assessing cellular morphology that allows for a more accurate overall assessment of hematopoietic cell lines, while core biopsies are the best for assessing overall cellularity, inflammatory lesions, and myelofibrosis.1 The ability to correctly identify hematopoietic abnormalities requires a knowledge of the normal morphology and distribution of bone marrow cellular elements.
There is a paucity of published information regarding avian bone marrow relative to mammalian marrow. Bone marrow cytology has been evaluated in neonatal and juvenile chickens, but samples were collected by scooping marrow directly from medullary cavities during necropsy and, thus, cellular yields might not be directly comparable with samples collected via current clinical aspiration techniques.2, 3 Reports of bone marrow cell morphology have been published for several other avian species, including the partridge (Alectoris Chukar),4 duck,5 pheasant (Phasianus colchicus),6 black-headed gull (Larus ridibundus),7 and Japanese quail (Coturnix coturnix japonica).8 Bone marrow cell morphology has been described anecdotally for psittacine species,9, 10 but to the authors' knowledge, studies using defined morphologic criteria with quantification of hematopoietic cell differentials are lacking. Most hematologic abnormalities reported in captive psittacines have been attributed to infectious diseases or stress associated with blood collection and captivity11, 12; however, hematologic disorders such as nonregenerative anemia, immune-mediated disease, and hemic neoplasia, among others, could warrant bone marrow examination.
The goal of this study was to determine hematopoietic cell differential counts and granulocyte:erythroid (G:E) ratios in bone marrow aspirate specimens from clinically healthy Hispaniolan Amazon parrots. We also wanted to create a reference of hematopoietic precursor images to guide the diagnostic evaluation of bone marrow aspirates in psittacines.
2 METHODS
2.1 Bone marrow and peripheral blood collection and sample preparation
Thirty-two Hispaniolan Amazon parrots (Amazona ventralis) from research colonies at Louisiana State University (LSU, n = 24) and the University of California-Davis (UCD, n = 8) were used in the study. Both research colonies originated from the same group of birds in Puerto Rico and were maintained under similar environmental conditions. Birds were housed alone or in pairs, in temperature-controlled (22-23°C) stainless steel cages, on a 12-hour light schedule. Birds were fed Kaytee pellets and provided with water bottles. No concurrent experiments were ongoing during this study. Experimental protocols were approved by the institutional animal care and use committees at LSU and UCD. Physical examinations were performed on the birds under manual restraint prior to sample collection.
Birds were anesthetized using a facemask with 2%-5% isoflurane in 1.75 L/min oxygen followed by intubation with a 3-mm endotracheal tube. Anesthesia was monitored using a stethoscope and by measuring the respiratory rate. Birds were placed on a heating pad and received a dose of butorphanol (5 mg/kg IM) and meloxicam (0.5 mg/kg IM) for analgesia.
The proximal tibiotarsus of one leg was aseptically prepared. A 1.5-inch, 22-gauge spinal needle was inserted into the tibiotarsus in a normograde manner parallel to the cortex from the tibial plateau approximate to the cnemial crest. The patella was pushed medially to avoid damaging tendons while inserting the needle. The stylet was removed, and a 3-mL syringe coated with 1% EDTA was attached. (In a pilot study on four pigeons (Columba livia), we found that 1% EDTA yielded superior cytologic samples compared with 1000 U/mL of heparin or no anticoagulant). Mild suction was applied until several drops of marrow were aspirated. If the initial aspiration did not yield any samples, the needle was repositioned by advancing or withdrawing it slightly while applying suction.
Drops of aspirated bone marrow were placed on 1-3 glass microscope slides, which were tilted to allow excess blood to run off. A squash prep technique was used to smear retained bone marrow spicules. Slides were quickly air-dried with a hairdryer or manual motion and stored at room temperature for 2-3 hours until stained with a modified Wright's stain using an automated slide stainer (Hematek, Siemens Inc; or Aerospray Hematology Pro, Wescor, Inc).
Peripheral blood samples were collected during the same anesthetic episode from the right jugular vein, placed in EDTA tubes (BD Microtainer, Becton Dickinson and Co.), and kept on wet ice until transferred to a refrigerator (4°C). A CBC was performed within 4 hours of collection. Packed cell volumes (PCVs) were determined using the microhematocrit method. Total WBC counts were determined using a hemocytometer with phloxine B13 (LSU) or Natt & Herrick's solution10 (UCD). Absolute leukocyte differentials were determined by counting 100 leukocytes on modified Wright's-stained smears. Differential leukocyte counts were determined by multiplying the percentage of each leukocyte type by the total WBC count.
2.2 Bone marrow cytologic assessments
Bone marrow samples were screened for cellularity and quality. Samples were considered adequately cellular if unit particles or a monolayer of hematopoietic cells was observed.9, 10 Samples were excluded if smears were excessively hemodiluted, too thick to visualize cell morphologies, or if a majority of the cells were lysed. Differential counts were obtained by examining 1-3 good-quality smears from each bird.
A 500-cell differential count was considered adequate for diagnostic bone marrow assessments, although 1000-cell differential counts could increase precision.1, 9, 10 In a preliminary assessment, 500- and 1000-cell differential counts of hematopoietic cells were compared on aspirates from two of the birds by a senior clinical pathology resident (DS) and a board-certified clinical pathologist (MC); differential cell counts did not differ, such that 500-cell differentials were used for the remaining samples. Representative images of hematopoietic cell stages were captured (Olympus DP25 digital camera with DP2-BSW imaging software, Center Valley, PA, USA) and used to guide differential cell counting. All smears were evaluated by a single individual (DS).
Erythroid precursors were classified as rubriblasts, prorubricytes, basophilic rubricytes, early and late polychromatophilic rubricytes, and polychromatophilic erythrocytes (Figure 1). Granulocytic precursors were classified as myeloblasts, progranulocytes, myelocytes, metamyelocytes, bands, and mature granulocytes (Figure 2). Heterophil, eosinophil, and basophil myelocytes, metamyelocytes, bands, and segmented cells were counted separately. Cells of the thrombocyte lineage could not be reliably identified and were not counted. Macrophages/monocytes, lymphocytes, plasma cells, and osteoblasts also were counted as part of the 500-cell differential count. Differential count results were expressed as percentages. A G:E ratio was calculated for each sample by dividing the percentage of all granulocytic lineage cells (including mature granulocytes) by the number of all erythroid lineage cells (excluding mature erythrocytes).9 Descriptive statistics (mean, SD, median, minimum, and maximum) were reported for each cell type (Excel 2011, Microsoft Corporation, Redmond, WA, USA).
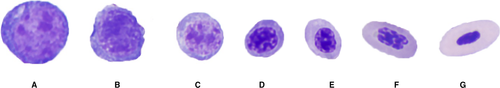
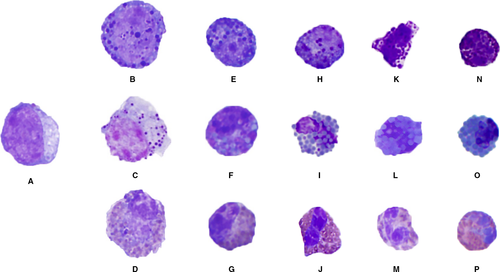
3 RESULTS
Of the 32 bone marrow samples collected and prepared, 17 were of sufficient cellularity and quality for inclusion into the study (13 from LSU and four from UCD). Color plates and descriptions of hematopoietic cell morphologies were recorded for referencing (Figures 1 and 2). Differential cell counts were tabulated (Table 1). Iron was not observed in any of the samples, although Prussian blue staining was not performed. Low numbers of macrophages, lymphocytes, and plasma cells, and rare osteoclasts were observed. The mean (± SD) G:E ratio was 0.4 ± 0.2 (median 0.4, range 0.1-0.9).
| Lineage | Cell type | Mean ± SD (%) | Median (%) | Minimum-maximum (%) |
|---|---|---|---|---|
| Erythroid | Rubriblast | 2.1 ± 0.6 | 2.0 | 1.0-3.4 |
| Prorubricyte | 5.1 ± 1.0 | 5.0 | 2.8-6.8 | |
| Basophilic rubricyte | 6.1 ± 1.9 | 6.6 | 4.0-11.2 | |
| Early polychromatophilic rubricyte | 20.3 ± 6.3 | 19.7 | 12.5-31.4 | |
| Late polychromatophilic rubricyte | 23.3 ± 5.4 | 23.0 | 15.6-33.0 | |
| Polychromatophilic erythrocyte | 11.5 ± 5.1 | 11.6 | 1.2-18.8 | |
| Total erythroid | 68.9 ± 8.6 | — | — | |
| Granulocytic | Myeloblast | 0.8 ± 0.6 | 0.6 | 0.2-2.7 |
| Progranulocyte | 2.6 ± 1.7 | 2.2 | 0.6-5.4 | |
| Myelocyte | ||||
| Heterophil | 2.6 ± 2.0 | 2.0 | 0.5-7.9 | |
| Eosinophil | 0.3 ± 0.1 | 0.0 | 0.0-0.5 | |
| Basophil | 0.4 ± 0.1 | 0.0 | 0.0-0.6 | |
| Metamyelocyte | ||||
| Heterophil | 3.2 ± 2.1 | 2.7 | 0.8-7.8 | |
| Eosinophil | Rarea | Rare | Rare | |
| Basophil | Rare | Rare | Rare | |
| Band | ||||
| Heterophil | 6.5 ± 3.4 | 5.9 | 2.2-12.0 | |
| Eosinophil | Rare | Rare | Rare | |
| Basophil | Rare | Rare | Rare | |
| Mature granulocyte | ||||
| Heterophil | 11.9 ± 5.2 | 10.8 | 2.4-18.6 | |
| Eosinophil | 0.5 ± 0.3 | 0.4 | 0.0-1.0 | |
| Basophil | Rare | Rare | Rare | |
| Total granulocytic | 28.1 ± 3.8 | — | — | |
| Other | Monocyte/macrophage | 0.3 ± 0.2 | 0.2 | 0-0.8 |
| Lymphocyte | 1.1 ± 0.7 | 1 | 0.2-3.0 | |
| Plasma cell | 0.5 ± 0.3 | 0 | 0-0.6 | |
| Osteoclast | Rare | Rare | Rare | |
- a Rare indicates < 0.1%.
Birds were deemed clinically healthy based on history and physical examination. CBCs were available for 15 of the 17 birds (11 from LSU and four from UCD) (Table 2). With minor exceptions (two birds had mildly decreased lymphocyte counts, two birds had mildly increased heterophil counts, and four birds had mildly decreased PCVs), CBC results were largely within expected reference limits for the population of captive Hispaniolan parrots at LSU.14
| Analyte (unit) | Mean ± SD | Median | Minimum-maximum | Reference valuesa |
|---|---|---|---|---|
| PCV (%) | 50 ± 5 | 48 | 44-60 | 47-60 |
| Total WBCs (/μL) | 9029 ± 2199 | 9217 | 6111-12 235 | 5800-15 900 |
| Heterophils (/μL) | 5346 ± 1680 | 4847 | 3390-9066 | 2300-7400 |
| Lymphocytes (/μL) | 2458 ± 1122 | 2111 | 1269-4690 | 1400-8100 |
| Monocytes (/μL) | 923 ± 383 | 775 | 435-1774 | 300-2300 |
| Eosinophils (/μL) | 184 ± 109 | 157 | 34-393 | 0-1000 |
| Basophils (/μL) | 118 ± 95 | 90 | 0-290 | 0-1000 |
- Abbreviations: SD, standard deviation.
- a From a population of 20 captive, nonanesthetized, Hispaniolan Amazon parrots.14
4 DISCUSSION
Hematopoietic cells of both granulocytic and erythroid lineages at all stages of development could be reliably identified via morphologic assessment of modified Wright's-stained bone marrow aspirate smears from Hispaniolan Amazon parrots. Bone marrows contained predominantly late-stage erythroid precursors; however, cells of the thrombocyte lineage could not be consistently identified. Low numbers of lymphocytes, plasma cells, and monocytes/macrophages were identified in bone marrow aspirates in this study, similar to findings reported in bone marrow aspirates from other avian species.8 These findings, and the color plates provided, will be useful for future clinical and investigative assessment of hematopoiesis in Amazon parrots, and potentially other psittacines.
While stages of thrombocyte development have been described in birds,9, 10, 15 we were unable to differentiate these precursors from erythroid precursors reliably. Thrombocytopenia is infrequently diagnosed in birds, in part due to challenges in enumerating clumped thrombocytes in smears. Thrombocytopenia has high clinical significance and has been documented in birds undergoing chemotherapy16 and as a complication of other medical interventions, notably fenbendazole toxicity.10 Cytochemical staining or immunophenotyping might help to distinguish cells of the thrombocyte and erythroid lineages in avian bone marrow, although technical issues with antibody cross-reactivity remain.17 Future research to identify cells of the thrombocyte lineage is warranted.
The predominance of erythroid cells resulted in G:E ratios <1.0, as has been reported in previous bone marrow studies of other avian species. Bone marrow from newly hatched to 8-week-old chicks had similar G:E ratios; however, mature erythrocytes were included in the bone marrow differentials and accounted for 38%-60% of all cells counted, such that these results are not comparable with those in our study.2 In a study of Japanese quails,8 the mean G:E ratio was 0.37, with a predominance of early polychromatophilic rubricytes, similar to our results. Pheasants6 and ducks5 were reported to have mean G:E ratios of 1.24 and 1.0, respectively; values higher than those in our study and similar to those expected in mammalian bone marrow.9 In this study, and possibly other studies relying on morphology for cell classification, some thrombocyte precursors also could have been misclassified as erythroid precursors, contributing to an erythroid predominance. In studies where thrombocyte precursors have been counted from a variety of different avian species, they accounted for <3% of nucleated cells.2, 6, 18, 19 While this might not be accurate, as cells were not verified with cytochemical stains, and morphologic identification is not definitive, this low percentage appears to be consistent with expectations for the thrombocyte series.
The unique histologic organization of avian bone marrow could also explain the predominance of erythroid cells in psittacines and other birds. Avian erythrocytes and thrombocytes develop intravascularly in the lumen of medullary sinuses, while granulocytes develop in the extravascular hematopoietic cords and must migrate through the sinus wall to enter the circulation.20 This segregation of erythroid and granulocytic elements can result in sampling bias when bone marrow is aspirated. Additionally, small sample sizes have been used to generate “normal” G:E ratios in other species,21-23 such that the true significance of small interspecies differences remains uncertain.
A few (n = 4) of the parrots in this study had mildly decreased PCVs compared with reference values, which could be attributed to circulatory alterations associated with isoflurane anesthesia, as has been previously described in other species.24, 25 The reference population of parrots had not been anesthetized for blood collection, and other sample-related, environmental, or physiologic differences with the study population could have accounted for the few CBC results slightly outside of the reference limits.14 Additionally, a small sample size was used to generate the reported reference values, such that confidence intervals around the reference limits are likely wide, affecting sensitivity and specificity. Thus, any potential CBC abnormalities were mild and likely attributable to pre- or post-analytical factors. This, together with the lack of clinical evidence of disease, made the probability of underlying hematologic disease very low.
A noteworthy observation our study confirmed the presence of different granule types and morphologies in avian progranulocytes. In contrast to progranulocytes in mammals, which by definition, contain only primary granules, psittacine progranulocytes also occasionally contained secondary granules, perhaps indicative of a later developmental stage. Previous morphologic descriptions of progranulocytes in birds described granules ranging from orange to basophilic to metachromatic, and as spherical or ring-shaped.9, 10, 15 It has been suggested that granule morphology might represent different intermediary stages of granulocyte development, or possibly even a unique organelle.15 Evaluation of the ultrastructure and chemical composition of progranulocyte granules could facilitate a more definitive classification of these cells; however, other features of avian progranulocytes allow them to be readily identified as an early, proliferative stage of granulocyte development, and a more specific classification might not alter the diagnostic assessment of avian bone marrow.
A limitation of this study was the small sample size, which precluded the calculation of reference intervals. Additionally, bone marrow smears from almost half of the birds sampled in our study were excessively hemodiluted, and therefore, inadequate for cytologic evaluation. Different techniques for preparation of bone marrow aspirates have been described1; in the authors' experience, the petri-dish protocol concentrates hematopoietic cells the best and facilitates separation of marrow spicules from blood in the squash prep. Furthermore, care must be taken to avoid applying excessive suction when aspirating or excessive pressure when smearing bone marrow samples to prevent cell rupture. In avian, and possibly other species, the choice of anticoagulant is an important consideration. Heparin is often used as an anticoagulant in avian and reptilian blood because of the cell lysis in EDTA in some species and to maximize sample volume; however, in the pilot for this study, heparin resulted in more cell lysis in bone marrow samples compared with EDTA.
In conclusion, our findings provide morphologic and quantitative data for hematopoietic cells in bone marrow aspirates from clinically healthy Hispaniolan Amazon parrots. These findings will be a valuable resource for the diagnostic evaluation of clinical bone marrow samples from Amazon parrots, and potentially, other psittacines.
ACKNOWLEDGMENTS
The authors thank Dr Terry Campbell (Emeritus Professor, Department of Clinical Sciences, Colorado State University) and Dr Nicole Stacy (Department of Large Animal Clinical Sciences, University of Florida) for their input on the classification of hematopoietic cells in the photographic color plates. We also thank Dr Joao Brandao (Assistant Professor, Veterinary Clinical Sciences, Oklahoma State University) and Tracy Drazenovich (Research Assistant, University of California-Davis) for assistance in bone marrow collection, Mal Hoover (Medical illustrator, Kansas State University) for her help in reformatting our photographic color plates, the Kaytee Avian Foundation for their support of the Amazon parrot colony at Louisiana State University, and the Richard M. Schubot Parrot Welfare and Wellness Program at the University of California-Davis.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to study design, data analysis and interpretation, and preparation and approval of the manuscript. DG and HB contributed additionally to sample collection. DS contributed additionally to sample evaluation and preparation of the photographic color plates.




